Abstract
S-Acylation (commonly referred to as S-palmitoylation) is a post-translational modification consisting in the covalent attachment of an acyl chain to a cysteine residue of the target protein. The lability of the resulting thioester bond gives S-acylation an essential characteristic: its reversibility. S-acylation dynamically regulates different aspects in the life of a protein (including stability, localization, interactome, and function) and, thus, plays critical roles in cellular physiology. For long, the reversibility of S-acylation has been neglected and thereby its potential as a regulatory mechanism for protein function undervalued. Thanks to technological advances, the field has now entered its golden era. A great diversity of interesting targets is being identified, the physio-pathological importance of the modification is starting to be revealed, structural information on the enzymes is becoming available, and the regulatory dynamics are gradually being understood. Here we will review the most recent literature in the S-acylation field, with a special focus on the molecular aspects of the modification, its regulation, and its consequences.
Introduction
Protein function can be modulated by a plethora of post-translational modifications. Some of these modify physical–chemical properties, such as the addition of charges by phosphorylation or increasing the hydrophobicity by the addition of lipids. This review focuses on S-acylation, a modification conserved among eukaryotes that consists in the covalent attachment of a long acyl chain (typically but not exclusively palmitoyl-CoA) to specific cysteine residues on the target protein. While most other protein long-chain lipidations (e.g. N-terminal myristoylation, prenylation, and O-acylation) result in stable bond formation, S-acylation is reversible, allowing dynamic fine-tuning the properties of the modified protein (Jiang et al. Citation2018). Chemically, the thiol group of the target cysteine residue reacts with the carbonyl carbon of the lipid moiety forming a thioester bond, which is more labile than its oxyester counterpart (Castro Citation1999). In a biological context, the S-acylation reaction is catalyzed by a family of enzymes named protein S-acyltransferases (PATs) whereas the reverse reaction, deacylation, is catalyzed by the so-called acyl protein thioesterases (APTs) or deacylases () (Tsutsumi et al. Citation2008; Won et al. Citation2018). Recently, long-chain N-acylation on lysine residues has also been shown to be reversible (with members of the sirtuin family catalyzing the deacylation reaction), and thus to have the potential of dynamically regulate protein properties (Feldman et al. Citation2013; Bheda et al. Citation2016; Jing et al. Citation2017). This review focuses exclusively on long-chain cysteine S-acylation, commonly referred to as S-palmitoylation. Other types of protein lipidation are reviewed elsewhere (Lin et al. Citation2012; Jiang et al. Citation2018).
Figure 1. Protein S-acylation. (A) Scheme of the protein S-acylation reaction. The acyl moiety from an acyl-CoA is covalently bound to the thiol group of a cysteine residue through a thioester bond. The direct reaction is catalyzed by DHHC S-acyltransferases and the reverse reaction is catalyzed by acyl protein thioesterases. The attached acyl chain is typically C16:0 but other chain length and saturation are possible (variable “n”). (B) Schematic representation of DHHC S-acyltransferases, highlighting their membrane topology and conserved motifs (see text for details). While most of the members of the family have four transmembrane domains (TMDs) as shown here, up to six TMDs are found in some of them. (C) Membrane topology for human zDHHC22 as predicted using TMHMM v2.0 DTU Bioinformatics, Denmark. Similar results are obtained using the predictor TMpred (ExPASy, SIB, Switzerland) (not shown) (see color version of this figure at www.tandfonline.com/ibmg).
