Abstract
Aminocarboxylic acid (monoamine-based) chelating agents such as GLDA, MGDA, NTA, and EDG are widely used in a variety of products and processes. In the European Union, based on the Green Deal and the Chemicals Strategy for Sustainability (CSS), there is an increasing tendency to speed up chemical hazard evaluation and to regulate chemicals by grouping substances based on molecular structure similarity. Recently, it was proposed to group polycarboxylic acid monoamines, hydroxy derivatives and their salts with monovalent cations, and to consider all group members as potential carcinogens based on the official CLP classification of one group member, viz. NTA, which is classified as suspected carcinogen Cat. 2. In this review, we show that a grouping approach for harmonized classification and labeling based on molecular structure alone, disregarding existing animal test data as well as current scientific and regulatory knowledge, would result in incorrect classification. Using such a simplistic, although considered pragmatic approach, classification of all group members upfront would not improve protection of human health. Instead, it could not only lead to unnecessary additional vertebrate animal testing but also to onerous and disproportionate restrictions being placed on the use of these valuable substances; some of these even being considered as green chemicals.
1. Introduction
Because of a general opinion that hazard evaluation of every individual chemical is taking a long time it has been proposed in the European Chemical Strategy for Sustainability (CSS) to use grouping approaches in order to speed up the regulatory process, viz. to regulate groups of chemicals with potential similar mode of action and endpoints, based on similarity of molecular structure.
Several grouping proposals have been published by ECHA since then, as so-called assessments of regulatory needs (ARN) which, although these are stated not to have direct regulatory consequences, strongly determine the potential regulatory roadmap for assessment by ECHA (see also Natsch et al. Citation2023).
Recently, the European Chemicals Agency (ECHA) also proposed a group Polycarboxylic acid monoamines, hydroxy derivatives and their salts with monovalent cations, based on the presence of a monoamino backbone and mono- to poly-carboxylic moieties (ECHA Citation2022). According to this ARN, this group would consist of 25 chelating substances. Chelating substances are used to bind multivalent metal ions in a wide variety of industrial and consumer applications. In this group of 25 substances, only six substances have a full REACH registration and publicly available information; seven substances have a full registration but without publicly available information; six substances are registered as NONS (thus also no publicly available information); three substances are registered as intermediate (thus no repeated exposure study data required); two only have a classification and labeling notification (thus not in use and no data available); and for one substance production has been ceased. As can be seen in the ARN document, all sorts of molecular structures (including phenyl groups) have been included but there is no underlying toxicological mechanistic reason given why the common presence of the selected structural determinants should lead to a common toxicological outcome of these molecules.
However, the fundamental basis for grouping is of course a common mechanism of action and similar endpoints. In contrast to ECHA’s ARN approach, also, Annex XI of the European REACH regulation 1907/2006/EU clearly defines toxicological groups with an emphasis on similar toxicological properties as a result of structural similarity or a common metabolite. This definition is based on the toxicological knowledge that in specific cases molecules with similar functional reactive groups, and similar overall structure (or common metabolites) lead to similar apical outcomes in case these structural features are the basis for a shared toxicological mode of action (Natsch et al. Citation2023).
Also, in contrast to ECHA’s ARN grouping approach, ECHA’s Read-Across Assessment Framework (RAAF; ECHA Citation2017) indicates: any read-across approach must be based on structural similarity between the source substance(s) (reference substances for which tests have been conducted) and target substance(s) (other substances that have not been tested). However, structural similarity alone is not sufficient to justify the possibility of predicting properties of the target substance(s) by read–across and a read-across hypothesis needs to be provided. The substantial requirements for chemical grouping and transparency in the read-across hypothesis are key (Wohlleben et al. Citation2023). This review summarizes why a grouping approach based on structural similarity (alone) as done in the ARN cannot be justified for this specific group of chelating agents.
The chemicals in this group can be considered (weak) chelating agents. The ability of chelating agents to remove and add ions to a solution, thus by affecting mineral balance, is the main mechanism whereby these chemicals exert their effects. For this paper, we have selected the four structures with available toxicity information viz. EDG, NTA, MGDA, and GLDA. EDG and MGDA have been REACH registered only as sodium salts, whereas NTA and GLDA have been registered both as sodium salt and acid. The structural formulas of these four chelating agents are indicated in .
Table 1. Monoamine based, aminocarboxylic acid chelating agents.
In the EU, NTA has a harmonized classification as carcinogen cat. 2 with a specific concentration limit of 5% due to low potency; NTA is listed by IARC as carcinogen cat. 2B. Regarding MGDA, it was indicated in the ARN that the available carcinogenicity study raises some uncertainties. For GLDA it was mentioned there is no data available to allow a conclusion on the tumorigenic potential but that data from repeat dose toxicity studies indicate the same target system/organ as NTA (i.e. the kidney) and that therefore, a carcinogenic potential cannot be excluded.
In the next sections a short summary is provided on structural and physicochemical properties, information on toxicokinetics and solubility, repeat dose toxicity, carcinogenicity, and genotoxicity data (where available) of each of the substances, and an assessment of urinary biomarkers of kidney toxicity for NTA, MGDA, and GLDA.
2. Physicochemical and structural considerations
All members of this aminocarboxylic acid (monoamine-based) chelants group have a molecular structure with a monoamine backbone with two (EDG) or three acetic acid groups attached to the nitrogen. GLDA has one additional COOH group (). It is the presence of multiple carboxylic acid groups and the amine that provides chelating agents with their unique metal ion chelating or sequestering properties.
Under neutral pH conditions all carboxylic groups of the chelates are deprotonated, whereas all amine groups are protonated, which leads to a betaine-type structure and results in the presence of a monovalent anion EDG1−, bivalent anions NTA2− and MGDA2−, and a trivalent anion GLDA3−. Due to the repelling nature of similar charges, the anionic arms of the chelates can be assumed to be maximally stretched in solution in the absence of multivalent metal-ions. The same applies to the stronger chelate EDTA.
By definition Denticity is the number of atoms in a ligand that can bind to the central metal ion; in the presence of multivalent metal-ions, the aminocarboxylates immediately form complexes releasing the protons bound to the nitrogen atom. For NTA and MGDA the number of atoms is four, so these are tetradentate ligands; for EDTA this is generally six, thus EDTA is a hexadentate ligand. For EDG and GLDA this is less clear: Although EDG also seems to have 4 atoms that could bind the metal ion, in practice the ethanol group (CH2CH2OH) is not suitable to bind the central metal ion except at very high pH. Thus, EDG will only bind the metal ion via the N atom and two COO− groups and as such will be a tridentate ligand. GLDA has the N atom and four COO− groups but in practice it seems that the most far away COO− group does not bind the central metal ion which means that GLDA would also act as a tetradentate ligand, just like MGDA and NTA; this is also reflected by comparable complex (thermodynamic) formation constants for these three chelants (). However, compared to the hexadentate chelating agent EDTA, the monoamine based chelates NTA, MGDA, and GLDA are moderate chelating agents, whereas EDG is only a weak chelating agent. Note that this table has a logarithmic scale; as an example, for zinc the complexing power of the weak/moderate chelates is several orders of magnitude lower (ca. 100,000× or more) than for EDTA which influences their physiological effects as well.
Table 2. Thermodynamic stability constants (log K values).
