Abstract
The potential carcinogenicity of talc has been evaluated in many studies in humans and experimental animals published in the scientific literature over the last several decades, with a number of these studies reporting no associations between talc exposure and any type of cancer. In order to fully understand the current state of the science regarding the potential for talc to induce human cancers, we conducted a comprehensive and systematic review of the available experimental animal and mechanistic evidence (in conjunction with a systematic review of the epidemiology evidence in a companion analysis) to evaluate whether it supports talc as being carcinogenic to humans. We considered study quality and its impact on the interpretation of results and evaluated all types of cancer and all exposure routes. We also evaluated the evidence on the potential for talc to migrate in the body to potential tumor sites. We identified seven experimental animal carcinogenicity studies and 11 mechanistic studies of talc to systematically review. We found that several of the experimental animal carcinogenicity studies of talc have limitations that preclude their sensitivity to detect increases in tumor incidence. Regardless, the studies cover multiple exposure routes, species, and exposure durations, and none indicate that talc is a carcinogen in experimental animals except in rats under conditions of extremely high exposure that likely resulted in lung particle overload, a nonspecific effect of high exposures to poorly soluble particles, and not from any carcinogenic properties of talc. Lung particle overload leading to lung tumor formation has only been observed in rats and not in any other species, including humans. The mechanistic studies indicate that talc is not genotoxic or mutagenic, but can induce some effects that could be events on a possible pathway to carcinogenicity, mainly at high exposures or in in vitro studies with exposures of unclear relevance in vivo, but these effects are not consistent across studies and cell types. This systematic review of the experimental animal carcinogenicity and mechanistic evidence for talc indicates that an association between talc exposure and cancer is not expected in humans. Talc carcinogenicity is not plausible in any species except rats, and only when the exposure conditions are high enough to induce lung particle overload, which is not relevant to human exposures.
Introduction
Talc is a plate-like mineral composed mainly of magnesium, silicon, and oxygen that is mined for its use in industrial, cosmetic, and pharmaceutical products (IARC Citation2010; Fiume et al. Citation2015; Borm Citation2023). Industrial-grade talc contains varying proportions of other minerals and can contain impurities such as iron and aluminum (IARC Citation2010; Fiume et al. Citation2015). Cosmetic-grade talc is generally >98% talc, and baby powder available in the United States (US) since the 1990s, as well as pharmaceutical-grade talc, generally contain >99% talc (IARC Citation2010).
Talc can form an asbestiform habit (i.e. a shape that is similar to asbestos fibers) when plates of non-asbestiform talc elongate to form long, thin, ribbon-like fibers with mean length/diameter ratios up to 100:1 for talc fibers longer than 5 µm (IARC Citation2010; Johnson Citation2020). However, asbestiform talc and other asbestiform fibers, besides the six asbestiform fiber types regulated as asbestos (chrysotile, crocidolite, amosite, tremolite, anthophyllite, and actinolite), are not asbestos and, similar to non-asbestiform minerals, they do not confer asbestos-related cancer risks (IARC Citation2010; Pierce et al. Citation2017; Miller et al. Citation2024). Some studies suggested that baby powder sold in the US prior to 1976 contained certain types of asbestos, i.e. asbestiform fibers such as tremolite, actinolite, and anthophyllite (IARC Citation2010; Johnson Citation2020; Ierardi et al. Citation2021), but the analyses to identify these fibers likely used unreliable or inaccurate methodologies for distinguishing asbestos from other fibers (Fiume et al. Citation2015; Pierce et al. Citation2017; Sanchez et al. Citation2023; Miller et al. Citation2024). An analysis of historic samples from cosmetic talc products manufactured prior to 1976 using currently accepted analytical methods that can distinguish between asbestiform and non-asbestiform habits (X-ray diffraction and polarized light microscopy with dispersion staining, as well as transmission electron microscopy with selected area electron diffraction and energy dispersive X-ray analysis) did not detect any asbestiform minerals (Pierce et al. Citation2017).
In 1976, specifications for cosmetic-grade talc that required no detectable asbestos fibers were developed (Fiume et al. Citation2015), and analyses of samples from more recently manufactured talc-containing retail products have been conducted by the US Food and Drug Administration (US FDA) using similar methods as Pierce et al. (Citation2017). Although some of these analyses reported the presence of certain types of asbestos in a small number of samples, the manufacturer of some of the positive samples used its own independent laboratory to test the samples and could not confirm the US FDA findings (Johnson Citation2020). The most recent analyses of talc-containing product samples by US FDA did not detect asbestos fibers in any of the samples (US FDA Citation2021, Citation2022, Citation2024).
Despite talc being considered a poorly soluble particle of low toxicity (Oberdörster Citation1995a; ILSI Citation2000; Driscoll Citation2022; Mundt et al. Citation2022), there have been a large number of studies on the potential carcinogenicity of talc exposure in humans and experimental animals published in the scientific literature over the past several decades. While some of these studies reported associations between talc exposure and increased incidences of certain types of cancer (particularly ovarian cancer and respiratory cancers), many studies did not report associations with any type of cancer (Goodman et al. Citation2020). It is also unclear whether there are any biologically plausible modes of action for the carcinogenicity of talc in humans or whether talc particles can migrate from the site of exposure to other sites in the body and induce tumor development.
The International Agency for Research on Cancer (IARC) classified inhaled talc not containing asbestos or asbestiform fibers as not classifiable as to its carcinogenicity (group 3), and perineal use of talc-based body powder as possibly carcinogenic to humans (group 2B) (IARC Citation2010). These classifications are based on what IARC (Citation2010) stated was inadequate evidence for human carcinogenicity of inhaled talc not containing asbestos or asbestiform fibers, limited evidence for human carcinogenicity of perineal use of talc-based body powder, and limited evidence for experimental animal carcinogenicity of talc not containing asbestos or asbestiform fibers. It is unlikely that the asbestiform fibers mentioned in the classification for inhaled talc include asbestiform talc (which, as noted above is not asbestos), as the talc in the inhalation studies cited by IARC (Citation2010) was described as being analyzed for "asbestos" or for either asbestiform or non-asbestiform amphiboles, such as tremolite, with no mention of asbestiform talc. IARC (Citation2010) also considered evidence on potential mechanisms by which talc exposure could cause cancer in humans, noting that inflammation may be operative in the effects of talc in the respiratory tract and that human and experimental animal studies provide weak to no evidence of retrograde transport of perineal talc exposure through the reproductive tract to the ovaries.
We are not aware of any other agency classifying talc as a known, suspected, probable, or possible human carcinogen, though the Canadian government conducted a screening assessment of talc and identified ovarian cancer as a potential concern for human health from perineal exposure to talc (Environment and Climate Change Canada and Health Canada Citation2021), and the National Institute of Public Health and the Environment of the Netherlands recently proposed to the European Chemicals Agency that talc should be classified as a category 2 carcinogen (i.e. suspected human carcinogen) under the Globally Harmonized System of Classification and Labelling of Chemicals, based on limited evidence from human and animal studies (Netherlands RIVM Citation2023). Talc has not been considered for listing in the National Toxicology Program’s (NTP's) Report on Carcinogens (NTP Citation2021), nor has it been evaluated for carcinogenicity under the United States Environmental Protection Agency’s (US EPA's) Integrated Risk Information Program (IRIS).
In order to fully understand the current state of the science regarding the potential for talc to induce human cancers, we conducted a comprehensive and systematic review of the available experimental animal and mechanistic evidence (in conjunction with a systematic review of the epidemiology evidence in a companion analysis) to evaluate whether it supports talc as being carcinogenic to humans. In this analysis, we considered study quality and its impact on the interpretation of results and evaluated all types of cancer and all exposure routes. We also evaluated the evidence on the potential for talc to migrate in the body to potential tumor sites. This review, along with our companion analysis of the epidemiology evidence (Boon et al. Citation2024), represents the most comprehensive and systematic review of the evidence on the potential for talc to cause any type of cancer under any exposure conditions.
Methods
This review was conducted using established best practices for systematic reviews, i.e. using the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) checklist (PRISMA Citation2024b), and the reporting of this review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (PRISMA Citation2024a).
We developed the methods for this review to address the principal question of whether the experimental animal and mechanistic literature support an association between exposure to talc and cancer in humans. This review includes relevant experimental animal and mechanistic literature evaluating talc exposure by any route and published in English through 18 January 2024. The review includes an evaluation of all potential types of cancer, with tumor incidence as the specific outcome for experimental animal carcinogenicity studies and any key event for a potential carcinogenic mechanism as the specific outcome for mechanistic studies. The protocol for this systematic review was registered at Open Science Forum as "Protocol for a Systematic Review of the Experimental Animal and Mechanistic Evidence for the Potential Human Carcinogenicity of Talc" (https://osf.io/y3mgj).
Article eligibility criteria
The identification of individual experimental animal carcinogenicity and mechanistic studies was guided by a population, exposure, comparator, outcomes, and study design (PECOS) statement for each stream of evidence. The PECOS statement for the experimental animal carcinogenicity studies is: In non-human mammals (P), what is the incidence of tumors (O) after repeated exposure to talc (E) compared to no exposure (C), as observed in primary experimental studies (S)? The corresponding inclusion and exclusion criteria for the experimental animal carcinogenicity studies are provided in .
Table 1. PECOS elements and corresponding inclusion and exclusion criteria for experimental animal carcinogenicity studies.
The PECOS statement for the mechanistic studies is: In any biological in vivo or in vitro test system (P), are there biologically plausible key events associated with carcinogenicity (O) after exposure to talc (E) compared to no exposure (C), as observed in primary experimental studies (S)? The corresponding inclusion and exclusion criteria for the experimental animal carcinogenicity studies are provided in .
Table 2. PECOS elements and corresponding inclusion and exclusion criteria for mechanistic studies.
As it is of interest to evaluate the potential for talc to migrate in the body to potential tumor sites, we also conducted literature searches for review articles on this topic to supplement our analysis. These include reviews published in English with evidence on talc toxicokinetics and migration, as well as publicly available gray literature (i.e. sources not found in standard, peer-reviewed literature databases, such as scientific and regulatory agency reviews and technical reports) on potential migration of talc, and supplemented the evaluation with primary studies where needed.
Information sources, search strategy, and study selection
We conducted separate literature searches for experimental animal and mechanistic studies published through 18 January 2024, using PubMed and Scopus. and indicate the search terms used to identify the experimental animal and mechanistic studies, respectively.
Table 3. PubMed and Scopus search strategies for experimental animal carcinogenicity studies.
Table 4. PubMed and Scopus search strategies for mechanistic studies.
Titles, abstracts, and full article texts, as needed, of the relevant studies identified from the systematic literature search for experimental animal and mechanistic studies were independently screened by two reviewers. Eligible studies were selected based on the PECOS statements and corresponding inclusion and exclusion criteria; non-eligible studies were excluded and the reasons for doing so were documented after full-text review. Any disagreement between the two reviewers was noted and resolved through discussion, after which a final list of studies for inclusion in the systematic review was generated. The study screening process was managed using the systematic review software tool Covidence (https://www.covidence.org/), which captured records of the study screening and reasons for study exclusion. The reference lists of the included articles and those of relevant reviews identified during the study selection process were also screened for potentially relevant studies to include in this review.
Reviews on talc toxicokinetics and migration were selected from the reviews identified using the search strategies above, and additional targeted searches were conducted for published reviews and gray literature using search terms related to toxicokinetics and migration.
Data extraction
For each included experimental animal or mechanistic study, one reviewer independently extracted the data (e.g. study characteristics, study results) and evaluated study quality (as discussed below), and this was checked for accuracy by a second reviewer. Collected data were stored and organized in Excel tables. Any disagreement between the two reviewers was noted and resolved through discussion. The study characteristics and results were tabulated and the characteristics were used as the basis for an evaluation of study quality.
Study quality evaluation
We evaluated study quality to assess how reliable the results of each experimental animal and mechanistic study are for addressing the principal research question. For each individual study included in the review, specific aspects were ranked as higher or lower quality according to the pre-specified criteria across several domains. This process is based on the study evaluation criteria for animal toxicological and in vitro studies presented in the US EPA Office of Research and Development (ORD) Staff Handbook for Developing IRIS Assessments (US EPA Citation2022). The domains encompass criteria for evaluation of potential risk of bias (RoB, i.e. systematic error or deviation from the truth) or sensitivity (i.e. level of confidence in an effect being truly detected and the potential for false negative results) (US EPA Citation2022).