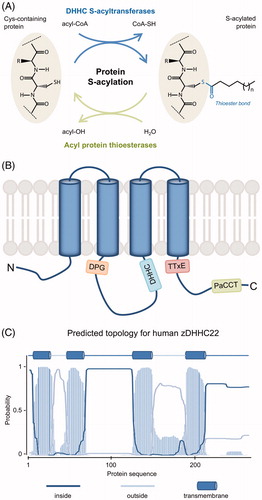
Protein S-acylation was first reported in 1979 (Schmidt and Schlesinger Citation1979) and since then hundreds to thousands of protein substrates have been identified in eukaryotes, including soluble and transmembrane proteins from most subcellular compartments (Blanc et al. Citation2015). S-Acylation of soluble proteins confers increased membrane affinity and thus controls protein localization and switches cellular mobility of the protein from diffusion to vesicular trafficking mode. One of the best-studied examples is S-acylation of Ras proteins, which regulates their trafficking between the Golgi apparatus and the plasma membrane (Linder and Deschenes Citation2007). Transmembrane proteins, despite their inherent membrane anchorage, also frequently undergo S-acylation, which may initiate conformational changes (e.g. tilting of transmembrane domains), regulate recruitment of the protein to specific membrane domains, promote association into protein complexes, or protect from other post-translational modifications such as ubiquitination thus preventing degradation (Abrami et al. Citation2008; Blaskovic et al. Citation2013). Membrane proteins undergoing S-acylation include channels and pumps (e.g. BK channels and sodium/potassium ATPase), many G protein-coupled receptors (e.g. rhodopsin), chaperones (e.g. calnexin), plasma membrane receptors (e.g. transferrin receptor, c-Met, LRP6, TEM8, EGF receptor), proteins involved in synaptic signaling (e.g. PSD-95, AMPA receptor), among others (Chamberlain and Shipston Citation2015; Coleman et al. Citation2016; Fukata et al. Citation2016; Collins et al. Citation2017; Howie et al. Citation2018).
For decades, the extent of protein S-acylation has been underestimated in particular due to the technical challenges associated with the identification of enzymes and substrates. Moreover, the reversibility of S-acylation has been neglected and thereby its potential as a regulatory mechanism for protein function undervalued. Thanks to technological advances, the field has now entered its golden era. A great diversity of interesting targets is being identified, the physio-pathological importance of the modification is starting to be revealed, structural information on the enzymes is becoming available, and the regulatory dynamics are gradually being understood. Here we will review the most recent literature in the S-acylation field. We will first describe the available methods, their advantages and drawbacks. Subsequently, we have chosen to focus on the molecular aspects of both the modification and its consequences, and the regulation of the modification. Excellent reviews covering earlier research and/or focusing on specific aspects such as S-acylation in cancer or in brain function have been recently published and thus these topics will only briefly be mentioned here (Chamberlain and Shipston Citation2015; Cho and Park Citation2016; Fukata et al. Citation2016; Gottlieb and Linder Citation2017; Lanyon-Hogg et al. Citation2017; De and Sadhukhan Citation2018). Likewise, this review focuses on S-acylation in mammals. The reader is referred to other reviews focused on S-acylation in plants (Hemsley Citation2009; Hurst and Hemsley Citation2015), parasites (Brown et al. Citation2017; Ritzefeld et al. Citation2017), or fungi (Santiago-Tirado and Doering Citation2016).
Tools to study protein S-acylation
A variety of different methods to study S-acylation have been developed, each addressing different aspects of the modification. In this review, we provide a description of each method highlighting the main pitfalls, limitations, and common misinterpretations.