Acid (H+) or monovalent salt (Na+) chelates are considered uncomplexed chelates and are called “empty” chelates. When no hydrogens have been substituted (NTA and GLDA acid), the chelant exists as an inner salt or zwitterion. The uncomplexed chelants (the acids) would be expected to add H + ions to media (which would lead to decreased pH) and would chelate metals present in their milieu based on affinity (). The highest affinity is for Fe3+, the lowest affinity for Mg2+. The order of affinity for the metal ions is the same for all five chelants, but with EDG showing the lowest values, NTA, MGDA, and GLDA showing comparable values, and with the highest values for EDTA as indicated above.
For a physiological system the conditional complex-formation constants are more important compared to the above mentioned thermodynamic stability constants ().
Table 3. Conditional complex building constants for EDG, NTA, MGDA, GLDA and EDTA.
All metal complexes pass through a more or less pronounced maximum of the conditional complex-formation constants as a function of the pH. Thus, the specificity for certain metal ions in the physiological compartment can only be approximated by using the pH-dependent, conditional stability constants. With increasing pH, ionization increases and the formation of certain complexes is enhanced. Physiological pH values, however, are between pH 1–2 in the stomach (pH 3–4 in the rat stomach), pH 6–8 in the digestive tract (intestines), pH 4 in cell compartments such as lysosomes, pH 7.4 in blood plasma, and pH 6.5 in urine. Therefore, the nature of metal-complexes may change during passage through the body. Concentrations of chelates in physiological compartments are low under normal use conditions so that sufficient metal ions are available for complexation. However, in experimental studies with laboratory animals at very high (artificial) doses of chelates, it is possible to exceed the available amounts of multivalent metal ions in feed, drinking water or the body fluids (e.g. saliva, gastric juice, blood, bile) and thus to induce a metal ion depletion.
Mineral homeostasis in a physiological, mammalian system is highly complex. Therefore, the impact of a high dose of a chelating substance on such a system, in which several substrate-cations, such as calcium and zinc, are present at the same time and in different concentrations, is hardly predictable. Here, thermodynamic complex formation constants to single metal ions can only be used to approximate general differences between different chelating agents; a closer approximation whether chelating agents are selective for certain ions in a physiological situation requires a closer look at the conditional stability constants as well as the respective physiological compartment. It is known, for example, that disturbance of the mineral homeostasis, i.e. a selective ion-deficiency, such as zinc deficiency, can induce severe effects depending on the extent and the duration of the deficiency. To estimate whether chelating agents, such as EDG, NTA, MGDA, and GLDA would have different abilities or potencies to induce a disturbance in essential metal homeostasis (e.g. zinc) in a complex physiological system, conditional stability constants toward ions such as calcium ions, which are present at a high concentration in such environments, have to be taken into account.
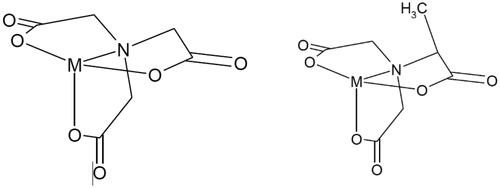
In addition, when comparing molecular size also, regarding MGDA and NTA, the additional methyl group of MGDA is expected to stick out, enlarging the three-dimensional structure of the complex (enlarging the stereochemistry ), which may result in steric hindrance influencing the characteristics of the substance, including intestinal absorption. It was not further investigated which factor, viz. size and shape or stereochemistry, would play the most important role.
Figure 1. Metal-complexes of NTA (left panel) and MGDA (right panel). Due to steric hindrance the additional methyl group in MGDA is expected to stick out enlarging the overall three-dimensional size of the metal complex.
Calcium is abundantly available in blood and urine which means that this calcium will be readily bound by the chelates. However, regarding solubility, there is a clear difference between these chelates when bound to calcium. EDG-Ca and NTA-Ca are not soluble, whereas MGDA-Ca and GLDA-Ca are. Solubility in water was measured at 37 °C and pH 7 and the results are indicated in (column 1).
Table 4. Solubility of Ca-bound chelating agents.
The solubility of MGDA-Ca and NTA-Ca was confirmed in a second experiment by solubility studies, mimicking the physiological situation in the urinary tract. To simulate the concentration of chelate in the presence of physiological Ca-ions in the urinary tract, 0.4% of either MGDA-Ca or NTA-Ca was stirred in open vessels at neutral pH at 37 °C so that the water could evaporate over time and resulted in metal-complexes in solution. As soon as any opacities were observed the concentration of chelate was determined (, column 2), showing that MGDA-Ca has a ca. 30-fold higher solubility than NTA-Ca. The lower solubility of NTA-Ca, compared to MGDA-Ca, may be related to the fact that in case of higher symmetry (NTA) it fits better in a crystal structure, and as such will be less soluble.
To assess crystallinity, samples of the Ca-chelates, after grinding, were placed in a standard sample holder for roentgen diffraction (XRD). The databases searched for the model compounds were the inorganic crystal structure database (ICSD) and the crystallography open database (COD). NTA-Ca and EDG-Ca were highly crystalline, 88 and 89%, respectively in contrast to GLDA-Ca with ca. 10% and MGDA-Ca with ca. 6%.
Overall, from the physicochemical and structural considerations the following conclusions can be drawn:
Molecular and complex charge: EDG < NTA ∼ MGDA < GLDA ∼ EDTA
Complexing power: EDG < NTA ∼ MGDA ∼ GLDA ≪ EDTA
Molecular size and shape: EDG < NTA ∼ MGDA < GLDA < EDTA
Complex shape and size (stereochemistry): EDG < NTA < MGDA < GLDA ∼ EDTA
Solubility of the Ca-chelate: EDG ∼ NTA ≪ MGDA ∼ GLDA ≪ EDTA
Crystallinity of the Ca-chelate: EDG ∼ NTA ≫ GLDA ∼ MGDA
3. Toxicokinetics (incl. SPECIES differences)
Dermal absorption of these empty chelates is negligible due to their size and charged structures. Upon inhalation, the large particle size leads to deposition in the upper airways and clearance via the gastrointestinal tract. Oral absorption rates have been determined for all aminocarboxylate-based chelating agents in rats and for some of the chelates also in other species; there are marked differences:
EDG: In a rat feeding study EDG was readily absorbed with 85% of the ingested dose voided in the urine and less than 10% recovered in feces (Anonymous Citation1985).
NTA: Upon dietary administration, NTA was absorbed to a lower rate than upon bolus administration. In humans and rhesus monkeys, the intestinal absorption was 12 and 14%, respectively, whereas 23% was absorbed in rabbits and 40–80% of NTA in rats and dogs depending on the type of administration (Budny Citation1972; Budny and Arnold Citation1973; BASF Citation1997a; SCCS Citation2010).
MGDA: Male rats receiving MGDA via gavage (single administration of 25 and 500 mg/kg bw; or seven consecutive administrations of 500 mg/kg bw/day) showed urinary recovery rates of 17% at the low dose, 21% at the high dose, and 33% using the repeated high dose scheme. Equimolar doses of NTA resulted in urinary recovery rates of 54, 41, and 48% (BASF Citation1997b, Citation2015).
GLDA: Urine samples collected in rats at the end of a 90-day oral gavage study showed levels of 2 and 1.3% of the nominal intake in males and females, respectively (Anonymous Citation2007). An absorption study (single gavage dose of 1000 mg/kg bw) in rats revealed an absorption up to 5% (Anonymous Citation2014).