The domains for experimental animal studies (either tumor incidence or in vivo mechanistic studies), as well as the specific criteria for each domain, are presented in , with the RoB domains in italics and the sensitivity domains in regular font. These criteria are primarily based on the ORD Staff Handbook (US EPA Citation2022), with the addition of a criterion regarding whether statistical analyses were conducted, which is based on other study quality criteria for experimental animal studies (NTP Citation2015; Waspe et al. Citation2021). For the criterion of chemical administration and characterization, we considered talc purity of at least 95%, with no evidence of asbestos fiber content when using appropriate analytical methods such as electron microscopy or X-ray diffraction along with light or phase-contrast microscopy, as adequate. For the criterion of endpoint measurement, we considered a sample size of 50 animals/sex/group appropriate for evaluation of tumor incidence, based on Organization for Economic Co-operation and Development (OECD) guidelines for carcinogenicity bioassays (OECD Citation2018), and of 5 animals/group for evaluation of mechanistic endpoints. For the criterion of sensitivity, we considered an exposure duration and observation period of 2 years to be appropriate and sensitive for evaluation of tumor incidence, whereas these factors needed only to be sufficient to observe the mechanistic endpoint of interest to be appropriate.
Table 5. Study quality domains and criteria for experimental animal studies.
The domains for in vitro mechanistic studies, as well as the specific criteria for each domain, are presented in , with the RoB domains in italics and the sensitivity domains in regular font. These criteria are primarily based on the ORD Staff Handbook (US EPA Citation2022), with the addition of a criterion regarding whether statistical analyses were conducted, which is based on other study quality criteria for in vitro studies (Waspe et al. Citation2021). As with the experimental animal studies, we considered talc purity of at least 95% with no evidence of asbestos when using appropriate analytical methods as adequate for the criterion of chemical administration and characterization. For the criterion of endpoint measurement, we considered replicates of three (triplicate) to be adequate.
Table 6. Study quality domains and criteria for in vitro mechanistic studies.
We considered all of the study quality criteria when interpreting the results of individual studies and the body of literature as a whole.
Evidence synthesis and integration
We assessed the results of studies within each stream of evidence in the context of their methodological strengths and limitations, as determined from the evaluation of study quality. We then synthesized the evidence within the experimental animal carcinogenicity stream by evaluating the following considerations for synthesis of animal studies from the ORD Staff Handbook (US EPA Citation2022): consistency, magnitude of effect, exposure–response relationships, and coherence. We synthesized the evidence within the mechanistic stream by evaluating the plausibility and human relevance of potential carcinogenic mechanisms for talc using the principles of the International Program on Chemical Safety (IPCS) Mode of Action (MOA)/human relevance framework (Boobis et al. Citation2008; Meek et al. Citation2014), considering the consistency, essentiality, temporality, exposure–response relationships, biological concordance, and human relevance of key mechanistic events.
We assessed the overall plausibility of causality for an association between talc and human carcinogenicity from integrating the evidence across the experimental animal carcinogenicity and mechanistic streams, considering coherence, biological plausibility, and human relevance, and taking study quality into account. We also considered the conclusions from our companion review regarding the plausibility of causality from the epidemiology evidence.
We assessed the strength of the overall evidence for the potential human carcinogenicity of talc as the overall plausibility of causality for an association between talc and human cancers from the evidence integration described above. We also identified data gaps and uncertainties, and assessed the level of confidence in the conclusions regarding plausibility of causality.
Results for experimental animal carcinogenicity studies
Literature selection
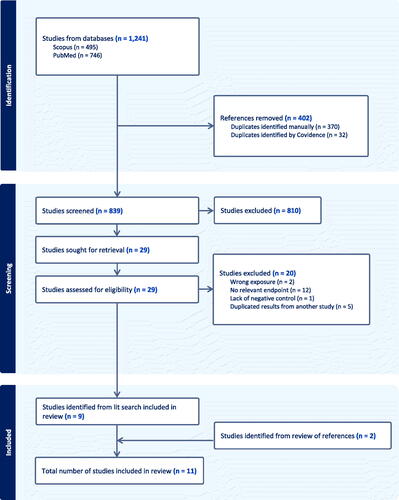
The literature search for experimental animal carcinogenicity studies yielded 853 results in PubMed and 1496 results in Scopus. After removing duplicates, 1772 results remained for further screening. After review of titles and abstracts to exclude studies that did not meet our inclusion and exclusion criteria (i.e. wrong species, not an in vivo study, wrong exposure, lack of untreated control group, no relevant endpoint, not a primary study, and not published in English), we identified 13 studies for full-text review, plus one additional study identified from reviewing reference lists of relevant studies and reviews. After full text review, we identified seven experimental animal carcinogenicity studies for inclusion in this review. The study selection process and results are shown in . The characteristics of the experimental animal carcinogenicity studies are summarized briefly below and presented in .
Figure 1. Literature search and study selection flowchart for experimental animal carcinogenicity studies.
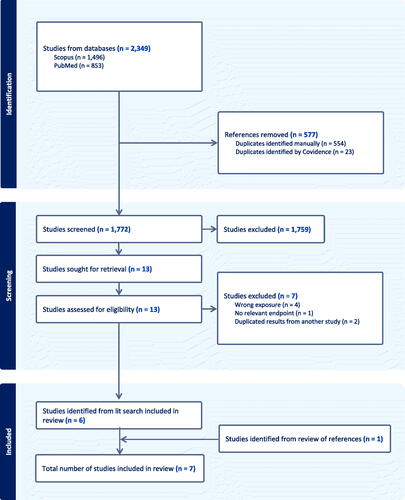
Table 7. Characteristics of experimental animal carcinogenicity studies.
Four studies evaluated tumor incidence in rats, one study evaluated tumor incidence in mice, and two studies evaluated tumor incidence in hamsters. The studies used various exposure routes and durations, including inhalation exposure for up to 1 year (Wagner et al. Citation1977, Citation1979; Wehner, Zwicker, et al. 1977) or for 2 years (NTP Citation1993); oral exposure for a duration of 5 months (Wagner et al. Citation1977); weekly exposure by intratracheal (IT) instillation for 18 weeks (Stenback and Rowland Citation1978); intravaginal or perineal exposure for 12 weeks (Keskin et al. Citation2009); and intraperitoneal (IP) exposure for 4 weeks (Pott et al. Citation1974). Two of the identified studies reported preliminary (Wagner et al. Citation1977) and final (Wagner et al. Citation1979) results for the same inhalation study. All studies evaluated multiple tumor endpoints, with lung, liver, kidney, stomach, and uterine tumors being the most commonly evaluated.
Study quality evaluation
The study quality ratings for each experimental animal carcinogenicity study across the study quality domains are summarized in .
Table 8. Study quality ratings for experimental animal carcinogenicity studies.
The NTP (Citation1993) inhalation bioassays in rats and mice were rated as having higher quality across all study quality domains. Details of these studies are reported in the NTP Technical Report 421, "Toxicology and Carcinogenesis Studies of Talc (CAS No. 14807-96-6) in F344/N Rats and B6C3F1 Mice (Inhalation Studies)" (NTP Citation1993), which is available at: https://ntp.niehs.nih.gov/sites/default/files/ntp/htdocs/lt_rpts/tr421.pdf. The cover page, foreword, list of contributors, and table of contents are provided in Appendix A. The research documented in the report was conducted at the Lovelace Biomedical and Environmental Research Institute which, at the time the research was conducted, was owned by the US government.
The NTP bioassays were conducted by a multi-disciplinary team of eight scientists from Lovelace according to NTP guidelines for carcinogenicity bioassays in small rodents. A deviation from the guidelines was that rats were exposed to talc for near lifetime durations (i.e. until mortality in any exposure group reached 80%) instead of the 2-year duration typically used in NTP carcinogenicity bioassays. The pathology slides were reviewed by a Lovelace pathologist, a quality assessment pathologist, and, ultimately, a Pathology Working Group consisting of 14 pathologists assembled by the NTP to ensure consistency in the diagnosis of lesions.
The NTP (Citation1993) study had an adequate number of animals (50/sex/group) that were randomized on the basis of weight and sex, addressed attrition bias, and used appropriate statistical methods. The talc test substance was from Pfizer’s Barretts, Montana mine and its purity was assessed at ∼98% with no detection of asbestos fibers by polarized light and transmission electron microscopy. Aerosol concentrations of talc were measured daily in each exposure chamber and were within ±8% of target concentrations. However, NTP (Citation1993) reported problems maintaining proper exposure concentrations throughout the 2-year study duration, but concluded that these differences did not preclude the ability to draw conclusions on the carcinogenicity of talc.
The remaining studies were rated as having lower quality across several or most study quality domains. Of these studies, three did not discuss whether allocation to experimental groups was random (Pott et al. Citation1974; Stenback and Rowland Citation1978; Keskin et al. Citation2009) and none reported blinding of investigators to study groups or consensus-based histopathology evaluation. With the exception of the study by Pott et al. (Citation1974), experimental conditions across all study groups were deemed to be similar and risk of confounding was low. Two studies did not adequately address attrition (Wagner et al. Citation1977, Citation1979; Wehner, Zwicker, et al. 1977). Two of the studies did not report the purity of the talc used (Pott et al. Citation1974; Keskin et al. Citation2009), whereas the studies by Wagner et al. (Citation1977, Citation1979) reported talc purity of <95% and the study by Wehner, Zwicker, et al. (1977) reported a purity of at least 95%, but neither of the studies reported the analytical methods for evaluating whether asbestos or other fibers were detected. Most of the studies used objective and valid assessments of histopathology to evaluate tumor incidence, with the exception of the study by Pott et al. (Citation1974), which lacked specific information on how endpoints were evaluated. Three studies (Pott et al. Citation1974; Wagner et al. Citation1977, Citation1979; Stenback and Rowland Citation1978) used an unpreferred method of data presentation, by pooling data for males and females in each exposure group or for all tumor types combined into one total tumor rate. Only one of the studies (Keskin et al. Citation2009) used statistical methods to assess the significance of the tumor incidences. Finally, all studies, except for the inhalation study by Wagner et al. (Citation1977, Citation1979), used short exposure durations (i.e. <1 year), which did not allow for the detection of tumors that might have developed during later windows of exposure, resulting in few or no tumors being observed. In addition, the sample sizes were small in many of the studies (<50/sex/group), and none provided a power calculation with justification of sample size.
Evaluation of study results
The results of the experimental animal carcinogenicity studies are summarized in .
Table 9. Results of experimental animal carcinogenicity studies.
Two small studies with short exposure durations evaluated tumor development in several different tissues. Keskin et al. (Citation2009) reported no tumors or preneoplastic changes in the ovaries or other reproductive tissues in rats examined immediately after intravaginal or perineal (aerosol) administration of 100 g talc in saline solution for only 12 weeks. There was a statistically significant increase in infections and inflammation in the genital tissues of talc-exposed rats compared to unexposed or saline-exposed controls, though such infections were also observed in some of the control animals and may have been unrelated to the talc exposure. The authors noted that pelvic inflammatory diseases generally are not associated with an increased risk of ovarian cancer in humans. Wagner et al. (Citation1977) fed rats 100 g talc over a 5-month period and reported a stomach leiomyosarcoma in one treated rat and uterine sarcomas in two treated rats but did not conduct statistical analyses of the results. There were no tumors in any of the other tissues examined in treated or control rats over their lifetime such as liver, lung, peritoneum, intestine, or kidney. Wagner et al. (Citation1977) did not consider the two uterine sarcomas to be related to the oral exposure to talc based on their location and the fact that they had observed uterine sarcomas in 3/126 control rats in a previous study. It is also unclear whether the stomach leiomyosarcomas were related to the talc treatment, as the authors also reported malignant tumors in the digestive organs of 3/126 controls from the previous study.
In another study with a short exposure duration, Pott et al. (Citation1974) exposed rats to 25 mg talc using IP injection for 4 weeks and reported no tumors in control animals and one tumor (incidence rate of 2.5%) in treated animals over their lifetime. The specific tumor type was not identified, but the tumors observed in the study overall were described by the authors as "nearly all" mesothelioma. The authors tested other substances, including glass and chrysotile asbestos, and these fibrous dusts had much higher tumor rates of 57.5% and 37.5%, respectively. There were no statistical analyses of the results, and the study was poorly reported, leading to ratings of low quality in all of the study quality domains except for attrition (), as the reported tumor rate included all animals in the study.
Several studies evaluated tumor incidence after exposure of talc to the respiratory tract. One of these studies used IT instillation to expose hamsters to 3 mg talc once per week for 18 weeks and then observed the animals over their lifetime (Stenback and Rowland Citation1978). The talc was of 93–97% purity but the authors stated that they could not exclude the possibility that the talc contained some asbestos (though they did not confirm the presence of asbestos). There were low incidences of several benign tumors in both control and treated animals (though no statistical analyses were conducted), as well as lymphomas in control animals only, but no malignant tumors reported in the respiratory tract or other tissues examined in treated animals.