Radiolabeling
The oldest and probably most accepted method to study protein S-acylation is metabolic labeling using radioactive palmitic acid (O’Brien and Zatz Citation1984; Hancock Citation1995). It consists of incubating tissue culture cells with radioactive palmitic acid in starvation medium for a certain period that can be adjusted according to the aims of each experiment. Both [3H]-palmitic acid and [14C]-palmitic acid can be used but the latter has remained limited given biosafety issues. After labeling, cell lysis is followed by immunoprecipitation of the protein of interest (endogenous or ectopically expressed), SDS-PAGE, and fluorography (Waterborg and Matthews Citation1994). To confirm that the acyl chain is bound to the protein through a thioester bond (i.e. that the modification being considered is S-acylation and not N- or O-acylation), a lane including a comparable amount of protein sample treated with hydroxylamine solution (to selectively hydrolyze the thioester bond and thus lose the radioactive signal) needs to be included in the same gel as a control. The time of exposure by fluorography varies from a few days up to months depending on the abundance of the protein, and the efficiency of the labeling and immunoprecipitation. The required time of exposure and the use of radioactive material are the most obvious disadvantages of this technique. It is important to realize that metabolic labeling only reports on newly or dynamically S-acylated proteins. That is, long-lived, stably S-acylated proteins that do not exchange the endogenous fatty acid by the exogenously added labeled one will remain invisible to this technique. Also, when adding radioactive palmitate (C16:0), S-palmitoylation will preferentially be monitored even though cells may metabolize the labeled fatty acid. No conclusion can be drawn regarding other chain lengths, thus it cannot be excluded that modification with other acyl chains (e.g. C18:0 or C16:1) would be more efficient. Besides, metabolic labeling does not report on the fraction of the total protein population that is S-acylated, nor does it provide information on the number of acylation sites and their occupancy (see below). The true power of this method resides in its ability to study dynamics. Indeed (i) it allows quantitative determination of the kinetics of fatty acid incorporation and it is compatible with pulse-chase experiments; and (ii) it allows quantitative comparisons of different conditions such as silencing or overexpression of S-acylation-related enzymes, cysteine point mutations, or treatment with inhibitors or other drugs.
Metabolic labeling using bioorthogonal chemistry
A non-radioactive, faster alternative to radiolabeling is based on the use of click-chemistry analogs of fatty acids. The metabolic labeling is performed using azide- or alkyne-modified fatty acids (e.g. 17-octadecynoic acid) (Kostiuk et al. Citation2008; Charron et al. Citation2009; Hannoush and Arenas-Ramirez Citation2009; Martin and Cravatt Citation2009). Cell labeling and lysis can be directly followed by immunoprecipitation of the protein of interest or by the so-called click reaction (a copper-catalyzed 1,3-dipolar cycloaddition). One important advantage of this technique compared to radiolabeling is that the click handle not only allows for detection of the protein of interest (based on fluorescence or chemiluminescence) but also for affinity pull-down of the cellular pool of labeled proteins (usually based on a biotin–streptavidin interaction) and global analysis of protein S-acylation when combined with mass spectrometry (MS)-based proteomics. Metabolic labeling with clickable fatty acids has also been followed by fluorescent labeling on cells and visualization by microscopy (Gao and Hannoush Citation2014). A detailed review on the use of click-chemistry-based probes for protein S-acylation has been recently published (Gao and Hannoush Citation2018). While a recent study has elegantly shown that fatty acid azides are good mimics of endogenous fatty acids and can be efficiently taken up and metabolized by cells (Greaves et al. Citation2017), this might not be true for all click-chemistry analogs and all tested conditions, so conclusions should be drawn with additional care.
Metabolic labeling using click-chemistry analogs, of course, faces the same limitations as the above-described radiolabeling approach. Therefore, it is highly recommended to combine these “lipid-centric” methods with the so-called “cysteine-centric” (Chamberlain and Shipston Citation2015) or “hydroxylamine-switch” (Won et al. Citation2018) methods described below.