EDTA: Only 5–20% EDTA was absorbed in rats after a single gavage administration of 50 mg/kg bw. The amount absorbed in 24 h determined from the quantity of the compound found in the tissues and urine varied from 2 to 18%, most of the values being in the range of 2–4%. About 99% of the EDTA absorbed appeared unchanged in the urine. An additional i.v. study with kidney ligation showed a steady state of EDTA in the blood, which supports that renal excretion is the only pathway of EDTA removal from the body (Foreman et al. Citation1953).
In conclusion oral absorption of the chelating agents differs significantly: ∼85%, ∼50%, ∼25%, ∼5%, and ∼4% for EDG, NTA, MGDA, GLDA, and EDTA, respectively; this order in oral absorption rate is in full alignment with the increase in size and/or charge, and thus there is a clear correlation between the membrane permeability of an aminocarboxylate-based chelate and its size, shape, and charge.
Besides the differences in absorption among the chelates there may also be species differences per chelate resulting in different plasma concentration which is the most relevant parameter for the renal findings described below. Regarding NTA, which—besides EDG—is the smallest and has the highest oral absorption rate, an oral dose of 20 mg/kg bw in dogs resulted in a plasma peak level of 15 mg/L whereas in humans a ∼130-fold lower dose of 100–170 µg/kg bw resulted in a peak of only 6.5 µg/L which is 2300-fold lower than in dogs and thus reflecting a species difference in bioavailability of a factor ∼20 (Michael and Wakim Citation1971; Budny Citation1972; Budny and Arnold Citation1973; Rubin and Martell Citation1980; Anderson et al. Citation1985). This may indeed indicate a lower sensitivity of humans compared to dogs and rats; however, it is not clear whether the percentage absorbed is related to species differences only or also due to quite different dosing amounts.
4. Short overview of repeated dose toxicity, mutagenicity and carcinogenicity data
See for a summary of these data .
Table 5. Repeat dose toxicity data and data on genotoxicity of EDG, NTA, MGDA and GLDA.
4.1. EDG-Na2
There is no chronic/carcinogenicity study available for EDG-Na2. Oral administration of EDG-Na2 to rats for 90 days revealed a NOEL of 41 mg/kg bw/day based on reversible histopathological kidney findings observed in high dose group animals. EDG-Na2 was non-mutagenic in all the tests performed.
4.2. NTA-Na3
NTA-Na3 and NTA-Na3.H20 (CAS No. 1866-53-8; considered equivalent to NTA-Na3) were administered to mice and rats in various carcinogenicity studies.
In summary, NTA is carcinogenic in both sexes of rats and mice via the oral route. NTA-Na3 associated development of tumors was mainly seen in the urinary system, where NTA-Na3 induced primary tumors of different origin at several locations in the urinary tract. Tumors in the rat kidney originated from the tubular-cell epithelium and from the pelvic transitional cell epithelium. Tumors from the transitional cell type were also found in the ureter and the urinary bladder. In mice, tumors originated from the renal tubular epithelium and occasionally from the renal pelvis. Results derived from ten in vitro and two in vivo genotoxicity tests showed that NTA does not appear to exert genotoxic effects (https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14301/7/7/1). In certain target tissues (e.g. kidney) high NTA concentrations may form NTA-Zn, and in doing so deplete zinc, for which an aneuploidic effect may not be excluded. Therefore, a direct genotoxic mechanism of NTA-Na3 carcinogenesis could not be demonstrated and as such a threshold could be derived. The lowest dose of NTA-Na3 causing one bladder papilloma was established as 92 mg/kg bw/day (NCI Citation1977); doses of NTA-Na3 required to induce a significant increase of renal tumors are ca 10-fold higher (depending on the type of the study).
4.3. MGDA-Na3
MGDA did not cause an increased incidence of neoplastic lesions in the test animals and can thus be considered non-carcinogenic. Non-neoplastic lesions of higher severity consisted of chronic nephropathy, cytoplasmatic vacuolization, pigment storage in tubular epithelial cells, (multi)focal tubular hyperplasia, and (multi)focal ectasia of ducts or vessels in the papillary region. The observed renal effects, such as vacuolation and hyperplasia in the renal epithelium can be considered reversible as shown also for NTA (Myers et al. Citation1982). No treatment-related findings were noted in the ureter. Urine sediment examination revealed an increased number of red blood cells in males of the high dose group whereas in NTA-treated rats, an increased number of unidentified crystals (females) and a higher number of erythrocytes and leukocytes were found (BASF Citation2006). MGDA-Na3 was shown non-mutagenic in several tests.
4.4. GLDA-Na4
In the repeated (90-day) oral toxicity study, there was no evidence of an effect on kidney function and no morphological correlate was obtained for the slightly higher absolute and relative kidney weight in males at this dose. Given the minimal findings at the high dose, a NOAEL of 300 mg/kg bw/day was established (Anonymous Citation2007). GLDA-Na4 was found to be non-mutagenic in several tests.
5. Kidney biomarker study
A kidney biomarker study was performed to compare the renal toxicity of NTA versus GLDA and MGDA using urinary biomarkers and renal cell proliferation as early predictors of nephrotoxicity (HIHS Citation2013). HIHS was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) and the study protocol was approved by the HIHS Institutional Animal Care and Use Committee (IACUC). Groups of male Wistar rats (10/group) were administered 1000 mg/kg bw/day of each of the sodium salts of NTA, MGA or GLDA via oral gavage for 28 days. The sodium salt of EDTA (EDTA-Na4; CAS No. 64-02-8) also at 1000 mg/kg bw/day, was used as negative control, and water as vehicle control. Endpoints evaluated included clinical observations, body weights, food and water consumption, urinalysis, gross pathology, kidney weights and histopathology, urinary protein biomarkers, and renal cell proliferation.
Mild diarrhea was observed in animals of all groups. Two animals of the NTA group were euthanized as moribund on Study Day 13. Mean body weight and food consumption were statistically significantly lower in the NTA group during the first two weeks; water consumption was reduced in the first week.
There were no changes in urine volume and pH between the groups. Mean urine sodium concentrations were statistically significantly elevated but comparable in all test groups at the weekly measurements (ca. 2.4–2.8× control; ). Mean urine calcium levels were statistically significantly higher in the NTA and MGDA groups (1.7× and 2.1×, respectively); mean calcium level was unchanged in the GLDA (1.0×) and EDTA (0.9×) groups. Throughout the study, a statistically significant lower mean urine magnesium level was observed in the NTA, MGDA, and EDTA groups (0.3×, 0.7×, and 0.6×, respectively). Urine zinc concentrations were higher at each weekly examination; these ranged from 2.4× to 7.5× compared to control with fold-changes being the highest in the NTA and MGDA groups and the lowest in the GLDA group.
Table 6. Levels of (metal)ions in urine compared to controls (biomarker study; HIHS Citation2013).
Levels of urine total protein and LDH were statistically significantly higher at some time points in the NTA group. The higher value of LDH on Day 28 in the EDTA group was statistically significant as were the higher values of urine total protein, ALP and LDH in the MGDA group (data not shown).