Two inhalation studies with relatively large sample sizes and with exposure durations up to 300 days or 1 year and observation throughout the lifetime of the animals reported no evidence that talc induces tumors in rodents. Wagner et al. Citation1977, Citation1979) evaluated lung tumors in rats exposed to 10.8 mg/m3 talc for 3, 6, or 12 months and reported lung fibrosis but no malignant tumors such as lung adenocarcinoma or mesothelioma. Lung adenomas were observed in two treated animals and one control animal in the 12-month exposure group, but these were noted to be likely incidental (though the authors did not conduct any statistical analyses of the results). Superfine chrysotile asbestos, used as a positive control, induced lung fibrosis to a similar extent as talc but also induced mesothelioma in one animal in the 3-month exposure group and lung adenocarcinoma in one animal in the 6-month group and two animals in the 12-month group.
Wehner, Zwicker, et al. (1977) exposed hamsters to 37.1 mg/m3 talc for various amounts of time per day over 30 days, or to 27.4 mg/m3 for various amounts of time per day over 300 days, and reported a variety of tumor types in one or two animals in some of the treated and control groups. The authors stated that the incidences of the different histological types of tumors were not related to treatment, though no statistical analyses were conducted. There were no primary neoplasms found in the respiratory tract in any animals (only metastatic lung carcinomas in one treated and one control animal). Both treated and control animals also had inflammation and hyperplasia in both the larynx and trachea, and the authors stated that there were no significant differences in these effects between treatment groups and no dose–response relationships for any respiratory lesions.
The NTP (Citation1993) 2-year inhalation bioassay in rats and mice was rated as having higher quality across all the RoB and sensitivity study quality domains (). There were no statistically significant increases in tumor incidence for male or female mice, and NTP (Citation1993) concluded that there was no evidence of carcinogenic activity of talc in mice. In rats, there was a significantly increased incidence of adrenal pheochromocytomas in males and females of the high-exposure group (18 mg/m3 talc), though these increases are likely not talc-related, as pheochromocytomas can result from stress and hypoxia, the spontaneous incidence of these tumors in rats is high in the strain that was used by NTP (Citation1993), and these tumors have not been reported in other particle inhalation studies except those conducted by NTP (Goodman Citation1995; IARC Citation2010). Female rats in the high-exposure group also had a significantly increased incidence of lung alveolar/bronchiolar carcinomas or lung alveolar/bronchiolar adenomas or carcinomas combined, and these results led NTP (Citation1993) to conclude that there was "clear evidence of carcinogenicity" of talc in female rats. The lung alveolar/bronchiolar carcinomas were observed in female rats in the high-exposure group beginning 828 days after the start of exposure. There was no increase in the incidence of lung tumors in male rats (for which only one alveolar/bronchiolar carcinoma was observed in the high-exposure group at terminal sacrifice), and NTP (Citation1993) speculated that this may be attributable to the slightly shorter length of exposure in the male rats (113 weeks) compared to the female rats (122 weeks) or to unspecified molecular events involved in carcinogenesis that may differ qualitatively or quantitatively between sexes.
Despite the high-quality ratings of the NTP (Citation1993) bioassay in our study quality rating system, it has been criticized for one aspect of the study design. The talc exposure conditions for the high-exposure (18 mg/m3) group were very high, inducing a high talc lung burden and chronic lung toxicity, such as chronic granulomatous inflammation, interstitial fibrosis, hyperplasia, squamous metaplasia, and pulmonary function impairment. This indicates that the maximum tolerated dose (MTD) may have been exceeded (Oberdörster Citation1995b; Morrow et al. Citation1996), which limits the reliability of the results. Such high exposure would have resulted in lung particle overload, which is often observed in rats after prolonged, high exposures to highly insoluble low toxicity particles that overwhelm particle clearance mechanisms, such as through impairment of macrophage mobility, resulting in particle retention and accumulation (Oberdörster Citation1995a; Morrow et al. Citation1996; ILSI Citation2000; Warheit et al. Citation2016; Driscoll Citation2022; Mundt et al. Citation2022).
Lung particle overload can result in excessive lung burden, leading to particle-induced inflammation followed by tumor formation, even for noncarcinogenic particles (Oberdörster Citation1995b; ILSI Citation2000; Wehner Citation2002; Warheit et al. Citation2016; Driscoll Citation2022; Mundt et al. Citation2022; Borm Citation2023). Although the NTP (Citation1993) bioassay included unexposed controls, it did not include any negative or positive dust controls to assess the carcinogenicity of talc compared to other control dusts. It is also notable that for a 7-week period beginning at week 11 of the study, the talc concentration for the 18 mg/m3 exposure group ranged from 30 to 40 mg/m3, which may have worsened the likely lung particle overload conditions in this group (Wehner Citation2002). Multiple investigators and agencies (including Dr. Jay Goodman, a member and Principal Reviewer of the NTP Board of Scientific Counselors Technical Reports Review Subcommittee that reviewed the NTP talc bioassay technical report) consider the lung tumors observed in female rats in the NTP (Citation1993) bioassay to be attributable to lung particle overload (Carr Citation1995; Goodman Citation1995; Oberdörster Citation1995b; Wehner Citation2002; Musser Citation2014). Oberdörster (Citation1995b) noted that increased lung tumor incidence from lung particle overload has been observed in both male and female rats exposed to various particle types and is therefore not a sex-specific phenomenon, but that some studies did report a higher incidence of tumors in female rats, which may be explained by an earlier response in females compared to males.
The NTP (Citation1993) bioassay also used micronized talc to reduce the particle size of the talc inhaled by the rats to mass median aerodynamic diameters in the range of 2.7–3.2 μm. The average particle size of typical cosmetic talc ranges from 4 to 15 μm (Fiume et al. Citation2015), and the size of the particles in the other experimental animal carcinogenicity studies of talc ranged from 4 to 25 μm (). After inhalation, smaller particles deposit in the deeper, alveolar region of the respiratory tract where they are subject to longer retention times because this region lacks the mucociliary clearance ability of the bronchial region. Thus, compared to talc particle sizes in other cosmetic talcs or in the other talc carcinogenicity studies, the micronized talc used by NTP (Citation1993) likely deposited deeper in the lung and had a longer retention time, higher lung burden, and greater potential to elicit adverse effects in the lungs.
Overall, several of the experimental animal carcinogenicity studies of talc have limitations that preclude their sensitivity to detect increases in tumor incidence. Regardless, the studies cover multiple exposure routes, species, and exposure durations, and none indicate that talc is a carcinogen in experimental animals except in rats under conditions of extremely high exposure that likely resulted in lung particle overload, a nonspecific effect of high exposures to particles such as talc, and not to any carcinogenic properties of talc.
Results for mechanistic studies
Literature selection
The literature search for mechanistic studies yielded 746 results in PubMed and 495 results in Scopus. After removing duplicates, 839 results remained for further screening. After review of titles and abstracts to exclude studies that did not meet our inclusion and exclusion criteria (i.e. wrong species, wrong exposure, lack of untreated control group, no relevant endpoint, not a primary study, not published in English), we identified 29 studies for full-text review, plus two additional studies identified from reviewing reference lists of relevant studies and reviews. After full text review, we identified 11 mechanistic studies for inclusion in this review. The study selection process and results are shown in . The characteristics of the experimental animal and in vitro mechanistic studies are summarized briefly below and presented in and , respectively.
Table 10. Characteristics of in vivo mechanistic studies.
Table 11. Characteristics of in vitro mechanistic studies.
In the three in vivo mechanistic studies that were identified for this review, talc was administered via IT instillation (Beck et al. Citation1987), inhalation (Shim et al. Citation2015), or oral gavage (Litton Bionetics, Inc Citation1974). Beck et al. (Citation1987) evaluated polymorphonuclear neutrophil (PMN) and macrophage counts in the lungs of hamsters after a single exposure to talc. Shim et al. (Citation2015) assessed macrophage infiltration and oxidative stress in the lungs of rats exposed to talc for 4 weeks. Litton Bionetics, Inc (Citation1974) conducted a dominant lethal assay in rats and evaluated chromosome aberrations in rat bone marrow cells after a single exposure or daily doses over five days. Litton Bionetics, Inc (Citation1974) also conducted in vitro mechanistic studies, evaluating chromosome aberrations in human embryonic lung cells and mutagenicity in bacterial and yeast strains.
Of the other eight in vitro mechanistic studies identified for this review, one assessed genotoxicity (specifically, unscheduled DNA synthesis (UDS) and sister chromatid exchange (SCE)) in rat pleural mesothelial cells (Endo-Capron et al. Citation1993); two evaluated neoplastic transformation in various human ovarian cell lines (Buz’Zard and Lau Citation2007; Harper et al. Citation2023); two evaluated inflammatory cytokine release from human or rabbit respiratory tract cells (Acencio et al. Citation2007; Ghio et al. Citation2012); three evaluated reactive oxidative species (ROS) production or response in human respiratory cell lines (Ghio et al. Citation2012), human ovarian cell lines (Buz’Zard and Lau Citation2007), or mouse macrophages (Mandarino et al. Citation2020); one measured cell proliferation in a colony-forming efficiency assay in hamster and rat respiratory cells (Wylie et al. Citation1997); and one measured cell proliferation and release of the inflammatory biomarker CA-125 from human macrophages and human female reproductive tract cells (Wylie et al. Citation1997; Fletcher et al. Citation2019).
Study quality evaluation
The study quality ratings for each in vivo and in vitro mechanistic study across the study quality domains are summarized in and , respectively.
Table 12. Study quality ratings for in vivo mechanistic studies.
Table 13. Study quality ratings for in vitro mechanistic studies.
The three in vivo mechanistic studies were rated as having higher quality across most of the study quality domains, though only the study by Litton Bionetics, Inc (Citation1974) specified the random allocation of animals to experimental groups. All three in vivo studies did not report blinding or other measures to reduce observational bias, but all were rated as higher quality for the absence of variation across treatment groups, reporting results for all outcomes, adequately addressing attrition, and using an appropriate study design to detect the outcomes of interest. Talc purity was not reported by Beck et al. (Citation1987) or Litton Bionetics, Inc (Citation1974), but was reported as asbestos-free and 96.7% pure by Shim et al. (Citation2015), who analyzed the talc using field emission scanning electron microscopy. All three studies were rated as higher quality for endpoint measurement based on using appropriate methods, negative control groups, and an adequate sample size, though Beck et al. (Citation1987) used only 3–4 animals per dose group for the time-course study (but used six per group for the dose–response study). In addition, while Litton Bionetics, Inc (Citation1974) used an adequate number of animals in the dominant lethal assay, only 3–5 animals per dose group per time point were used to assess chromosomal aberrations.
The in vitro mechanistic studies were also rated as having higher quality across most of the study quality domains, with the exception of the study by Litton Bionetics, Inc (Citation1974). All studies were rated as higher quality for observational bias, absence of variation across treatment groups, reporting results for all outcomes, and sensitivity of the experiment to detect the outcomes of interest, except that Litton Bionetics, Inc (Citation1974) quantified results using light microscopy without blinding, rather than using automated approaches, and did not report any measures to reduce observational bias. In addition, the evaluation of SCE (but not UDS) by Endo-Capron et al. (Citation1993) and of neoplastic transformation (but not ROS production) by Buz’Zard and Lau (Citation2007) also relied on microscopy without use of automated approaches or standard laboratory kits. Multiple studies did not report the purity of the talc used (Litton Bionetics, Inc Citation1974; Acencio et al. Citation2007; Buz’Zard and Lau Citation2007; Ghio et al. Citation2012; Mandarino et al. Citation2020), two studies used talc of low purity (Endo-Capron et al. Citation1993; Wylie et al. Citation1997), and while Harper et al. (Citation2023) and Fletcher et al. (Citation2019) did not specifically report the purity of the talc they used, they did report using Johnson & Johnson baby powder, and such brands of cosmetic talc are known to be of high (∼99%) purity (Fiume et al. Citation2015). Three studies (Litton Bionetics, Inc Citation1974; Acencio et al. Citation2007; Ghio et al. Citation2012) did not report the number of replicates used in their assays, and one study (Mandarino et al. Citation2020) used only duplicates. All studies were rated as high quality for results presentation, except for the study by Litton Bionetics, Inc (Citation1974), which did not conduct statistical analyses for any in vitro endpoints. In addition, while Buz’Zard and Lau (Citation2007) reported the results of statistical analyses for the neoplastic transformation assay and ROS production in neutrophils, the authors did not report the results of statistical analyses comparing ROS production in treated ovarian cells to unexposed control cells.