Acyl-biotin exchange (ABE) and acyl resin-assisted capture (Acyl-RAC)
ABE and Acyl-RAC are two related, extensively used methods to study protein S-acylation (Drisdel and Green Citation2004; Forrester et al. Citation2011). These cysteine-centric methods provide a snapshot of the cellular pool of S-acylated proteins. Briefly, these methods are based on three main steps, once the protein sample is obtained: (1) blocking of all free thiol groups, (2) selective cleavage of thioester bonds using a hydroxylamine solution at neutral pH, and (3) capture of the proteins with newly freed thiol groups either by biotinylation and subsequent streptavidin-based capture (ABE) or directly by thiopropyl beads-mediated pull-down (Acyl-RAC). These methods allow for western blot-based detection of the proteins of interest (notably more than one protein can be analyzed at the same time) or can be easily coupled to MS-based proteomics. Together with bioorthogonal labeling, these methods have proved to be a valuable source of information towards the global analysis of protein S-acylation, as described below (Blanc et al. Citation2015; Sanders et al. Citation2015). Moreover, these methods can be applied to all kind of protein samples, including tissues from animal models and other biological samples. Several caveats need, however, to be highlighted. A main drawback of these methods is that the capture of proteins is based on the selective cleavage of thioester bonds; thus, enzymes forming non S-acylation-related thioester bonds will be picked up as false positives for S-acylation. These include ubiquitin conjugases and activating enzymes, subunits of the pyruvate dehydrogenase, fatty acid synthase, and other metabolic enzymes utilizing phosphopantetheine prosthetic groups or acting on acyl-CoA substrates (Roth et al. Citation2006; Kang et al. Citation2008). Additional false positives can be detected due to incomplete blockade of free thiol groups during the first step of the protocol. It is, moreover, important to note that this method does not discriminate a protein with one S-acylation site from one with multiple S-acylation sites. Thus, for a protein with several S-acylated residues, mutation of individual cysteines (or downregulation of cysteine-selective PATs) might not be translated into a reduced signal as the remaining cysteines would still be available for pull-down. Besides, capture efficiency might be influenced by a variety of factors, such as protein relative abundances and hydrophobicity. Therefore, these methods provide only semi-quantitative information. Finally, quantification requires the parallel analysis of a known amount of input sample. All in all, these thioester-based methods are very powerful and allow for a relatively easy and fast identification of S-acylation candidates in a variety of samples but careful analysis of the results should be performed to avoid overinterpretations when intended to obtain quantitative information.
Monitoring multi-S-acylation using a PEG-induced mass shift assay
None of the above methods provide information regarding the number of S-acylation sites, nor the abundance of the modified molecules. Therefore, the Fuller group developed a PEG switch assay (Howie et al. Citation2014). This assay is a modification of the Acyl-RAC method where protein samples are treated with 5 kDa or 10 kDa PEG-maleimide during neutral hydroxylamine incubation to allow for PEGylation of the freed cysteine residues. The Hang and Fukata groups have proposed modified versions of this method, which they called acyl-PEG exchange (Percher et al. Citation2016) or APEGS (Yokoi et al. Citation2016). In all these methods, for every reacted cysteine, PEGylation leads to a mass shift that can be observed by SDS-PAGE and western blotting. If a protein has two S-acylation sites, this would allow the detection of three bands, additionally providing some information on the relative abundance of doubly, singly, and non-acylated species. Note that even the singly modified species can be a mixed population (Dallavilla et al. Citation2016). Also, PEGylation efficiency might be affected by the distance between sites, and thereby potential steric hindrance, and by labeling probabilities. Therefore, PEGylation is particularly useful to estimate the fraction of non-acylated protein.
Given the specific advantages and disadvantages of the different methods available to monitor S-acylation (), a combination of at least one lipid-centric and one cysteine-centric methods is required to obtain a reliable view of the S-acylation status of a given protein.
Table 1. Methods to study protein S-acylation. See text for more details.