With respect to urine protein biomarkers, the mean level of kidney injury molecule 1 (Kim-1), a sensitive and early predictive biomarker of kidney injury/repair, was statistically significantly higher in the NTA and MGDA groups when compared to the control group. The mean urine CLU level was statistically significantly higher in the NTA group, as well as the mean levels of CLU, CAL, OST, and rat EGF-R in the MGDA group. None of these biomarkers were increased in the GLDA group. Additionally, there was a statistically significant higher value of GST-alpha in the NTA and MGDA groups at all weekly measurements, and in the GLDA group on days 7, 14, and 21. GST-alpha levels ranged from 2.2- to 7.3-fold of control means with the highest change in the NTA group and the lowest in the GLDA group ().
At necropsy, minimal gross lesions were observed. A statistically significant higher mean relative kidney weight was observed in the NTA and MGDA groups; the increase ranged from 15 to 25% above control mean values. Results of kidney histopathology did not reveal evidence of test substance-related effects. With respect to renal cell proliferation, a statistically significantly higher mean BrdU labeling index of cortical proximal tubular cells was observed in the NTA group; a higher mean index was also observed in the MGDA group but statistical significance was not reached ().
In summary, the NTA group exhibited decreases in body weight gain, food and water consumption, and urinary magnesium, and increases in kidney weights, levels of urine calcium, total protein, LDH, Kim-1, CLU, and GST-alpha. The NTA group also showed a statistically significant increase in renal proximal tubule cell proliferation. Several of these findings albeit less pronounced were also noted in the MGDA group; some of the above renal effects were seen in the EDTA group. None of these changes were seen in the GLDA group except for a smaller increase in GST-alpha.
6. Discussion and conclusion
NTA was classified as suspected human carcinogen (Cat. 2; H351) due to its non-genotoxic and threshold-based mechanism, viz. concentration-dependent cytotoxicity in the urinary tract. For low potency carcinogens specific concentration limits of 1–5% have been proposed depending on differences in species sensitivity (human < experimental animals) and high T25-values. In case of NTA both prerequisites were fulfilled to a sufficient degree; hence, in the EU a regulatory SCL of 5% was set for mixtures containing NTA.
In experimental animals, based on all investigations on NTA (subchronic, chronic, and mechanistic studies) an overall NOAEL of 15 mg/kg bw/day was established. Tumorigenic doses were at least 10-fold higher; in rat feeding studies no enhanced tumor incidences were obtained at 0.2% NTA in the diet (92 mg/kg bw/day; Nixon et al. Citation1972). In a subsequent feeding study, NTA caused one kidney adenoma and one bladder papilloma at 355 mg/kg bw/day and a steep increase in the dose-response relationship between 526 and 1053 mg/kg bw/day. Dietary studies in mice did not show tumorigenic effects at dose levels of 169 and 339 mg/kg bw/day. The lowest dose level showing tumors (kidney) was about 100 mg/kg bw/d in a drinking water study (Goyer et al. Citation1981; Anderson et al. Citation1985).
In terms of classical hazard assessment, however, drinking water administration of NTA-Na3 enhanced the vulnerability of the animals in an artificial manner. Whereas in NTA-Na3 feeding studies the animals doubled their drinking water consumption in response to sodium and NTA was diluted in the primary urine, this compensatory mechanism is blocked when NTA-Na3 solution was the only fluid supply; thus, the animals had an alkaline solution as their only drinking source. Because the kidney is strongly involved in the pH regulation of the whole organism, the NTA-Na3 exposure consistently resulted in a high urinary pH and, in consequence, a higher NTA binding to calcium under alkaline conditions (Leibold et al. Citation2002). Therefore, under those artificial conditions and as explained above, NTA would exert a stronger cytotoxic and carcinogenic activity. Overall, dose levels in the order of 50 mg NTA/kg bw/day did not result in increased tumor incidences.
As indicated in the ARN, according to ECHA (Citation2022), all group members would have common target organ toxicity (kidney, urinary tract) with potential for carcinogenicity based on positive carcinogenicity studies with NTA and indications for some substances showing hyperplasia in the same target organ; and thus, also according to ECHA, due to structural similarity and the common observed effects, all substances would need to be considered as potential cat. 2 carcinogens. Even, for MGDA, for which a negative carcinogenicity study is available, it was concluded that [.] in spite of the uncertainties concerning the potential for carcinogenicity for MGDA, the available information indicates potential for repeated dose toxicity (STOT RE (kidney)) due to findings in a combined chronic and carcinogenicity study (OECD TG 453), showing chronic nephropathy in most males and females with highest gradings in animals treated with top dose, comparing to the positive control substance. In conclusion, the test item showed specific renal toxicity, it was less toxic than the positive substance administered for comparison. Moreover, the test item was not carcinogenic, whereas the positive substance caused tumors in renal pelvis and testes. However, similarity on the target organ (urinary system) for both test and positive control substances, indicates a similar mode of action and potency differences could be taken into account. On that basis, classification potential as Carc. 2 cannot be excluded [.].
Regarding the testes tumors seen in the positive control (NTA) of this study (BASF Citation2006) it should be noted these were adenomas and not carcinomas, and it was not the only type of tumors noted in long-term studies with NTA: phaeochromocytomas and hepatocellular adenomas were observed in female rats and adenocarcinomas of the preputial glands in males (NCI Citation1977), and pituitary tumors in male rats (Goyer et al. Citation1981). However, only one sex was affected in each case, these tumor types were not consistently found among all studies and were observed at doses already within the nephrotoxic range, most of these tumor types occur spontaneously, and the increases were not statistically significant and dose-response relationships were absent. For NTA, a reproducible spectrum of tumors was only found in the kidneys and epithelia of the lower urinary tract.
Nevertheless, according to the agency, presence of hyperplasia (“common observed effects”) in the same target organ (kidney) and structural similarity would be sufficient to conclude (although with “some remaining uncertainty”) a potential for carcinogenicity? And “potency differences could be taken into account” even if a full-blown carcinogenicity study with MGDA has shown no carcinogenicity at levels above 1000 mg/kg bw/day? Moreover, similarity in target organ would indicate a similar mode of action?
There could be an initial concern because EDG, NTA, and MGDA have all shown kidney toxicity ().
Figure 2. Overview of urinary tract findings induced by EDG, NTA, MGDA, GLDA and EDTA. *Read-across to NTA as Ca-microcrystal formation is likely due to the very low solubility of EDG-Ca and because marked renal toxicity was noted in vivo.
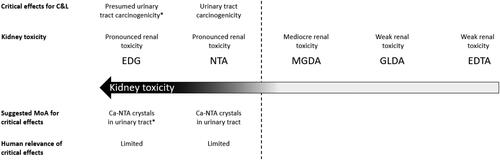
The kidney toxicity induced by GLDA at the testing limit dose of 1000 mg/kg bw/day in the 90-day study was very limited and not even corroborated by histopathological renal findings. As mentioned above, even for MGDA which structure is closest to NTA, there were marked differences in complex size and shape, complexing power, oral absorption rates and related maximum plasma peaks. And, thus, although the target organ for systemic toxicity is similar for NTA and MGDA, the severity of renal toxicity was much lower for MGDA. Even more obvious, no kidney tumor formation was observed in a combined chronic toxicity/carcinogenicity study at a dose higher than the testing limit dose of 1000 mg MGDA/kg bw/day.