Evaluation of study results
The results of the in vivo and in vitro mechanistic studies are summarized in and , respectively.
Table 14. Results of in vivo mechanistic studies.
Table 15. Results of in vitro mechanistic studies.
Talc did not induce chromosome aberrations in vitro or in vivo; mutations in bacterial or yeast cells in vitro or in a host-mediated assay; or mutations in the dominant lethal assay in rats (Litton Bionetics, Inc Citation1974), though the study did not include statistical analyses for any of these assays except the dominant lethal assay, and the number of replicates was not reported for the in vitro assays. Talc was also negative for genotoxicity in in vitro assays for UDS and SCE (Endo-Capron et al. Citation1993). Each of these studies used outdated methods that are not considered reliable for the prediction of genotoxicity, however.
The results were inconsistent in the two studies that evaluated the ability of talc to induce neoplastic transformation by measuring the ability of cells to grow suspended in soft agar. Buz’Zard and Lau (Citation2007) reported that talc increased the growth of ovarian granulosa cells but that growth of ovarian epithelial cells had a reverse dose–response relationship, with the greatest effect at the lowest dose (5 μg/mL), and a significant decrease in growth compared to controls at the highest dose (100 μg/mL). By contrast, Harper et al. (Citation2023) reported increased growth after talc exposure in two different ovarian epithelial cell lines, but not in peritoneal fibroblast cells. Harper et al. (Citation2023) used a very high dose of talc (500 μg/mL), but neither study discussed the relevance of the high in vitro concentrations that they used to plausible talc exposures in vivo. In addition, the findings of neoplastic transformation are not consistent with the lack of genotoxicity and mutagenicity of talc.
Several studies evaluated specific events on a possible pathway of talc carcinogenicity. This pathway involves macrophage infiltration, chronic inflammation, and ROS formation leading to cell injury, repair, and proliferation, which can increase the likelihood of mutations in DNA as secondary effects and lead to tumor development (Lynch et al. Citation2022, Citation2023; Borm Citation2023). Beck et al. (Citation1987) reported increased macrophages in the lungs of hamsters 14 days after exposure to 37.5 mg/kg-day industrial-grade talc via IT instillation, but not at earlier time points, and increased PMN counts in the lungs that decreased over time and approached control levels 7 and 14 days after exposure to 37.5 mg/kg-day talc. This dose, given as a large bolus to the lungs via IT instillation, would result in an immediate lung burden of approximately 4.5 mg, or 6 mg talc/g lung. The authors did not report the purity of the talc used, but a later study by Sato et al. (Citation2020) evaluated the composition of the same talc sample used by Beck et al. (Citation1987) and reported that the sample consisted of 78% talc particulate and 22% fibrous talc, with no detection of asbestos fibers. Sato et al. (Citation2020) also reviewed the pathology slides from Beck et al. (Citation1987) and confirmed that talc exposure induced lung inflammation in the animals. Shim et al. (Citation2015) also reported macrophage infiltration in the lungs of rats exposed to 50 or 100 mg/m3 talc via inhalation for 4 weeks.
Studies that evaluated the effects of talc on levels of markers of ROS production and oxidative stress reported increased expression of the oxidative stress response marker (i.e. antioxidant) superoxide dismutase 2 (SOD2), but not glutathione peroxidase (GPx), in rat lung after exposure to a high concentration (100 mg/m3) talc (Shim et al. Citation2015); increased ROS production in two types of mouse macrophage cell lines, but not a third type, except in the presence of a high dose of estradiol (Mandarino et al. Citation2020); and an increase in ROS production that was transient at lower talc doses in PMNs (Buz’Zard and Lau Citation2007). Buz’Zard and Lau (Citation2007) also observed decreased ROS production in talc-exposed ovarian epithelial and granulosa cells, but did not conduct any statistical analyses of these results compared to unexposed controls. Ghio et al. (Citation2012) reported a time- and concentration-dependent increase in ROS in human mesothelial and bronchial epithelial cells exposed to talc, as well as increased release of the inflammatory cytokine interleukin (IL)-8, but not IL-6, from these cells. Acencio et al. (Citation2007) also reported an increased release of IL-8, as well as two other inflammatory cytokines, transforming growth factor (TGF)-β and vascular endothelial growth factor (VEGF), in talc-exposed rabbit pleural mesothelial cells. Fletcher et al. (Citation2019) reported an increase in release of CA-125, an inflammatory marker, in human ovarian and fallopian tube epithelial cells, but not in human macrophages, 72 h after exposure to high purity cosmetic talc.
Fletcher et al. (Citation2019) also reported that exposure to cosmetic talc increased cell proliferation in human ovarian and fallopian tube epithelial cells, as well as macrophages, within 24 h of exposure. Wylie et al. (Citation1997) reported inconsistent results for the effects of talc on proliferation of hamster tracheal epithelial cells and rat pleural mesothelial cells. These authors used three different types of fibrous talc of low purity and reported either no effects or statistically significant decreases in proliferation of these cells after exposure to various concentrations of the three types of talc, with no dose–response relationships.
In summary, the mechanistic studies indicate that talc is not genotoxic or mutagenic, but was positive in assays of neoplastic transformation in certain cell types but not others. The studies also provide some evidence of macrophage infiltration and a transient increase in PMN counts in the lungs of animals after high IT or inhalation exposures, as well as mixed effects on ROS production and oxidative stress response in vitro depending on the cell type. Expression and release of certain inflammatory markers were also observed for some cell types exposed to talc in vitro, and cell proliferation was increased after talc exposure in several human cell lines but not in rodent respiratory cells. Together, the studies indicate that talc can induce some effects that could be events on a possible pathway to carcinogenicity, mainly at high exposures or in in vitro studies with exposures of unclear relevance in vivo, but these effects are not consistent across studies and cell types.
Evaluation of migration studies
Several studies have evaluated whether talc can migrate from the site of exposure to other organs or tissues in the body, with most of these studies measuring talc or other particle types in female reproductive tissues. For example, multiple studies investigated the presence of talc in normal ovarian tissue or ovarian tumor samples collected from women undergoing surgery.
Henderson et al. (Citation1971) reported talc in 5/12 normal ovarian tissue samples and 10/13 ovarian tumor samples, but the authors did not report whether they took precautions to prevent contamination of the samples from the use of talc-containing surgical gloves. Henderson et al. (Citation1979) attempted to avoid talc contamination sources and reported talc in three normal ovarian tissue samples from healthy women and three ovarian adenocarcinoma samples, but there were no apparent differences in talc content between the healthy women and cancer patients, and the highest amount of talc was measured in a sample from normal ovarian tissue. Mostafa et al. (Citation1985) reported "crystalline foreign particles" composed largely of magnesium and silicon in 9% of normal ovarian samples from 100 women with pelvic disease but did not report precautions to avoid contamination from gloves during surgery or tissue processing, and did not identify the particles specifically as talc. Heller et al. (Citation1996) identified talc particles in normal ovarian tissue from 24 women, but the talc particle counts were similar among exposed (those who reported applying talc powder to the perineum or underwear) and unexposed women and were not correlated with the estimated level of talc use in the exposed women.
Cramer et al. (Citation2007) reported a case study of talc in samples from the pelvic lymph nodes of one woman with ovarian cancer, but did not evaluate talc from lymph nodes of either unexposed or healthy controls. Other studies by the same research group reported talc in pelvic lymph nodes and reproductive tissues of women with ovarian cancer who were asked whether they had a history of perineal talc use (McDonald, Fan, Welch, Cramer, Godleski Citation2019; McDonald, Fan, Welch, Cramer, Stearns, et al. Citation2019). McDonald, Fan, Welch, Cramer, Stearns, et al. (Citation2019) reported talc in pelvic lymph nodes of 22 ovarian cancer patients in which the mean talc concentration was higher in those that reported perineal talc use than those who did not. Nine of the 10 women who reported perineal talc use also reported use of talc on other parts of the body, however, introducing other exposure routes besides perineal application (such as inhalation), so this study does not indicate that talc applied specifically to the perineal area can migrate to the pelvic lymph nodes (Goodman et al. Citation2020). McDonald, Fan, Welch, Cramer, Godleski (Citation2019) also reported increased talc counts in pelvic lymph node, cervix, uterine corpus, fallopian tube, and ovary samples from five women with ovarian cancer who reported a history of perineal talc use compared to six ovarian cancer patients who did not report talc use. The same number and types of tissues were not analyzed in the control and exposed subjects; for example, only ovary and fallopian tube tissues were analyzed in controls, and the lack of measurement of talc in lymph node tissue from controls is an important limitation given that lymph nodes had the highest talc concentrations in the exposed group.
Johnson et al. (Citation2020) used polarized light microscopy and scanning electron microscopy with energy dispersive X-ray analysis to compare the size of talc particles in various reproductive tissues from 11 ovarian cancer patients with a history of perineal talc use to talc particles in either newly purchased or partially used samples of cosmetic talc powder. The authors concluded that the cosmetic talc particles were consistent with those in the tissues based on the particle size and dimensions. The presence of similarly-sized particles does not indicate that the talc in the tissues came from the same type of powder, however. The authors did not evaluate the same types of tissues across the patients or analyze the same number of particles across the patients, and there were no unexposed control patients, thus limiting the interpretation of the results regarding the potential source of the talc particles.
Overall, the studies of the presence of talc in ovarian tissue samples had small numbers of subjects, did not show any consistent correlation between reported perineal talc use and concentrations of talc in tissues, and cannot identify the source or exposure route of the talc, which could have come from contamination in some cases (Fiume et al. Citation2015; Goodman et al. Citation2020; Lynch et al. Citation2023). In addition, the presence of talc in the tissues of ovarian cancer patients does not indicate that talc was the cause of or even associated with their cancer.
Several studies evaluated retrograde particle transport through the female reproductive tract in humans and experimental animals, though none of the human studies specifically examined talc, nor did they examine particle transport after external application of particles to the perineal area. The particles were generally in solution and placed directly into the vagina of women who were undergoing various types of abdominal or gynecological surgeries. Egli and Newton (Citation1961) reported carbon particles had migrated from the vagina to the fallopian tubes in two out of three women in which oxytocin was administered to facilitate transport, whereas De Boer (Citation1972) reported that the cervix was a barrier to transport and that carbon particles placed in the vagina were transported to the fallopian tubes in only one of 37 patients. Two studies used intravaginal application of radiolabeled albumin microspheres and reported that some of these particles reached the ovaries or fallopian tubes (Venter and Iturralde Citation1979; Kunz et al. Citation1996), but these studies did not account for the potential leaching of radioactivity from the particles (Fiume et al. Citation2015). Sjösten et al. (Citation2004) evaluated migration of starch particles from powdered gloves in 60 patients undergoing hysterectomies who had intravaginal exams prior to the surgery and reported higher concentrations of these particles in the cervix, uterus, and fallopian tubes one day after the exam compared to those without an exam, and in the cervix and uterus (but not fallopian tubes) four days after the exam.
The studies of retrograde particle transport in humans do not provide strong evidence that talc can migrate from the vagina to the ovaries. The women in the studies were surgical patients with restricted movement during the procedures and some investigators used deliberate measures to encourage transport of particles, so the study conditions may not be applicable to healthy women (IARC Citation2010; Fiume et al. Citation2015; Goodman et al. Citation2020). The studies did not evaluate talc specifically, and in all but one study, the particles were in solution and placed directly in the vagina which is not relevant to human exposures of dry talc particles external to the body (Goodman et al. Citation2020).
Most of the studies of retrograde transport of particles in experimental animals used talc, though none examined transport after external application of talc specifically to the perineal area. Phillips et al. (Citation1978) administered radiolabeled suspensions of talc intravaginally to three rabbits and reported no migration of talc to the ovaries three days later. In another study in rabbits, Edelstam et al. (Citation1997) reported starch particles in the peritoneal cavity, cervix, uterus, and fallopian tubes after intravaginal administration, but there were no differences in particle counts between exposed and unexposed control animals. Henderson et al. (Citation1986) found talc particles in the ovaries of rats after intravaginal doses of talc suspensions, whereas Boorman and Seely (Citation1995) reported no talc particles in the ovaries of rats from the NTP (Citation1993) cancer bioassay, even though these authors stated that the rats were exposed to talc aerosol at concentrations high enough to cover their fur and cage bars and provide ample opportunity for perineal, respiratory, and oral exposure.