Global profiling of S-acylproteomes
The implementation of MS-based high-throughput approaches to study protein S-acylation has significantly contributed to the development of the field by providing S-acylation candidates for further follow-up studies as well as statistics on the cellular processes or compartments enriched in S-acylated proteins. To date, a total of 40 S-acylproteomics studies on several species (including unicellular eukaryotes, plants, and mammals) have been published. From these, 17 studies are based on lipid-centric approaches using click-chemistry (Martin and Cravatt, Citation2009; Yount et al. Citation2010; Wilson et al. Citation2011; Jones et al. Citation2012; Li et al. Citation2012; Martin et al. Citation2012; Zhang et al. Citation2013; Chesarino et al. Citation2014a; Colquhoun et al. Citation2015; Foe et al. Citation2015; Morrison et al. Citation2015; Peng and Hang Citation2015; Santiago-Tirado et al. Citation2015; Serwa et al. Citation2015; Hernandez et al. Citation2016; Sobocińska et al. Citation2018; Thinon et al. Citation2016), whereas 23 studies employ cysteine-centric approaches (mainly ABE or Acyl-RAC) (Roth et al. Citation2006; Kang et al. Citation2008; Yang et al. Citation2010; Dowal et al. Citation2011; Emmer et al. Citation2011; Forrester et al. Citation2011; Merrick et al. Citation2011; Ivaldi et al. Citation2012; Jones et al. Citation2012; Marin et al. Citation2012; Hemsley Citation2013; Wan et al. Citation2013; Ren et al. Citation2013a; Wei et al. Citation2014; Gould et al. Citation2015; Morrison et al. Citation2015; Caballero et al. Citation2016; Fang et al. Citation2016; Pinner et al. Citation2016; Upadhyay et al. Citation2016; Collins et al. Citation2017; Edmonds et al. Citation2017; Shen et al. Citation2017). The comparative analysis of the available S-acylproteomics studies represents a valuable source of information and a powerful hypothesis-generating engine. Particularly useful is the comparison of S-acylation candidate datasets obtained using complementary approaches (lipid- and cysteine-centric), which significantly increases the confidence of the analysis. SwissPalm, a comprehensive, manually curated database has been developed to compile all available studies on protein S-acylation, both proteomics-based and single protein studies, and facilitate comparisons (Blanc et al. Citation2015). The second version of SwissPalm (www.swisspalm.org) includes a total of 661 articles reporting on the potential S-acylation of almost 10,000 proteins from 55 organisms. Moreover, several user-friendly tools allow for the direct comparison of an input protein dataset with one or more of the available S-acylproteomics studies and a deeper exploration of the database.
DHHC protein S-acyltransferases
DHHC protein S-acyltransferases (DHHC PATs) were discovered decades after the first report on protein S-acylation, even after the identification of protein thioesterases, the enzymes catalyzing the reverse reaction. Genetic studies in yeast led to the identification of the multipass transmembrane proteins Erf2/Erf4 and Akr1 as the enzymes responsible for S-acylation of Ras2 and Yck2, respectively (Bartels et al. Citation1999; Lobo et al. Citation2002; Roth et al. Citation2002). The S-acyltransferase activity of Erf2 and Akr1 was shown to be dependent on an Asp-His-His-Cys (DHHC) motif present within a ∼50 residue-long cysteine-rich domain (CRD), giving the family its name and allowing the identification of S-acyltransferases in other organisms (Huang et al. Citation2004; Keller et al. Citation2004; Fukata et al. Citation2006; Mitchell et al. Citation2006). The DHHC S-acyltransferase family in yeast is composed of five (Schizosaccharomyces pombe) to seven (Saccharomyces cerevisiae) members and this number goes up to 24 in mammals. Other model organisms such as Caenorhabditis elegans and Drosophila melanogaster also have about 20 predicted DHHC PATs (Bannan et al. Citation2008; Edmonds and Morgan Citation2014; Fukata et al. Citation2016). Whether all members of the family are expressed and functional in each organism remains to be established. It is, however, intriguing that the number of enzymes is roughly conserved, suggesting that the diversity originated early in evolution and has been maintained.