In the kidney biomarker study (HIHS Citation2013), the chelating agents NTA, MGDA, and GLDA were not tested at equimolar doses but tested at the limit dose of 1000 mg/kg bw/day; this limit is applicable because there is no foreseeable human exposure for these chelates indicating no need for a higher dose level to be tested. EDTA was used as negative control. Based on molecular weight, 1000 mg/kg bw/day corresponds to 3.89 mmol/kg bw/day for NTA, 3.69 mmol/kg bw/day for MGDA, 2.85 mmol/kg bw/day for GLDA and 2.63 mmol/kg bw/day for EDTA (MW: 380). Based on the percentage oral absorption (50% for NTA, 25% for MGDA, and ∼5% for GLDA/EDTA, respectively; see Section 4) internal doses would be maximally 1.95 mmol/kg bw/day for NTA, 0.92 mmol/kg bw/day for MGDA, 0.14 mmol/kg bw/day for GLDA and 0.13 mmol/kg bw/day for EDTA, or in other words, compared to NTA, a two-times lower internal molar dose for MGDA and 15-times lower molar doses for GLDA and EDTA. It should also be noted that these chelating agents are not (bio)transformed and excreted fast (no bioaccumulation).
So, based on the calculated 15-times higher internal molar dose, there might be a clear explanation for the higher kidney toxicity of NTA versus GLDA/EDTA as in this biomarker study kidney toxicity of GLDA and EDTA was very limited; the same applies to the higher kidney toxicity of NTA versus MGDA (two-times higher internal molar dose). Also, the 15-times higher internal molar dose for NTA and the seven-times higher internal molar dose of MGDA can explain the 2- to 3-fold higher excretion of zinc in the urine compared to GLDA and EDTA () despite the much higher binding affinities of GLDA and EDTA for zinc compared to NTA and MGDA ().
As indicated above, mineral homeostasis in a physiological, mammalian system underlies complex mechanisms, influenced by the chelating substance with its binding affinities in the respective microenvironment, but also on the amounts of several substrate-cations present, such as calcium and zinc. Concurrence with calcium ions is of high importance because blood calcium levels in rats are approximately 100-fold higher than zinc levels in untreated animals ().
Table 7. Levels of sodium, calcium and zinc in blood and urine of untreated rats.
Correspondingly, the recommended daily allowances in rats for calcium (RDA 5000 mg/kg feed) are approximately 400-fold higher compared to zinc (RDA 12 mg/kg feed); however, in practice calcium levels are ∼100-fold higher because standard diets generally contain 50-60 mg Zn/kg diet as to accommodate for higher zinc requirements in pregnant rats (NRC 1995). However, in situations in which the extracellular zinc pools are depleted, e.g. after high doses of the chelating agents which have a higher affinity for zinc, calcium-complexes are formed instead which are excreted renally (Havlicek Citation1967; Michael and Wakim Citation1973; Cohen and Guilmette Citation1976; Anderson Citation1981; Powell et al. Citation1999). On a molar basis, sodium, calcium and zinc levels in urine were comparable to those in blood ().
Toxicogenomic approaches have identified protein biomarkers of renal cell injury/repair as early predictors of kidney toxicity prior to changes in kidney histopathology (Hoffmann et al. Citation2010; Ozer et al. Citation2010; Harpur et al. Citation2011). In the 28-day biomarker study (HIHS Citation2013), there were numerous significant early indicators of kidney injury in rats administered NTA, less so for MGDA, not for GLDA and EDTA, and no changes in kidney histopathology. Mean relative kidney weight was increased, and several urine analytes were altered in the NTA group, and urine protein biomarkers, such as Kim-1, CLU, and GST-alpha were statistically significantly higher (). Kim-1 and CLU are inducible proteins compared to GST-alpha which is a constitutively expressed protein. Also, renal cell proliferation was significantly higher following NTA administration (), though kidney histopathology did not indicate microscopic evidence of cell injury in this 28-day study.
Table 8. Levels of kidney biomarkers in urine compared to controls (biomarker study; HIHS Citation2013).
Table 9. Mean labeling indices (LI) for kidney cortical proximal tubular cells (biomarker study; HIHS Citation2013).
Hoffmann et al. (Citation2010) stated that urinary Kim-1 and CLU reflect changes in gene/protein expression and histopathological alterations in the kidney in the absence of functional changes and further concluded that Kim-1 and CLU are early and sensitive noninvasive markers of renal injury. Kim-1 and CLU are approved by the US-FDA to evaluate kidney damage in animal studies. Results of the current kidney biomarker study are in agreement with the statements made by Harpur et al. (Citation2011) and Hoffmann et al. (Citation2010). The usefulness of GST-alpha as a biomarker of chemical-induced renal toxicity was reviewed by Harpur et al. (Citation2011). Overall, this urinary protein biomarker study showed that NTA, and MGDA to a lesser extent, caused clear and significant early renal cell toxicity and renal cell proliferation but without microscopic evidence of renal cell injury. The order of highest to lowest severity of renal findings for the test substances studied for 28 days was NTA > MGDA≫GLDA = EDTA.
Thus, in this 28-day study in which rats were administered an equivalent absolute dose of MGDA and NTA, kidney toxicity and renal injury biomarker results were slightly less for MGDA, likely because of the at least two-times lower internal molar dose. However, the outcome of the two-year carcinogenicity studies with these two compounds was clearly different. Treatment-related kidney tumors were only found in rats dosed with NTA but not with MGDA: the lowest dose of NTA causing tumors was established as 92 mg/kg bw/day (NCI Citation1977) whereas no treatment-related tumors were noted at levels up to 1132 mg MGDA/kg bw/day in males, and up to 1317 mg MGDA/kg bw/day in females (BASF Citation2006). Also, no carcinogenicity was observed for EDTA at levels of up to close to 1000 mg/kg bw/day (see further below). This indicates that besides the different absorption rates and related plasma concentrations other factors may have played a role.
As indicated in Section 4, NTA-related tumors were not only found in the kidneys of rats; treatment with NTA also resulted in primary tumors at several locations in the urinary tract. Tumors in the rat kidney originated from the tubular-cell epithelium and from the pelvic transitional cell epithelium whereas tumors from the transitional cell type were also found in the ureter and the urinary bladder. Also, transitional cell hyperplasia was noted in the ureter and in the urinary bladder. According to IARC (Citation1999), the nephrocarcinogenic effects of NTA in rats and mice appear to be related to dose-dependent changes in Zn2+ homeostasis; the toxicity occurs at high doses and appears to be due to Zn2+ accumulation secondary to the chelating properties of NTA. Administration of NTA-Zn or Zn2+ accentuated the nephrotoxicity of NTA (IARC Citation1999). In the urinary biomarker study (HIHS Citation2013), NTA administration resulted in higher excretion of zinc in the urine. Also according to IARC, NTA has shown urothelial effects at doses higher than those required for nephrotoxicity and proliferative effects; and although the mechanism of induction of the urothelial effects is not known, they are not related to Zn2+ homeostasis but rather correlate with depletion of cellular calcium and possibly the formation of NTA-containing microcrystals (Anderson Citation1980; IARC Citation1999). In the biomarker study (HIHS Citation2013), NTA administration also resulted in higher urinary levels of calcium. Thus, also according to IARC (Citation1999), the renal and urothelial effects of NTA are associated with cellular toxicity and regenerative hyperplasia; its toxic, regenerative proliferative and tumorigenic effects occur only at high doses and no direct genotoxic effect appears to be involved. Moreover, this is corroborated by the abundance of calcium in urine compared to zinc, the higher excretion of calcium in the urine, and the higher preference of NTA to bind calcium (compared to Na). Consequently, the formation of high amounts of insoluble NTA-Ca microcrystals will act as a tumor promotor by inducing persistent inflammation due to chronic mechanical irritation and cytotoxicity. Such microcrystal formation has indeed been observed in urine sediments of NTA-treated rats (Anderson and Kanerva Citation1978; Anderson Citation1980; BASF Citation1997a, Citation2006). The facts that cytotoxicity and tumors were seen in identical locations in the kidneys, and in ureter and bladder, that inflammation and cytotoxicity are early events that lead to regenerative hyperplasia, and hyperplasia is often associated with tumor development, provide supporting evidence for this cytotoxic mode of action.