Two studies evaluated retrograde transport of talc in monkeys, whose reproductive tracts more closely resemble those of humans (Wehner et al. Citation1985). In these studies, a radioactive talc suspension was administered intravaginally as a single dose into two monkeys that were examined three days after administration (Wehner et al. Citation1985) or as 30 intravaginal doses over 45 days into six monkeys that were examined two days after the last dose (Wehner et al. Citation1986). In both studies, the monkeys were administered oxytocin to facilitate transport and were restrained with their pelvis elevated for 20 min after each administration, but talc was detected only in samples from the vagina and did not translocate beyond the application site to any of the other tissues that were studied (peritoneal lavage fluid, ovaries, fallopian tubes, or uterus).
Overall, the studies of transport of talc or other particles after intravaginal exposure in experimental animals do not provide evidence that talc, even in suspension and with measures taken to facilitate transport, can migrate from the vagina or external perineal area to the ovary or other tissues such as the uterus, fallopian tubes, or peritoneum (IARC Citation2010; Lynch et al. Citation2023).
Experimental animal studies of talc using other exposure routes also do not indicate that talc translocates from the site of exposure. Wehner, Wilkerson, et al. (Citation1977) exposed hamsters to 40–75 mg/m3 talc via inhalation for 2 h and reported that 6–8% of the inhaled talc was deposited in the alveoli, with a retention half-time in the alveoli of 7–10 days; after 132 days, alveolar clearance of the talc was essentially complete and there was no translocation of the talc to the liver, kidneys, or ovaries. In studies with oral exposure to talc in hamsters (Wehner, Tanner, et al. 1977) and in rats, mice, and guinea pigs (Phillips et al. Citation1978), there was no intestinal absorption and no translocation of talc from the gastrointestinal tract to the liver or kidneys. In addition, Wehner, Tanner, et al. (1977) reported no talc in the lungs, indicating that none of the orally administered talc suspension was aspirated or translocated to the lungs. IARC (Citation2010) reported on several studies in rats and rabbits with intrapleural instillation (i.e. talc pleurodesis) as the exposure route, and in these studies, talc was observed in several extrapulmonary organs in a manner that suggested that transport of talc from the pleura increases with increasing dose and decreasing talc particle size. Talc pleurodesis is also used in humans as a medical procedure in which talc is surgically deposited between the lung and the pleural space to produce an adhesion that prevents the build-up of fluid or air in the pleural space in patients with certain conditions such as malignant pleural effusion (Acencio et al. Citation2007). There have been no increased incidences of lung cancer or mesothelioma reported in talc pleurodesis patients (Wehner Citation2002; Baiu et al. Citation2019).
Evidence synthesis and integration
Experimental animal carcinogenicity evidence synthesis
Here, we synthesize the experimental animal carcinogenicity stream of evidence by evaluating consistency and magnitude of effects, exposure–response relationships, and coherence within and across the studies in this evidence stream.
The experimental animal carcinogenicity studies consistently indicate that talc exposure does not induce a significant increase in any malignant tumor type (or malignant and benign combined) across species and exposure routes, though many of these studies did not evaluate exposure durations of more than 1 year, which may not have been a long enough exposure period to induce tumors and observe increased tumor incidences. The intravaginal, perineal, oral, IP injection, and IT instillation exposure routes were utilized in only one study each, so an evaluation of consistency across these routes is not possible. Three studies utilized the inhalation exposure route, for which there was inconsistency within and across studies. While the 2-year cancer bioassay conducted by NTP (Citation1993) reported an increased incidence of adrenal pheochromocytomas in male and female rats and of lung tumors in female rats in the high dose group (18 mg/m3 talc), the pheochromocytomas were likely not talc-related (as discussed above) and the lung tumors in females were likely a result of lung particle overload, given that the exposure concentration was so high that the MTD was likely exceeded in this study. Lung tumors were not observed in mice in the NTP (Citation1993) study that were exposed to the same talc concentrations as the rats, nor in rats in the inhalation study by Wagner et al. (Citation1977, Citation1979), in which a lower concentration of talc aerosol was used (10.8 mg/m3) over a shorter duration (3, 6, or 12 months). There was also no increased incidence of adrenal pheochromocytomas or lung tumors in hamsters that inhaled higher concentrations of talc (37.1 or 27.4 mg/m3) but for shorter frequencies (up to 2.5 h per day) and durations (30 or 300 days) (Wehner, Zwicker, et al. 1977) than in the NTP (Citation1993) study.
The magnitude of the effects in the only study that reported statistically significant increases in tumor incidence (NTP Citation1993) was not high, particularly in the context of the incidence in the unexposed control group. Even though the incidence of adrenal pheochromocytomas was significantly increased in the high exposure groups of male and female rats (with a significant trend of increasing incidence with increasing exposure concentration), the incidence of these tumors was high in the control groups (53% in male controls and 27% in female controls) in the study, and this type of tumor typically has a high spontaneous incidence rate in the F344 rat strain that was used by NTP (Citation1993). This high spontaneous incidence is one of the reasons why these tumors are not considered to be related to the talc exposure. Lung alveolar/bronchiolar carcinomas were observed in female rats in the high exposure group at an incidence of 10% compared to unexposed controls that had an incidence of 0% for such tumors. When alveolar/bronchiolar carcinomas and adenomas were combined, the incidence increased to 26% in the high-exposure group females and 2% in unexposed control females, with a significant exposure–response trend. Overall, this indicates a modest increase in malignant lung tumors in female rats with an increasing exposure–response relationship under the likely lung particle overload conditions in the NTP (Citation1993) study.
There is coherence across the studies in showing that talc does not induce malignant tumors by any exposure route, except in one study (NTP Citation1993) in rats under lung particle overload conditions, a phenomenon to which rats appear to be particularly sensitive compared to other species. This is supported by the fact that in the same study, mice that inhaled the same concentration of talc as the rats for a similar duration did not develop an increased incidence of lung tumors. In addition, the fact that another rat inhalation study reported no lung tumors with a lower concentration of talc over a shorter duration (Wagner et al. Citation1977, Citation1979), and a hamster inhalation study reported no primary lung tumors with higher exposure concentrations of talc but with shorter frequencies and durations, also supports that the increased lung tumor incidence in the female rats in the NTP (Citation1993) study was attributable to lung particle overload. Because lung particle overload is a nonspecific effect of high exposures to particles, the increased lung tumor incidence in the female rats was likely not attributable to any inherent carcinogenic properties of the talc particles.
Mechanistic evidence synthesis
Here, we synthesize the mechanistic stream of evidence by evaluating the plausibility and human relevance of potential carcinogenic mechanisms for talc using the principles of the IPCS MoA/human relevance framework (Boobis et al. Citation2008; Meek et al. Citation2014), considering the consistency, essentiality, temporality, exposure–response relationships, biological concordance, and human relevance of key mechanistic events.
The mechanistic studies do not indicate that talc is genotoxic or mutagenic; therefore, we focused our synthesis of the mechanistic evidence on the results of studies that evaluated specific events on a hypothesized MoA of talc carcinogenicity involving macrophage infiltration, chronic inflammation, and ROS formation leading to cell injury, repair, and proliferation, which can increase the likelihood of DNA damage and mutations, as well as tumor development.
An evaluation of consistency considers whether the pattern of observations across species and test systems is what would be expected based on the hypothesized MoA (Meek et al. Citation2014). There are limited data available to assess the consistency of the key events in the hypothesized MoA for talc carcinogenicity, as each was evaluated in only a few studies. The in vivo studies provide evidence of macrophage infiltration in the lungs, as well as a transient increase in PMN counts, in hamsters and rats after high exposures to talc. There was also increased expression of one antioxidant (SOD2) but not another (GPx) in rat lungs after high inhalation exposure to talc. While the respiratory cells examined in vitro had increased ROS production and release of some inflammatory cytokines, proliferation was significantly decreased. In ovarian cells studied in vitro, ROS production was decreased but one inflammatory marker was increased, as was cell proliferation. In macrophages, there was an increase in ROS production, release of one inflammatory marker, and increased cell proliferation in vitro. This indicates a lack of consistency in patterns to be expected for each cell type, except for the limited amount of data on macrophages for which ROS production, release of the inflammatory marker CA-125, and cell proliferation were evaluated in one study each, and talc was associated with increases in each of these endpoints.
An evaluation of the essentiality of key events considers whether the sequence of events is reversible (i.e. later events are prevented) if dosing is stopped or if a key event is prevented (Meek et al. Citation2014). While some effects of talc were transient (increased lung PMN counts in vivo and increased ROS production in PMNs), the mechanistic studies were only designed to assess direct effects of talc on each key event and not to evaluate downstream effects after prevention of earlier key effects. As such, an evaluation of essentiality is not possible with the current database of mechanistic studies of talc.
An evaluation of temporality considers whether key events are observed in a hypothesized order (Meek et al. Citation2014). The study designs for the mechanistic studies of talc do not allow for evaluation of temporality. The in vivo study by Shim et al. (Citation2015) evaluated macrophage infiltration and expression of oxidative stress response markers in rats at the same time (after 4 weeks of inhalation exposure), although it is possible that these two events could occur simultaneously in the hypothesized MoA. The in vitro studies are not informative for temporality as they generally involve a single exposure to cells and evaluate endpoints at a single or few time points and cannot recapitulate the effects that can occur over time in whole organisms. Of those that evaluated more than one event in the hypothesized MoA at different time points, there was no evidence to support temporality. Although Buz’Zard and Lau (Citation2007) reported an increased neoplastic transformation of human ovarian cells 72 h after exposure, the same cell types did not have increased ROS production (an earlier key event) at 24 and 72 h after exposure. Fletcher et al. (Citation2019) evaluated a later key event (cell proliferation) 24 h after exposure of various cell lines and an earlier key event (release of an inflammatory marker) 72 h after exposure, though the earlier key event would be indicative of acute inflammation, not chronic inflammation, given the short duration of the in vitro study.
An evaluation of exposure–response relationships considers whether key events are observed at exposures below or similar to those associated with the end effect, which would support the hypothesized MoA (Meek et al. Citation2014). The end effect in this case, talc-induced tumor development, has only been observed in the lungs of rats in the NTP (Citation1993) carcinogenicity bioassay, with 2-year inhalation exposures to 18 mg/m3 talc. Shim et al. (Citation2015) evaluated macrophage infiltration and oxidative stress response in the lungs of rats exposed to 5, 50, or 100 mg/m3 talc for 4 weeks and reported increased macrophage infiltration with exposure to 50 or 100 mg/m3 talc, but not 5 mg/m3 talc, as well as increased oxidative stress response with exposure to 100 mg/m3 talc, but not 50 mg/m3 talc. This indicates that the earlier key events were only observed at higher exposures than the end effect of tumors, though it is unknown whether the key events would be observed at or near exposures to 18 mg/m3 in the study by Shim et al. (Citation2015), as they did not evaluate any exposures between 5 and 50 mg/m3 talc. In addition, any inflammatory response after 4 weeks of exposure would likely be indicative of acute inflammation, not chronic inflammation. In the NTP (Citation1993) bioassay itself, rats exposed to both the lower talc concentration of 6 mg/m3 and the carcinogenic concentration of 18 mg/m3 had chronic inflammation, accumulation of macrophages and neutrophils, and hyperplasia in the lung, indicating that these earlier events were observed at concentrations lower than or the same as the concentration associated with tumor development, which supports the hypothesized MoA.
An evaluation of biological concordance considers whether the hypothesized MoA conflicts with broader biological knowledge (Meek et al. Citation2014). The hypothesized MoA is analogous to a non-genotoxic MoA involving cytotoxicity leading to cell damage, inflammation, oxidative stress, cell repair and proliferation, secondary DNA damage and mutations, and tumor development. Talc, however, is generally considered to be of low toxicity, and the phenomenon of lung particle overload, to which rats are particularly sensitive, is consistent with the hypothesized MoA except that the inflammation and oxidative stress is a result of excessively high lung burdens that overwhelm particle clearance mechanisms, and not from an inherent cytotoxicity of talc (Oberdörster Citation1995a; Morrow et al. Citation1996; ILSI Citation2000; Warheit et al. Citation2016; Driscoll Citation2022; Mundt et al. Citation2022). As discussed above, talc-related lung tumors were only observed in rats in the high exposure group for which the MTD was likely exceeded, and not in mice or hamsters exposed to the same or higher inhalation concentrations of talc, indicating that the lung tumors in female rats in the NTP (Citation1993) bioassay were attributable to lung particle overload, and the hypothesized MoA does not conflict with this knowledge.