In humans, most DHHC S-acyltransferase family members have four transmembrane domains (), except for zDHHC13, zDHHC17, and zDHHC23, which are predicted to have six, and zDHHC4 and zDHHC24 predicted to have five. These last two cases are curious as they imply that the N- (zDHHC4) or C-terminus (zDHHC24) face the luminal/extracellular space, whereas in all other DHHC PATs, both ends of the protein face the cytosol. Even more oddly, zDHHC22 is predicted in Uniprot (www.uniprot.org) to have only two transmembrane domains. Online prediction tools (TMPRED, TMHMM) however identify two additional transmembrane segments, as shown in (embnet.vital-it.ch/software/TMPRED_form.html, www.cbs.dtu.dk/services/TMHMM/) (). Importantly, the DHHC-CRD always localizes to the cytosolic side thus restricting DHHC PAT-mediated S-acylation to the cytosolic environment. Beyond the CRD there is little sequence homology between different family members, except for three short conserved motifs: an Asp-Pro-Gly (DPG) sequence located at the N-end of the cytosolic loop containing the DHHC-CRD; a Thr-Thr-Xxx-Glu motif (TTxE, where x represents any amino acid) and a 16 residue-long Palmitoyltransferase Conserved C-terminal (PaCCT) motif, both located after the last transmembrane domain () (Mitchell et al. Citation2006; González Montoro et al. Citation2009). As discussed later, N- and C-terminal domains might also contain protein-protein interaction domains/motifs involved in substrate specificity.
Table 2. Structural specificities of human DHHC S-acyltransferases (DHHC PATs).
DHHC PATs have been found in most compartments of the endomembrane system. A reliable assessment of their subcellular distribution has however been hindered by the low protein copy number of these enzymes () and the lack of good antibodies against the endogenous proteins. Studies using tagged DHHC PATs indicate that in S. cerevisiae the seven members of the family distribute between the endoplasmic reticulum (ER) (Erf2, Swf1, and Pfa4), the Golgi apparatus (Akr1 and Akr2), the vacuole (Pfa3), and the plasma membrane (Pfa5) (Ohno et al. Citation2006). In human HEK293T cells, most ectopic overexpressed tagged DHHC PATs were found to localize to the ER and the Golgi apparatus, while a few were found at the plasma membrane (Ohno et al. Citation2006). ER retention was shown to occur through C-terminal KKxx-like motifs for zDHHC4 and zDHHC6 (Gorleku et al. Citation2011; Abrami et al. Citation2017). zDHHC2 and zDHHC5 were found to traffic through the endocytic system in a manner dependent on their C-terminus (Greaves et al. Citation2011; Brigidi et al. Citation2015). In synaptic spines, these two enzymes were found in vesicles and at the plasma membrane, where they regulate the localization and assembly of synaptic proteins into domains (Fukata et al. Citation2016). Finally, zDHHC8 and zDHHC13 were shown to regulate mitochondria architecture and function (Maynard et al. Citation2008; Shen et al. Citation2017), but mitochondrial localization was not established. Also, these DHHC PATs were found to have roles in other subcellular compartments (Fernández-Hernando et al. Citation2006; Huang et al. Citation2009; Singaraja et al. Citation2009; Lemonidis et al. Citation2014).
Table 3. Protein abundance (in copy number per cell) for enzymes involved in protein S-(de)acylation, as reported in quantitative proteomics studies.
Structure and mode of catalysis of DHHC PATs
DHHC PATs have been proposed to function by following a two-step mechanism (Jennings and Linder Citation2012) where the acyl-CoA provides the acyl chain to the catalytic DHHC cysteine to form a transient acyl-enzyme intermediate. This step is often referred to as “autoacylation”. This nomenclature is however somewhat confusing because several DHHC PATs undergo regulatory S-acylation on cysteines outside the DHHC motif () (Abrami et al. Citation2017; Collins et al. Citation2017). In a second step, the acyl moiety is transferred from the cysteine of the enzyme to that of the protein substrate. As discussed later, this two-step model has strong implications on the potential substrate recognition mechanisms.