This is in sharp contrast with the findings observed at the same absolute dose (and only about two-times lower internal molar dose) of MGDA, resulting in comparable, but slightly less, kidney toxicity in the biomarker study. MGDA administration also resulted in higher urinary zinc and calcium levels, but in contrast to NTA-Ca, MGDA-Ca is soluble and does not result in the formation of microcrystals. Thus, as indicated above, the mechanism of action of NTA to induce tumors in rats is based on a cytotoxic mode of action due to formation of insoluble microcrystals, a mechanism which does not occur with MGDA. At high doses MGDA induces kidney toxicity, but no tumors; at doses above the classification limits for STOT Repeated Exposure cat. 1 or 2 which are 10 and 100 mg/kg bw/day, respectively (for 90-day exposure duration).
In the biomarker study at 1000 mg/kg bw/day, EDTA and GLDA were only very slightly nephrotoxic which could, in first instance, be explained by the 15-times lower internal molar dose compared to NTA. However, EDTA administration has not resulted in the formation of treatment-related tumors in rats and mice (NTIS 1977); and EDTA-Ca is also well soluble. In the two-year feeding study in rats at two dose levels of 3750 ppm and 7500 ppm EDTA (corresponding to approximately 250 and 500 mg/kg bw/day) no substance related toxic effects were observed. The same study at the same dose levels (3750 ppm and 7500 ppm; corresponding to approximately 469 and 938 mg/kg bw/day) conducted with mice showed a NOAEL of 938 mg/kg bw/day (NTIS 1977). In addition, a 90-day feeding study of EDTA in rats revealed a NOAEL of 500 mg/kg bw/day (Wynn et al. Citation1970) when using 1, 5, or 10% (respectively 500, 2500, or 5000 mg/kg bw/day) EDTA in the diet for 90 days. The mid and high dose animals expressed a significant decrease of body weight and food consumption. Dose-dependent mortality was evident by 20% in the 5% and 60% mortality in the 10% group. In these groups animals exhibited diarrhea and were emaciated. Water consumption was increased. In the upper dose there was an intermittent decrease of hematocrit and hemoglobin levels, livers appeared to be pale but histological investigation failed to reveal any pathological alteration.
Regarding GLDA, very slight nephrotoxicity was noted at 1000 mg/kg bw/day in both the 28-day biomarker study and the 90-day repeated dose toxicity study (OECD TG 408), and GLDA-Ca is also well soluble. It is, therefore, clear that GLDA can be compared with EDTA, also in view of similar internal dosage at the same external dose, and thus concluded that GLDA has no carcinogenic properties in the same way as was shown for NTA.
In contrast, EDG-Ca, just like NTA-Ca, has shown kidney toxicity, is not soluble and is also forming microcrystals; as such the proposed self-classification as carcinogen cat. 2 in line with NTA is warranted.
In summary, this review has shown that grouping based on structure alone is quite simplistic; other factors play important roles. In this case there are clear differences in absorption from the gut after oral dosing (varying from less than 5–85%), thus clear differences in internal molar dose (up to 15-times higher at an absolute dose of 1000 mg/kg bw/day) and thus clear differences in kidney toxicity as shown in the biomarker study; the differences in absorption are related to molecular size, and not to the molecular structure as such (all being a monoamine-based chelating agent). However, the chelating property to bind calcium—either in blood and excreted as calcium-complex in urine or binding in urine itself—results in calcium-bound chelates with clear differences in solubility and formation of microcrystals; the latter inducing cytotoxicity at high doses resulting in tumor formation in the urinary tract.
We therefore conclude that the mechanism of action of NTA to induce tumors in rats and mice in the urinary tract is based on a cytotoxic mode of action by formation of microcrystals of very limited solubility and that cytotoxicity is an early lesion that leads to regenerative hyperplasia which is often associated with tumor development. Based on the possibility to form microcrystals, together with the available data as described above, we assume this will be the case for EDG too. However, this mode of action does not apply to MGDA, EDTA, and GLDA, as they do not form insoluble microcrystals. MGDA and EDTA have indeed shown to be non-carcinogenic up to the testing limit dose of ∼1000 mg/kg bw/day and even higher in the case of MGDA. And only very limited kidney toxicity was seen for GLDA at 1000 mg/kg bw/day in both a 90-day and 28-day biomarker study.
Finally, although ARN documents are legally non-binding and ECHA is always stressing in public presentations that such documents are only prepared to address potential concerns, viz. The purpose of the assessment of regulatory needs of a group of substances is to help authorities conclude on the most appropriate way to address the identified concerns for a group of substances [.]; yet these documents present the public impression of a conclusion about restrictions for groups or sub-groups of chemicals and hence may set a precedent for further binding actions (Natsch et al. Citation2023). The present case is an example where actually quite far-reaching conclusions on toxicity are taken just based on structural similarity alone while not taking true mechanistic knowledge into account. Thus, although the chelates discussed in this review showed structural similarity, their hazard profile regarding tumor formation is quite different and harmonized classification for carcinogenicity of these chelates is contraindicated based on the available evidence described above.
| Abbreviations | ||
| ALP | = | Alkaline phosphatase |
| ARN | = | Assessment of Regulatory Needs |
| CAL | = | Calbandin |
| CLP | = | Classification, Labeling and Packaging |
| CLU | = | Clusterin |
| CSS | = | Chemical Strategy for Sustainability |
| ECHA | = | European Chemical Agency |
| EDG | = | Ethanol diglycine |
| EDTA | = | Ethylenediaminetetraacetic acid |
| EGF-R | = | Epidermal growth factor - rat |
| GLDA | = | Glutamate diacetate |
| GST | = | Glutathione-S-transferase |
| HGPRT | = | Hypoxanthine-guanine phosphoribosyl-transferase |
| IARC | = | International Agency for Research on Cancer |
| Kim-1 | = | Kidney injury molecule 1 |
| LDH | = | Lactate dehydrogenase |
| MGDA | = | Methylglycine N,N-diacetic acid |
| NOAEL | = | No Observed Adverse Effect Level |
| NOEL | = | No Observed Effect Level |
| NONS | = | Notification of New Substances |
| NTA | = | Nitrilotriacetate |
| OST | = | Osteopontin |
| (Q)SAR | = | (Quantitative) Structure Activity Relationship |
Acknowledgements
The authors acknowledge the reviewers and the editor for their time and dedication in reviewing this work. They would also like to thank Dr. Arjen Reichwein (Nouryon) for valuable scientific and technical input to the section “Physicochemical and structural considerations.”