To evaluate the human relevance of the hypothesized MoA, we considered the exposure levels at which the key mechanistic events were observed and any fundamental differences between the test systems and humans. The in vivo mechanistic study using IT instillation as an exposure route (Beck et al. Citation1987) is not relevant to human inhalation of talc, as the dose is given as a large bolus that is deposited directly into the trachea, bypassing the upper respiratory tract and normal deposition and clearance, and does not allow for the slower deposition of the particles over time, as occur in humans with inhalation exposure. The in vivo study by Shim et al. (Citation2015) used inhalation as the exposure route, but saw effects on macrophage infiltration and oxidative stress responses in the lungs mainly at high exposure concentrations of 50 or 100 mg/m3, which is not relevant to human exposures to talc, even in heavily exposed miners and millers of talc who were generally exposed to very high concentrations, but with average concentrations ranging from 0.1 to 25.6 mg/m3 (Honda et al. Citation2002; Wild et al. Citation2002; Coggiola et al. Citation2003).
The in vitro studies of mechanistic events used short-term assays that can only evaluate acute effects under non-physiological conditions that do not address human-relevant exposure routes, talc particle clearance, or complex processes such as chronic inflammation and interactions between different cell types that enhance tumor formation. None of the in vitro studies related their exposure concentrations to in vivo doses, with the exception of the study by Acencio et al. (Citation2007). This study noted that the exposure concentration of talc administered to rabbit pleural mesothelial cells in culture, 25 μg/cm2 (equivalent to 125 μg/mL, based on the use of 25 cm2 tissue culture plates and 5 mL tissue culture media), is 100-fold lower than the concentration used in talc pleurodesis in humans (2500 μg/cm2 pleural membrane surface), though the authors stated that talc does not spread evenly over the pleural surface during pleurodesis, such that much of the surface is exposed to lower concentrations similar to those used in their study. While each of the key events in the hypothesized MoA is a physiological effect that can plausibly occur in humans in general, the human relevance of the studies of these events in response to talc exposure is unclear, as almost all used exposures much higher than those experienced by humans, or used exposure routes or test systems that are not physiologically relevant to humans.
Evidence integration across streams
Here, we assess the overall plausibility of causality for an association between talc and human carcinogenicity by integrating the evidence across the experimental animal and mechanistic streams, considering coherence, biological plausibility, and human relevance and taking study quality into account.
An evaluation of coherence considers whether all of the known facts related to observed effects from various evidence streams fit together in a logical manner. From our review, the only evidence that talc exposure induces malignant tumors is from one high-quality inhalation bioassay in which female rats, but not male rats or mice of either sex, had an increased incidence of lung tumors at a high exposure concentration of talc (NTP Citation1993). Other experimental animal carcinogenicity studies were smaller and of shorter duration and, thus, may have had a limited ability to detect statistically significant changes in tumor incidence if there were any, or if they had conducted statistical analyses of the results. The lung tumors observed in female rats in the NTP (Citation1993) inhalation bioassay are likely attributable to lung particle overload conditions given the high exposure concentration and more than 2-year duration of the study. Rats are particularly sensitive to lung particle overload compared to other species, and this is consistent with the lack of lung tumors observed in mice and hamsters exposed to talc by inhalation or IT instillation, or in rats exposed at lower inhalation concentrations or other exposure routes. It is also consistent with the lack of associations with lung cancer (and any other cancer) reported in the epidemiology studies of talc exposure, as described in our companion review (Boon et al. Citation2024). Regardless of study quality, the evidence from mechanistic studies for several key events in a hypothesized MoA for talc-induced carcinogenicity is not consistent with the lack of evidence of talc carcinogenicity in humans and experimental animals, except under lung particle overload conditions in rats.
An evaluation of biological plausibility considers whether there is compelling evidence for a biological mechanism of an effect. We evaluated the mechanistic studies that each reported on one or two key events in a hypothesized MoA for talc-induced cancer that is physiologically plausible in both experimental animals and humans, but the evidence does not indicate that these key events would take place in humans or experimental animals except under the high exposure conditions of lung particle overload. Taher et al. (Citation2019) noted that talc exhibits at least three of the "10 key characteristics of carcinogens" (KCCs) that were proposed by the IARC as a set of characteristics common to established human carcinogens (Smith et al. Citation2016). The three KCCs that talc exhibits in certain studies are induction of inflammation, induction of oxidative stress, and altered cell proliferation (Taher et al. Citation2019). While talc was shown to induce these mechanistic effects in some of the studies reviewed here, each effect on its own has only a speculative role in tumor induction, particularly when the epidemiological and experimental animal studies do not support carcinogenicity of talc except in rats under lung particle overload conditions. Just because talc can induce certain mechanistic effects in cells that are consistent with a biologically plausible hypothesized MoA for carcinogenicity does not provide evidence of carcinogenicity per se. For example, while ROS generation may be a key event in the hypothesized MoA for carcinogenicity, it is not an indication of carcinogenesis on its own, and may instead be a generic marker of cell perturbation or may be counteracted by antioxidants before resultant cell damage and carcinogenicity could occur. The three KCCs that talc exhibits in certain studies are also shared by many non-carcinogenic substances (Bus Citation2017; Goodman and Lynch Citation2017; Smith et al. Citation2021), mainly at high exposure concentrations where positive results for the characteristics would be expected anyway (Smith et al. Citation2021), so the fact that talc exhibits any of the KCCs does not automatically indicate it is carcinogenic, particularly in humans. However, the three KCCs that talc has been shown to exhibit are involved in the hypothesized MoA for carcinogenicity in rats under lung particle overload conditions, which requires high in vivo exposures to talc, and thus it is plausible that they could be involved as key events in this MoA if it is specific to lung particle overload.
The finding of lung tumors in female rats exposed to talc in one experimental animal study has little relevance for an association between talc exposure and cancer in humans. The epidemiology studies do not support a causal association, as indicated in our companion analysis (Boon et al. Citation2024), and while the only experimental animal study to report talc-induced tumors used the physiologically relevant exposure route of inhalation, there is strong evidence that the lung tumors observed in the study were attributable to lung particle overload and that this phenomenon is not relevant to humans. Talc is considered a poorly soluble particle of low toxicity, and exposures to other such particles have also induced lung tumors in rats under conditions of lung particle overload but have not induced lung tumors in other experimental animal species such as mice, hamsters, or non-human primates, even with lung burdens of particles that were similar to or greater than those that produced lung cancer in rats (ILSI Citation2000; Warheit et al. Citation2016; Mundt et al. Citation2022). Studies of human populations heavily exposed to coal dust particulates that resulted in lung burdens that would be considered high enough to induce lung particle overload conditions have also not reported any consistent evidence of increased risks of lung cancer (Oberdörster Citation1995a; ILSI Citation2000). In addition, epidemiology studies of workers exposed to high concentrations of other poorly soluble particles of low toxicity, such as carbon black and titanium dioxide, have also not reported elevated risks of lung cancer, even though they induced lung tumors at high exposure concentrations in rats (Warheit et al. Citation2016; Mundt et al. Citation2022). Differences in particle distribution between rats and primates also support the lack of human relevance of lung particle overload-induced tumors observed in rats. In humans and other primates, a greater proportion of deposited particles translocates to interstitial sites, whereas in rats, deposited particles remain on alveolar surfaces within lung macrophages where they can accumulate and greatly increase lung burden (Warheit et al. Citation2016; Mundt et al. Citation2022). Several scientific committees have stated that lung tumors in rats that occur only under lung particle overload conditions are not relevant to human cancer hazard or risk (Warheit et al. Citation2016; Driscoll Citation2022), and this is consistent with the lack of associations between talc exposure and cancers in the epidemiology literature.
Based on the integration across each of the evidence streams, we find that the overall plausibility of causality for an association between talc and human cancers is not strong, and in fact, carcinogenicity of talc is not plausible in any species except rats, and only when the exposure conditions are high enough to induce lung particle overload.
Discussion
We evaluated whether the evidence from experimental animal carcinogenicity and mechanistic studies of talc supports an association between talc exposure and human cancer. This review, along with our companion analysis of the epidemiology evidence, represents the most comprehensive and systematic review of the evidence on the potential for talc to cause cancer. Overall, we found that the reviewed studies do not support talc as a human carcinogen. The evidence indicates that talc carcinogenicity has only been observed in one study in rats under conditions of lung particle overload, which is not relevant to humans.
Although the one study that reported an increased incidence of rat lung tumors (NTP Citation1993) is the only high quality experimental animal carcinogenicity study that we identified, our conclusion that these tumors are specific to rat lung particle overload and have not been observed (and are not expected to be observed) in other species is well supported and has a moderate level of confidence. Additional studies would address some of the uncertainty regarding the limited number of high quality carcinogenicity studies with exposure durations long enough to allow detection of tumors and the fact that most of the mechanistic studies were conducted in vitro and cannot recapitulate physiological conditions in vivo. Ideally, experimental animal carcinogenicity studies with rats and another species exposed for 2 years to several low and high inhalation concentrations of talc, as well as negative and positive dust controls, and including interim sacrifices to evaluate the various key events associated with an MoA for lung particle overload (macrophage infiltration, inflammation, ROS formation, cellular injury, and cell proliferation) would confirm whether the lung tumors observed in rats are indeed attributable to particle overload conditions that are not relevant to other species, including humans.
A strength of this analysis is the evaluation of any type of cancer and any type of carcinogenic mechanism, as well as any exposure route, to ensure that this review captured the most relevant literature to address the question of talc carcinogenicity. Another strength is that we used established best practices for systematic reviews and incorporated established frameworks for study quality evaluation. We used these frameworks to systematically evaluate the quality of each of the included studies and incorporated aspects of study quality into the interpretation, synthesis, and integration of the results. We also summarized the study characteristics and results into detailed tables that forgo the need for detailed descriptions of every study, which streamlined the overall analysis. In addition, we evaluated the evidence on the potential for talc to migrate in the body to potential tumor sites to supplement our analysis of the carcinogenicity and mechanistic evidence.
Our conclusions are consistent with several other reviews that included evaluations of talc carcinogenicity, most of which focused on one type of cancer each (Fiume et al. Citation2015; Goodman et al. Citation2020; Ierardi et al. Citation2021; Lynch et al. Citation2022, Citation2023; Borm Citation2023), as well as with reviews and evaluations of lung particle overload (Goodman Citation1995; Oberdörster Citation1995a; Morrow et al. Citation1996; ILSI Citation2000; Warheit et al. Citation2016; Driscoll Citation2022; Mundt et al. Citation2022; Borm Citation2023).
Conclusions
This systematic review of the experimental animal carcinogenicity and mechanistic evidence for talc indicates that an association between talc exposure and cancer is not expected in humans. Talc carcinogenicity has only been observed in one study in rats under conditions of lung particle overload, which is not relevant to human exposures. Lung particle overload is a nonspecific effect of high exposures to poorly soluble particles, and lung tumors observed under such conditions are not indicative of any carcinogenic properties of the particles themselves. The mechanistic studies indicate that talc is not genotoxic or mutagenic, but can induce some effects that could be events on a possible pathway to carcinogenicity, mainly at high exposures or in in vitro studies with exposures of unclear relevance in vivo, but these effects are not consistent across studies and cell types. Our conclusion that the lung tumors observed in rats in one carcinogenicity study with talc are specific to rat lung particle overload has a moderate level of confidence, given that additional studies that address some of the remaining uncertainties would confirm whether the lung tumors observed in rats are indeed attributable to particle overload conditions that are not relevant to other species, including humans. In conclusion, we have found that talc carcinogenicity is not plausible in any species except rats, and only when the exposure conditions are high enough to induce lung particle overload, which is not relevant to human exposures.
| Abbreviations | ||
| GPx | = | glutathione peroxidase |
| IARC | = | International Agency for Research on Cancer |
| IL | = | interleukin |
| IP | = | intraperitoneal |
| IPCS | = | International Program on Chemical Safety |
| IRIS | = | Integrated Risk Information Program |
| IT | = | intratracheal |
| KCC | = | key characteristics of carcinogens |
| MOA | = | mode of action |
| MTD | = | maximum tolerated dose |
| NTP | = | National Toxicology Program |
| OECD | = | Organization for Economic Co-operation and Development |
| ORD | = | Office of Research and Development |
| PECOS | = | population, exposure, comparator, outcomes, and study design |
| PMN | = | polymorphonuclear neutrophil |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRISMA-P | = | Preferred Reporting Items for Systematic review and Meta-Analysis Protocols |
| RoB | = | risk of bias |
| ROS | = | reactive oxidative species |
| SCE | = | sister chromatid exchange |
| SOD2 | = | superoxide dismutase 2 |
| TGF | = | transforming growth factor |
| UDS | = | unscheduled DNA synthesis |
| US | = | United States |
| US EPA | = | United States Environmental Protection Agency |
| US FDA | = | United States Food and Drug Administration |
| VEGF | = | vascular endothelial growth factor |
Acknowledgements
The authors thank the Gradient staff members who provided essential assistance in preparing this paper: Megan Rosenberg for editorial assistance, Eric Fischbach for administrative assistance, and Holly Howes for assistance with the literature searches. We also thank Dr. Lorenz Rhomberg, an Advising Principal at Gradient, and the five external peer reviewers selected by the journal editor, whose identities were anonymous to the authors, for reviewing this paper and providing helpful comments that strengthened the quality of this paper.
Declaration of interest
All authors are employed by Gradient, an environmental science consulting firm founded in 1985 (https://gradientcorp.com/). Gradient conducts work for both private and government clients. Funding for this work was provided by Essential Minerals Association (EMA), an organization that represents the interests of companies that mine or process minerals (https://www.essentialminerals.org/). Gradient prepared this analysis with the intent of submitting it to the IARC Monographs Meeting 136: Talc and Acrylonitrile upon journal acceptance. The authors reserve the right to submit this accepted manuscript to other scientific or regulatory groups or legal proceedings.
This paper was drafted during the authors’ normal course of employment. The authors have sole responsibility for the writing and content of this paper. This paper represents the professional opinions of the authors and not necessarily those of EMA or its member companies. EMA and its member companies were not involved with the study design, analysis, interpretation of the evidence, or writing of this paper. EMA and its member companies did not have input on the substance of this paper and did not review this paper prior to its acceptance. This paper was not reviewed by anyone except the authors and the individuals listed in the Acknowledgments section prior to its acceptance.
Gradient has previously submitted comments to US EPA, US FDA, and Health Canada regarding issues related to talc measurements and epidemiology. These activities were funded by the Cosmetics Alliance (CA) Canada, the Industrial Minerals Association – North America (IMA-NA, now known as EMA), and the National Stone Sand and Gravel Association (NSSGA). CA Canada and IMA-NA also funded previous Gradient work on recall bias in talc ovarian cancer studies and a critical review of talc and ovarian cancer. Gradient recently published a paper on recall bias that was informed by some of this earlier work, but did not receive funding for the analysis or preparation of the paper for publication (Goodman JE, Espira LM, Zu K, Boon D. 2024. Quantitative recall bias analysis of the talc and ovarian cancer association. Glob Epidemiol. 7:100140).
Dr. Julie E. Goodman is on the Scientific Advisory Board of NSSGA. Dr. Goodman has also served as an expert witness in litigation involving talc. Drs. Goodman and Robyn L. Prueitt have served as scientific expert witnesses in litigation involving asbestos.
Supplemental material
Supplemental material for this article is available electronically at https://ntp.niehs.nih.gov/publications/reports/tr/400s/tr421.
References
- Acencio MM, Vargas FS, Marchi E, Carnevale GG, Teixeira LR, Antonangelo L, Broaddus VC. 2007. Pleural mesothelial cells mediate inflammatory and profibrotic responses in talc-induced pleurodesis. Lung. 185(6):343–348. doi: 10.1007/s00408-007-9041-y.
- Baiu I, Yevudza E, Shrager JB. 2019. Talc pleurodesis: a medical, medicolegal, and socioeconomic review. Ann Thorac Surg. 109(4):1294–1301. doi: 10.1016/j.anthroacsur.2019.08.104.
- Beck BD, Feldman HA, Brain JD, Smith TJ, Hallock M, Gerson B. 1987. The pulmonary toxicity of talc and granite dust as estimated from an in vivo hamster bioassay. Toxicol Appl Pharmacol. 87(2):222–234. doi: 10.1016/0041-008x(87)90284-5.
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. 2008. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 38(2):87–96. doi: 10.1080/10408440701749421.
- Boon D, Goodman JE, Colonna KJ, Espira LM, Prueitt RL. 2024. A systematic review of the epidemiology evidence on talc and cancer. Crit Rev Toxicol. doi: 10.1080/10408444.2024.2351081.
- Boorman GA, Seely JC. 1995. The lack of an ovarian effect of lifetime talc exposure in F344/N rats and B6C3F1 mice. Regul Toxicol Pharmacol. 21(2):242–243. doi: 10.1006/rtph.1995.1035.
- Borm PJA. 2023. Talc inhalation in rats and humans: a review and appraisal of available evidence. J Occup Environ Med. 65(2):152–159. doi: 10.1097/JOM.0000000000002702.
- Bus BJ. 2017. IARC use of oxidative stress as key mode of action characteristic for facilitating cancer classification: glyphosate case example illustrating a lack of robustness in interpretative implementation. Regul Toxicol Pharmacol. 86:157–166. doi: 10.1016/j.yrtph.2017.03.004.
- Buz’Zard AR, Lau BH. 2007. Pycnogenol reduces talc-induced neoplastic transformation in human ovarian cell cultures. Phytother Res. 21(6):579–586. doi: 10.1002/ptr.2117.
- Carr CJ. 1995. Talc: consumer uses and health perspectives. Regul Toxicol Pharmacol. 21(2):211–215. doi: 10.1006/rtph.1995.1030.
- Coggiola M, Bosio D, Pira E, Piolatto PG, La Vecchia C, Negri E, Michelazzi M, Bacaloni A. 2003. An update of a mortality study of talc miners and millers in Italy. Am J Ind Med. 44(1):63–69. doi: 10.1002/ajim.10240.
- Cramer DW, Welch WR, Berkowitz RS, Godleski JJ. 2007. Presence of talc in pelvic lymph nodes of a woman with ovarian cancer and long-term genital exposure to cosmetic talc. Obstet Gynecol. 110(2 Pt. 2):498–501. doi: 10.1097/01.AOG.0000262902.80861.a0.
- De Boer CH. 1972. Transport of particulate matter through the human female genital tract. J Reprod Fertil. 28(2):295–297. doi: 10.1530/jrf.0.0280295.
- Driscoll KE. 2022. Review of lung particle overload, rat lung cancer, and the conclusions of the Edinburgh Expert Panel – it’s time to revisit cancer hazard classifications for titanium dioxide and carbon black. Front Public Health. 10:907318. doi: 10.3389/fpubh.2022.907318.
- Edelstam GA, Sjösten AC, Ellis H. 1997. Retrograde migration of starch in the genital tract of rabbits. Inflammation. 21(5):489–499. doi: 10.1023/A:1027307612999.
- Egli GE, Newton M. 1961. The transport of carbon particles in the human female reproductive tract. Fertil Steril. 12:151–155. doi: 10.1016/s0015-0282(16)34084-5.
- Endo-Capron S, Renier A, Janson X, Kheuang L, Jaurand MC. 1993. In vitro response of rat pleural mesothelial cells to talc samples in genotoxicity assays (sister chromatid exchanges and DNA repair). Toxicol In Vitro. 7(1):7–14. doi: 10.1016/0887-2333(93)90107-G.
- Environment and Climate Change Canada and Health Canada. 2021. Screening assessment for talc (Mg3H2(SiO3)4) (Chemical Abstracts Service Registry Number 14807-96-6); [accessed 2024 Apr 24]. https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/screening-assessment-talc.html.
- Fiume MM, Boyer I, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG, Shank RC, Slaga TJ, et al. 2015. Safety assessment of talc as used in cosmetics. Int J Toxicol. 34(Suppl. 1):66S–129S. doi: 10.1177/1091581815586797.
- Fletcher NM, Harper AK, Memaj I, Fan R, Morris RT, Saed GM. 2019. Molecular basis supporting the association of talcum powder use with increased risk of ovarian cancer. Reprod Sci. 26(12):1603–1612. doi: 10.1177/1933719119831773.
- Ghio AJ, Soukup JM, Dailey LA, Richards JH, Turi JL, Pavlisko EN, Roggli VL. 2012. Disruption of iron homeostasis in mesothelial cells after talc pleurodesis. Am J Respir Cell Mol Biol. 46(1):80–86. doi: 10.1165/rcmb.2011-0168OC.
- Goodman J, Lynch H. 2017. Improving the International Agency for Research on Cancer’s consideration of mechanistic evidence. Toxicol Appl Pharmacol. 319:39–46. doi: 10.1016/j.taap.2017.01.020.
- Goodman JE, Kerper LE, Prueitt RL, Marsh CM. 2020. A critical review of talc and ovarian cancer. J Toxicol Environ Health B Crit Rev. 23(5):183–213. doi: 10.1080/10937404.2020.1755402.
- Goodman JI. 1995. An analysis of the National Toxicology Program’s (NTP) Technical Report (NTP TR 421) on the toxicology and carcinogenesis studies of talc. Regul Toxicol Pharmacol. 21(2):244–249. doi: 10.1006/rtph.1995.1036.
- Harper AK, Wang X, Fan R, Kirsch Mangu T, Fletcher NM, Morris RT, Saed GM. 2023. Talcum powder induces malignant transformation in normal human primary ovarian epithelial cells. Minerva Obstet Gynecol. 75(2):150–157. doi: 10.23736/S2724-606X.21.04989-7.
- Heller DS, Westhoff C, Gordon RE, Katz N. 1996. The relationship between perineal cosmetic talc usage and ovarian talc particle burden. Am J Obstet Gynecol. 174(5):1507–1510. doi: 10.1016/S0002-9378(96)70597-5.
- Henderson WJ, Hamilton TC, Baylis MS, Pierrepoint CG, Griffiths K. 1986. The demonstration of the migration of talc from the vagina and posterior uterus to the ovary in the rat. Environ Res. 40(2):247–250. doi: 10.1016/S0013-9351(86)80100-1.
- Henderson WJ, Hamilton TC, Griffiths K. 1979. Talc in normal and malignant ovarian tissue. Lancet. 1(8114):499. doi: 10.1016/S0140-6736(79)90861-4.
- Henderson WJ, Joslin CA, Turnbull AC, Griffiths K. 1971. Talc and carcinoma of the ovary and cervix. J Obstet Gynaecol Br Commonw. 78(3):266–272. doi: 10.1111/j.1471-0528.1971.tb00267.x.
- Honda Y, Beall C, Delzell E, Oestenstad K, Brill I, Matthews R. 2002. Mortality among workers at a talc mining and milling facility. Ann Occup Hyg. 46(7):575–585. doi: 10.1093/annhyg/mef075.
- [IARC] International Agency for Research on Cancer. 2010. IARC monographs on the evaluation of carcinogenic risks to humans volume 93: carbon black, titanium dioxide, and talc. International Agency for Research on Cancer (IARC), World Health Organization (WHO). IARC Monograph No. 93; [accessed 2024 Mar 29]. http://monographs.iarc.fr/ENG/Monographs/vol100C/mono93.pdf.
- Ierardi AM, Urban A, Marsh GM. 2021. A quantitative weight of evidence assessment of Hill’s guidelines for causal inference for cosmetic talc as a cause of mesothelioma. Toxicol Appl Pharmacol. 417:115461. doi: 10.1016/j.taap.2021.115461.
- [ILSI] ILSI Risk Science Institute Workshop Participants. 2000. The relevance of the rat lung response to particle overload for human risk assessment: a workshop consensus report. Inhal Toxicol. 12(1–2):1–17. doi: 10.1080/08958370050029725.
- Johnson KE, Popratiloff A, Fan Y, McDonald S, Godleski JJ. 2020. Analytic comparison of talc in commercially available baby powder and in pelvic tissues resected from ovarian carcinoma patients. Gynecol Oncol. 159(2):527–533. doi: 10.1016/j.ygyno.2020.09.028.
- Johnson NF. 2020. Inhalation toxicity of talc. J Aerosol Med Pulm Drug Deliv. 34(2):79–107. doi: 10.1089/jamp.2020.1609.
- Keskin N, Teksen YA, Ongun EG, Ozay Y, Saygili H. 2009. Does long-term talc exposure have a carcinogenic effect on the female genital system of rats? An experimental pilot study. Arch Gynecol Obstet. 280(6):925–931. doi: 10.1007/s00404-009-1030-3.
- Kunz G, Beil D, Deininger H, Wildt L, Leyendecker G. 1996. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Hum Reprod. 11(3):627–632. doi: 10.1093/HUMREP/11.3.627.
- Litton Bionetics, Inc. 1974. Mutagenic evaluation of talc. Rockville (MD): US Food and Drug Administration (US FDA); p. 144. Report Nos.: NTIS PB-245458, FDABF-GRAS-302.
- Lynch HN, Lauer DJ, Leleck OM, Freid RD, Collins J, Chen K, Thompson WJ, Ierardi AM, Urban A, Boffetta P, et al. 2023. Systematic review of the association between talc and female reproductive tract cancers. Front Toxicol. 5:1157761. doi: 10.3389/ftox.2023.1157761.