Declaration of interest
This paper was prepared as a reaction to ECHA’s Assessments of Regulatory Needs (ARN) in which several aminocarboxylic acid (monoamine-based) chelating agents were grouped based on molecular structure. The authors prepared the manuscript as a critical but constructive reflexion of the read-across hypothesis used in this ARN approach, making use of available information, including company-specific data, and knowledge of the respective substances. Preparation, review and editing of the manuscript was performed by all authors, and the paper did not receive any funding. All authors of this manuscript are working in the chemical industry producing chelating agents mentioned in this paper. These chelating agents have been REACH registered; NTA has been evaluated by IARC in 1999. The authors have sole responsibility for the manuscript; opinions and conclusions expressed within this manuscript are those of the authors.
References
- Anderson RL, Kanerva RL. 1978. Hypercalcinuria and crystalluria during ingestion of dietary Nitrilotriacetate. Food Cosmet Toxicol. 16(6):569–574. doi: 10.1016/S0015-6264(78)80225-9.
- Anderson RL, Kanerva RL. 1979. Comparisons of response of Fischer-344 and Charles River rats to 1.5% nitrilotriacetic acid and 2% trisodium nitrilotriacetate, monohydrate. Food Cosmet Toxicol. 17(2):137–140. doi: 10.1016/0015-6264(79)90211-6.
- Anderson RL. 1980. The relationship of insoluble nitrilotriacetate (NTA) in the urine of female rats to the dietary level of NTA. Food Cosmet Toxicol. 18(1):59–64. doi: 10.1016/0015-6264(80)90012-7.
- Anderson RL. 1981. The role of zinc in nitriloacetate(NTA)-associated renal tubular cell toxicity. Food Cosmet Toxicol. 19(5):639–650. doi: 10.1016/0015-6264(81)90516-2.
- Anderson RL, Bishop WE, Campbell RL. 1985. A review of the environmental and mammalian toxicology of nitrilotriacetic acid. Crit Rev Toxicol. 15(1):1–102. doi: 10.3109/10408448509023766.
- Anonymous. 1985. EDG. https://echa.europa.eu/registration-dossier/-/registered-dossier/11665/7/2/2 (unpublished report).
- Anonymous. 1986a. EDG. https://echa.europa.eu/registration-dossier/-/registered-dossier/11665/7/7/2/?documentUUID=3ad80f3a-9b9a-4ed0-8c7e-2af827bbc037) (unpublished report).
- Anonymous. 1986b. EDG. https://echa.europa.eu/registration-dossier/-/registered-dossier/11665/7/7/3 (unpublished report).
- Anonymous. 1987. EDG. https://echa.europa.eu/registration-dossier/-/registered-dossier/11665/7/6/2 (unpublished report).
- Anonymous. 1994. GLDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/2174/7/7/2 (unpublished report).
- Anonymous. 1995a. GLDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/2174/7/7/2/?documentUUID=11b419c0-93f6-48be-9219-03c63e21bdea (unpublished report).
- Anonymous. 1995b. GLDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/2174/7/7/2/?documentUUID=9f9831e5-0b94-455f-940e-765801c906c3 (unpublished report).
- Anonymous. 1995c. GLDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/2174/7/7/3 (unpublished report).
- Anonymous. 2007. GLDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/2174/7/6/2. (unpublished report).
- Anonymous. 2012. EDG. https://echa.europa.eu/registration-dossier/-/registered-dossier/11665/7/7/2/?documentUUID=083532ed-fb5a-425a-bd3a-e1acd14b7d31 (unpublished report).
- Anonymous. 2014. GLDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/2174/7/2/2 (unpublished report).
- BASF. 1995a. MGDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/15592/7/7/2/?documentUUID=e5bfffa1-49fc-4fed-88a6-0d7c56dc7524 (unpublished report).
- BASF. 1995b. MGDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/15592/7/7/2 (unpublished report).
- BASF. 1997a. NTA. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/12171/7/6/2/?documentUUID=59c44e58-8f45-464d-8c67-65358e3cfcff.
- BASF. 1997b. MGDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/15592/7/2/2/?documentUUID=c69c895d-8738-4d64-8336-68a42a2f35ad (unpublished report).
- BASF. 1997c. MGDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/15592/7/7/3. (unpublished report).
- BASF. 1998a. NTA. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14301/7/6/2/?documentUUID=5a2c5947-5e1d-404d-a283-c2e6b1cbfc3c.
- BASF. 1998b. MGDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/15592/7/6/2/?documentUUID=7180c783-d912-40eb-a5ce-3bcc6f8e8fcd (unpublished report)
- BASF. 2001. MGDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/15592/7/7/2/?documentUUID=a471f4a3-07b2-4b74-a507-c1e2194b2a66 (unpublished report).
- BASF. 2003. NTA. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14301/7/6/2/?documentUUID=ea1c99fc-f84e-4f2e-9eb4-b32864edbc28.
- BASF. 2006. MGDA. https://echa.europa.eu/registration-dossier/-/registered-dossier/15592/7/8 (unpublished report).
- BASF. 2015. MGDA; https://echa.europa.eu/registration-dossier/-/registered-dossier/15592/7/2/2/?documentUUID=d1ea6ff9-93df-4a0c-93a0-bf73373e4217 (unpublished report).
- Begum ZA, Rahman IMM, Tate Y, Egawa Y, Maki T, Hasegawa H. 2012a. Formation and stability of binary complexes of divalent ecotoxic ions (Ni, Cu, Zn, Cd, Pb) with biodegradable aminopolycarboxylate chelants (dl-2-(2- carboxymethyl)nitrilotriacetic acid, GLDA, and 3-hydroxy-2,2 ′ -iminodisuccinic acid, HIDS) in aqueous solutions. J Sol Chem. 41(10):1713–1728. doi: 10.1007/s10953-012-9901-9.
- Begum ZA, Rahman IMM, Sawai H, Tate Y, Maki T, Hasegawa H. 2012b. Stability constants of Fe(III) and Cr(III) complexes with dl-2-(2-carboxymethyl)nitrilotriacetic acid (GLDA) and 3-hydroxy-2,2′-iminodisuccinic acid (HIDS) in aqueous solution. J Chem Eng Data. 57(10):2723–2732. doi: 10.1021/je3005936.
- Budny JA. 1972. Metabolism and blood pressure effects of disodium nitrilotriacetate (Na2NTA) in dogs. Toxicol Appl Pharmacol. 22(4):655–660. doi: 10.1016/0041-008x(72)90293-1.
- Budny JA, Arnold JD. 1973. Nitrilotriacetate (NTA): human metabolism and its importance in the total safety evaluation program. Toxicol Appl Pharmacol. 25(1):48–53. doi: 10.1016/0041-008x(73)90161-0.
- Budny JA, Niewenhuis RJ, Buehler EV, Goldenthal EI. 1973. Subacute oral toxicity of trisodium nitrilotriacetate (Na3NTA) in dogs. Toxicol Appl Pharmacol. 26(1):148–153. doi: 10.1016/0041-008x(73)90095-1.
- Cohen N, Guilmette R. 1976. Biological effects of the enhanced excretion of Zinc after calcium diethylenetriaminepentaacetate chelation therapy. Bioinorg Chem. 5(3):203–210. doi: 10.1016/s0006-3061(00)82018-9.