- Lynch HN, Lauer DJ, Thompson WJ, Leleck O, Freid RD, Collins J, Chen K, Ierardi AM, Urban AM, Cappello MA, et al. 2022. Systematic review of the scientific evidence of the pulmonary carcinogenicity of talc. Front Public Health. 10:989111. doi: 10.3389/fpubh.2022.989111.
- Mandarino A, Gregory DJ, McGuire CC, Leblanc BW, Witt H, Rivera LM, Godleski JJ, Fedulov AV. 2020. The effect of talc particles on phagocytes in co-culture with ovarian cancer cells. Environ Res. 180:108676. doi: 10.1016/j.envres.2019.108676.
- McDonald SA, Fan Y, Welch WR, Cramer DW, Godleski JJ. 2019. Migration of talc from the perineum to multiple pelvic organ sites. Am J Clin Pathol. 152(5):590–607. doi: 10.1093/ajcp/aqz080.
- McDonald SA, Fan Y, Welch WR, Cramer DW, Stearns RC, Sheedy L, Katler M, Godleski JJ. 2019. Correlative polarizing light and scanning electron microscopy for the assessment of talc in pelvic region lymph nodes. Ultrastruct Pathol. 43(1):13–27. doi: 10.1080/01913123.2019.1593271.
- Meek ME, Palermo CM, Bachman AN, North CM, Lewis RJ. 2014. Mode of action human relevance (species concordance) framework: evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol. 34(6):595–606. doi: 10.1002/jat.2984.
- Miller E, Beckett EM, Cheatham D, Comerford CE, Lewis RC, Krevanko C, Mandava N, Pierce JS. 2024. A review of the mesotheliogenic potency of cleavage fragments found in talc. Toxicol Ind Health. 7482337241246924. doi: 10.1177/07482337241246924.
- Morrow PE, Haseman JK, Hobbs CH, Driscoll KE, Vu V, Oberdörster G. 1996. The maximum tolerated dose for inhalation bioassays: toxicity vs overload. Fundam Appl Toxicol. 29(2):155–167. doi: 10.1093/toxsci/29.2.155.
- Mostafa SA, Bargeron CB, Flower RW, Rosenshein NB, Parmley TH, Woodruff JD. 1985. Foreign body granulomas in normal ovaries. Obstet Gynecol. 66(5):701–702.
- Mundt KA, Santamaria AB, Thompson WJ, Bates CA, Boles C, Dotson GS, Yong M. 2022. Carcinogenicity of poorly soluble low toxicity particles: commentary on epidemiology as a risk assessment "Reality Check". Front Public Health. 10:920032. doi: 10.3389/fpubh.2022.920032.
- Musser SM. 2014. Letter to S. Epstein from University of Illinois. Chicago, Re: response to citizen petitions requesting that the Food and Drug Administration (FDA) require a cancer warning on cosmetic talc products. US Food and Drug Administration (US FDA). Report No.: 94P-0420, FDA-2008-P-0309-0001/CP.
- [Netherlands RIVM] Netherlands National Institute of Public Health and the Environment. 2023. CLH report: proposal for harmonised classification and labelling for talc (Mg3H2(SiO3)4) (EC No.: 238-877-9; CAS No.: 14807-96-6) (version number: 2.0). Submitted to European Chemicals Agency (ECHA).
- [NTP] National Toxicology Program. 1993. Toxicology and carcinogenesis studies of talc (CAS No. 14807-96-6) in F344/N rats and B6C3F1 mice (inhalation studies). Report No.: NTP Technical Report Series No. 421, NIH Publication No. 93-3152. 287.
- [NTP] National Toxicology Program. 2015. Office of Health Assessment and Translation (OHAT). OHAT risk of bias rating tool for human and animal studies; [accessed 2024 Mar 29]. https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf.
- [NTP] National Toxicology Program. 2021. Report on carcinogens. 15th ed.; [accessed 2024 Mar 29]. https://ntp.niehs.nih.gov/go/roc15.
- [OECD] Organisation for Economic Co-operation and Development. 2018. OECD guideline for the testing of chemicals: carcinogenicity studies. Report No.: OECD/OCDE 451.
- Oberdörster G. 1995a. Lung particle overload: implications for occupational exposures to particles. Regul Toxicol Pharmacol. 21(1):123–135. doi: 10.1006/rtph.1995.1017.
- Oberdörster G. 1995b. The NTP talc inhalation study: a critical appraisal focused on lung particle overload. Regul Toxicol Pharmacol. 21(2):233–241.
- Phillips JC, Young PJ, Hardy K, Gangolli SD. 1978. Studies on the absorption and disposition of 3H-labelled talc in the rat, mouse, guinea-pig and rabbit. Food Cosmet Toxicol. 16(2):161–163. doi: 10.1016/s0015-6264(78)80197-7.
- Pierce JS, Riordan AS, Miller EW, Gaffney SH, Hollins DM. 2017. Evaluation of the presence of asbestos in cosmetic talcum products. Inhal Toxicol. 29(10):443–456. doi: 10.1080/08958378.2017.1392656.
- Pott F, Huth F, Friedrichs KH. 1974. Tumorigenic effect of fibrous dusts in experimental animals. Environ Health Perspect. 9:313–315. doi: 10.1289/ehp.749313.
- PRISMA. 2024a. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA); [accessed 2024 Mar 29]. https://www.prisma-statement.org.
- PRISMA. 2024b. PRISMA for systematic review protocols (PRISMA-P); [accessed 2024 Mar 29]. http://www.prisma-statement.org/Extensions/Protocols.
- Sanchez MS, McGrath-Koerner M, McNamee BD. 2023. Characterization of elongate mineral particles including talc, amphiboles, and biopyriboles observed in mineral derived powders: comparisons of analysis of the same talcum powder samples by two laboratories. Environ Res. 230:114791. doi: 10.1016/j.envres.2022.114791.
- Sato E, McDonald SA, Fan Y, Peterson S, Brain JD, Godleski JJ. 2020. Analysis of particles from hamster lungs following pulmonary talc exposures: implications for pathogenicity. Part Fibre Toxicol. 17(1):20. doi: 10.1186/s12989-020-00356-0.
- Shim I, Kim HM, Yang S, Choi M, Seo GB, Lee BW, Yoon BI, Kim P, Choi K. 2015. Inhalation of talc induces infiltration of macrophages and upregulation of manganese superoxide dismutase in rats. Int J Toxicol. 34(6):491–499. doi: 10.1177/1091581815607068.
- Sjösten ACE, Ellis H, Edelstam GAB. 2004. Retrograde migration of glove powder in the human female genital tract. Hum Reprod. 19(4):991–995. doi: 10.1093/humrep/deh156.
- Smith CJ, Perfetti TA, Hayes AW, Berry SC, Trosko JE, King JA, Goodman JI, Begley CG, Dayan A. 2021. Categorizing the characteristics of human carcinogens: a need for specificity. Arch Toxicol. 95(8):2883–2889. doi: 10.1007/s00204-021-03109-w.
- Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, DeMarini DM, Caldwell JC, Kavlock RJ, Lambert P, et al. 2016. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect. 124(6):713–721. doi: 10.1289/ehp.1509912.
- Stenback F, Rowland J. 1978. Role of talc and benzo(a)pyrene in respiratory tumor formation. An experimental study. Scand J Respir Dis. 59(3):130–140.
- Taher MK, Farhat N, Karyakina NA, Shilnikova N, Ramoju S, Gravel CA, Krishnan K, Mattison D, Wen SW, Krewski D. 2019. Critical review of the association between perineal use of talc powder and risk of ovarian cancer. Reprod Toxicol. 90:88–101. doi: 10.1016/j.reprotox.2019.08.015.
- [US EPA] Environmental Protection Agency (US), Office of Research and Development (ORD), Center for Public Health and Environmental Assessment (CPHEA). 2022. ORD staff handbook for developing IRIS assessments. Washington (DC). Report No.: EPA/600/R-22/268.
- [US FDA] US Food and Drug Administration, Center for Food Safety and Applied Nutrition (CFSAN). 2021. CFSAN Constituent Update: FDA releases data from the agency’s 2021 testing of talc-containing cosmetic products for asbestos; [accessed 2024 Apr 17]. https://www.fda.gov/food/cfsan-constituent-updates/fda-releases-data-agencys-2021-testing-talc-containing-cosmetic-products-asbestos.
- [US FDA] US Food and Drug Administration, Center for Food Safety and Applied Nutrition (CFSAN). 2022. CFSAN constituent update: FDA releases data from the agency’s 2022 testing of talc-containing cosmetic products for asbestos; [accessed 2024 Apr 17]. https://www.fda.gov/food/cfsan-constituent-updates/fda-releases-data-agencys-2022-testing-talc-containing-cosmetic-products-asbestos.
- [US FDA] US Food and Drug Administration, Center for Food Safety and Applied Nutrition (CFSAN). 2024. CFSAN constituent update: FDA releases data from the agency’s 2023 testing of talc-containing cosmetic products for asbestos; [accessed 2024 Apr 17]. https://www.fda.gov/cosmetics/cosmetics-news-events/fda-releases-data-agencys-2023-testing-talc-containing-cosmetic-products-asbestos.
- Venter PF, Iturralde M. 1979. Migration of a particulate radioactive tracer from the vagina to the peritoneal cavity and ovaries. S Afr Med J. 55(23):917–999.
- Wagner JC, Berry G, Cooke TJ, Hill RJ, Pooley FD, Skidmore JW. 1977. Animal experiments with talc. In: Walton WH, McGovern B, editors. Inhaled Particles IV 1977. Proceedings of an International Symposium Organized by the British Occupational Hygiene Society; Sep 22–26; Edinburgh. Elmsford (NY): Pergamon Press. p. 647–654.
- Wagner JC, Berry G, Hill RJ, Skidmore JW, Pooley FD. 1979. An animal model for inhalation exposure to talc. In: Lemen R, Dement JM, editors. Dusts and disease: Proceedings of the Conference on Occupational Exposures to Fibrous and Particulate Dust and their Extension into the Environment. Park Forest South (IL): Pathotox Publishers, Inc.; p. 389–392.
- Warheit DB, Kreiling R, Levy LS. 2016. Relevance of the rat lung tumor response to particle overload for human risk assessment – update and interpretation of new data since ILSI 2000. Toxicology. 374:42–59. doi: 10.1016/j.tox.2016.11.013.
- Waspe J, Bui T, Dishaw L, Kraft A, Luke A, Beronius A. 2021. Evaluating reliability and risk of bias of in vivo animal data for risk assessment of chemicals – exploring the use of the SciRAP tool in a systematic review context. Environ Int. 146:106103. doi: 10.1016/j.envint.2020.106103.
- Wehner AP, Hall AS, Weller RE, Lepel EA, Schirmer RE. 1985. Do particles translocate from the vagina to the oviducts and beyond? Food Chem Toxicol. 23(3):367–372.
- Wehner AP, Tanner TM, Buschbom RL. 1977. Absorption of ingested talc by hamsters. Food Cosmet Toxicol. 15(5):453–455. doi: 10.1016/S0015-6264(77)80013-8.
- Wehner AP, Weller RE, Lepel EA. 1986. On talc translocation from the vagina to the oviducts and beyond. Food Chem Toxicol. 24(4):329–338. doi: 10.1016/0278-6915(86)90011-6.
- Wehner AP, Wilkerson CL, Cannon WC, Buschbom RL, Tanner TM. 1977. Pulmonary deposition, translocation and clearance of inhaled neutron-activated talc in hamsters. Food Cosmet Toxicol. 15(3):213–224. doi: 10.1016/S0015-6264(77)80392-1.
- Wehner AP, Zwicker GM, Cannon WC. 1977. Inhalation of talc baby powder by hamsters. Food Cosmet Toxicol. 15(2):121–129. doi: 10.1016/S0015-6264(77)80317-9.
- Wehner AP. 2002. Cosmetic talc should not be listed as a carcinogen: comments on NTP's deliberations to list talc as a carcinogen. Regul Toxicol Pharmacol. 36(1):40–50. doi: 10.1006/rtph.2002.1560.
- Wild P, Leodolter K, Réfrégier M, Schmidt H, Zidek T, Haidinger G. 2002. A cohort mortality and nested case-control study of French and Austrian talc workers. Occup Environ Med. 59(2):98–105. doi: 10.1136/oem.59.2.98.
- Wylie AG, Skinner HC, Marsh J, Snyder H, Garzione C, Hodkinson D, Winters R, Mossman BT. 1997. Mineralogical features associated with cytotoxic and proliferative effects of fibrous talc and asbestos on rodent tracheal epithelial and pleural mesothelial cells. Toxicol Appl Pharmacol. 147(1):143–150. doi: 10.1006/taap.1997.8276.