- European Chemical Agency [ECHA]. 2017. Read-Across Assessment Framework (RAAF). https://echa.europa.eu/documents/10162/13628/raaf_en.pdf/614e5d61-891d-4154-8a47-87efebd1851a.
- European Chemical Agency [ECHA]. 2022. Assessment of regulatory needs (ARN)—group name: polycarboxylic acid monoamines, hydroxy derivatives and their salts with monovalent cations. https://echa.europa.eu/documents/10162/45dde3f2-73a7-e456-078c-7021a30da8cd.
- [EU] European Union. 2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the registration, evaluation, authorisation and restriction of chemicals (REACH). https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20140410.
- Foreman H, Vier M, Magee M. 1953. The metabolism of C14-labeled ethylenediaminetetraacetic acid in the rat. J Biol Chem. 203(2):1045–1053. doi: 10.1016/S0021-9258(19)52375-4.
- Havlicek F. 1967. Metabolism and toxicity of therapeutic chelating agents. 3. Effect of EDTA and DTPA on the excretion of endogenous zinc. Strahlentherapie. 134(2):296–305.
- Goyer RA, Falk HL, Hogan M, Feldman DD, Richter W. 1981. Renal tumors in rats given trisodium nitrilotriacetic acid in drinking water for two years. J Natl Cancer Inst. 66(5):869–880.
- Harpur E, Ennulat D, Hoffman D, Betton G, Gautier JC, Riefke B, Bounous D, Schuster K, Beushausen S, Guffroy M, et al. 2011. Biological qualification of biomarkers of chemical-induced renal toxicity in two strains of male rats. Toxicol Sci. 122(2):235–252. doi: 10.1093/toxsci/kfr112.
- The Hamner Institutes for Health Sciences [HIHS]. 2013. The comparative renal toxicity of NTA versus GLDA and other chelates using urinary biomarkers and renal cell proliferation as early predictors of nephrotoxicity. Report No. 12006B (unpublished report).
- Hoffmann D, Adler M, Vaidya VS, Rached E, Mulrane L, Gallagher WM, Callanan JJ, Gautier JC, Matheis K, Staedtler F, et al. 2010. Performance of novel kidney biomarkers in preclinical toxicity studies. Toxicol Sci. 116(1):8–22. doi: 10.1093/toxsci/kfq029.
- The International Agency for Research on Cancer [IARC]. 1999. Nitrilotriacetic acid and its salts. IARC Monographs on the evaluation of carcinogenic risks to humans—some chemicals that cause tumours of the kidney or urinary bladder in rodents and some other substances. Vol. 73. Lyon, France: WHO; p. 385–399.
- Jayalakshmi S, Platel K. 2016. Compromised zinc status of experimental rats as a consequence of prolonged iron & calcium supplementation. Indian J Med Res. 143(2):238–244. doi: 10.4103/0971-5916.180221.
- Leibold E, Deckardt K, Mellert W, Potthoff-Karl B, Grundler O, Jäckh R. 2002. NTA and Fe(III)NTA: differential patterns of renal toxicity in subchronic studies. Hum Exp Toxicol. 21(8):445–452. doi: 10.1191/0960327102ht273oa.
- Martell AE, Smith RM. 2004. Critically selected stability constants of metal complexes, version 8.0 (NIST standard reference database 46).
- Merski JA. 1982. Alterations of renal tissue structure during a 30-day gavage study with nitrilotriacetate. Food Chem Toxicol. 20(4):433–440. doi: 10.1016/s0278-6915(82)80109-9.
- Michael WR, Wakim JB. 1971. Metabolism of nitrilotriacetic acid (NTA). Toxicol Appl Pharmacol. 18(2):407–416. doi: 10.1016/0041-008x(71)90133-5.
- Michael WR, Wakim JB. 1973. Effect of trisodium nitrilotriacetate (Na3NTA) on the metabolism of selected metal ions. Toxicol Appl Pharmacol. 24(4):519–529. doi: 10.1016/0041-008x(73)90213-5.
- Myers MC, Kanerva RL, Alden CL, Anderson RL. 1982. Reversibility of nephrotoxicity induced in rats by nitrilotriacetate in subchronic feeding studies. Food Chem Toxicol. 20(6):925–934. doi: 10.1016/S0015-6264(82)80230-7.
- Natsch A, Adamsson G, Rocha V. 2023. ECHA ARN documents: chemical grouping without a toxicological rationale. Arch Toxicol. 97(5):1433–1437. doi: 10.1007/s00204-023-03479-3.
- National Cancer Institute [NCI]. 1977. Bioassays of nitrilotriacetic acid (NTA) and nitrilotriacetic acid, trisodium salt, monohydrate (Na3NTA•H2O) for possible carcinogenicity. Testing Laboratory: Litton Bionetics Inc. Report No.: (NIH): 77-806.
- Nixon GA. 1971. Toxicity evaluation of trisodium nitrilotriacetate. Toxicol Appl Pharmacol. 18(2):398–406. doi: 10.1016/0041-008x(71)90132-3.
- Nixon GA, Buehler EV, Niewenhuis RJ. 1972. Two-year rat feeding study with trisodium nitrilotriacetate and its calcium chelate. Toxicol Appl Pharmacol. 21(2):244–252. doi: 10.1016/0041-008x(72)90067-1.
- National Research Council (US) [NRC]. 1995. Nutrient requirements of the laboratory rat–nutrient requirements of laboratory animals. 4th Rev ed. Washington, DC: Subcommittee on Laboratory Animal Nutrition, National Academies Press.
- National Cancer Institute (US) [NTIS]. 1977. Bioassay of trisodium ethylenediaminetetraacetate trihydrate (EDTA) for possible carcinogenicity. NTIS Technical Report Series No. 11.
- Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, Staedtler F, Mahl A, Grenet O, Roth DR, et al. 2010. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol. 28(5):486–494. doi: 10.1038/nbt.1627.
- Powell JJ, Burden TJ, Greenfield SM, Taylor PD, Thompson RP. 1999. Urinary excretion of essential metals following intravenous calcium disodium edetate: an estimate of free zinc and zinc status in man. J Inorg Biochem. 75(3):159–165. doi: 10.1016/s0162-0134(99)00054-9.
- Rubin M, Martell AE. 1980. The implications of trace metal-nitrilotriacetic acid speciation on its environmental impact and toxicology. Biol Trace Elem Res. 2(1):1–19. doi: 10.1007/BF02789031.
- Scientific Committee on Consumer Safety [SCCS]. 2010. Opinion on trisodium nitrilotriacetate (NTA). Report No.: SCCS/1391/10.
- Walker BE, Kelleher J. 1978. Plasma whole blood and urine zinc in the assessment of zinc deficiency in the rat. J Nutr. 108(10):1702–1707. doi: 10.1093/jn/108.10.1702.
- Wohlleben W, Mehling A, Landsiedel R. 2023. Lessons learned from the grouping of chemicals to assess risks to human health. Angew Chem Int Ed Engl. 62(22):e20221065162. doi: 10.1002/anie.202210651.
- Wynn JE, Van’t Riet B, Borzelleca JF. 1970. The toxicity and pharmacodynamics of EGTA: oral administration to rats and comparisons with EDTA. Toxicol Appl Pharmacol. 16(3):807–817. doi: 10.1016/0041-008x(70)90087-6.