In a recent seminal paper, the Banerjee group reported the first X-ray structures of DHHC PATs (human zDHHC20 and zebrafish zDHHC15), comprising the transmembrane and catalytic domains (Rana et al. Citation2018). The two enzymes display remarkably similar structures. The four transmembrane domains (TMDs) adopt a teepee-like structure (with the top facing the lumenal/extracellular compartments and the bottom facing the cytosol), positioning the DHHC active site right at the cytosolic aqueous-membrane interface (). This location is consistent with and might explain the fact that cytosolic cysteine residues close to TMDs are so far the best S-acylation site candidates (Blanc et al. Citation2015). Concerning the zinc ions known to bind to the CRD, the X-ray structures reveal that they play critical structural roles, allowing the correct positioning of the DHHC active site residues and other residues involved in the catalytic site hydrogen bond network.
Figure 2. X-ray crystal structures of DHHC S-acyltransferases. (A) Cartoon representation of the X-ray crystal structures of human zDHHC20 (cyan, PDB 6BMN) and zebrafish zDHHC15 (blue, PDB 6BMS) highlighting their strong overlap. (B) Cartoon representation of human zDHHC20 covalently bound to 2-bromopalmitate (PDB 6BML). The 2-bromopalmitate molecule is shown as red and yellow spheres and the surface of the protein is depicted to highlight the insertion of the lipid into the hydrophobic cavity formed by the four transmembrane domains of the protein. (C) Cartoon and sticks representation of the active site and acyl chain cavity of human zDHHC20 covalently bound to 2-bromopalmitate (PDB 6BML). The four residues forming the DHHC motif are shown in cyan, the acyl chain of 2-bromopalmitate is shown in yellow and the hydrophobic residues contributing to the acyl chain binding cavity (as described in Rana et al. Citation2018) are shown in green. Highlighted in pink is residue Thr241 (from the TTxE motif) forming a hydrogen-bonding network with the catalytic residues (dashed lines). Zn ions are shown as spheres in all representations. The disposition of the protein relative to the membrane is approximately indicated by light brown lines. Figure created using Pymol v0.99 (DeLano Scientific, San Carlos, CA) (see color version of this figure at www.tandfonline.com/ibmg).
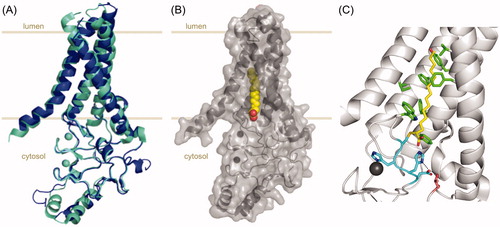
The most striking feature of this structure is the hydrophobic cavity stretching from the active site into the membrane (). All four TMDs contribute to this cavity, which accommodates the acyl chain to be transferred to the substrate. Through structure-guided mutagenesis, the authors proposed that the cavity acts as a “hydrocarbon ruler”, its geometry determining the acyl chain length preferences of the enzyme (Wyckoff et al. Citation1998). This structural analysis explains the experimentally observed acyl chain preferences of zDHHC3 and zDHHC7 and confirms the predictions made by the Chamberlain group in this respect (Greaves et al. Citation2017). Rana, Kumar et al. propose an enzymatic mechanism based on their structural observations, which is consistent with the two-step model. They show that the first two residues of the DHHC motif form a hydrogen-bonded pair and speculate that this histidine residue could initially activate the DHHC cysteine for the nucleophilic attack on the carbonyl-thioester of the acyl-CoA molecule and later on activate the acyl-enzyme thioester for acylation of the protein substrate.
For some DHHC PATs, such as the yeast Swf1 and Pfa4 and the human zDHHC17, acyl-enzyme intermediates could not be detected (González Montoro et al. Citation2015; Greaves et al. Citation2017; Verardi et al. Citation2017), raising the possibility of an alternative mechanism in which the first histidine residue of the DHHC motif would favor the direct nucleophilic attack of the protein substrate to the acyl-CoA (González Montoro et al. Citation2015). Whether or not an acyl-enzyme intermediate forms, it seems that the first histidine residue of the DHHC motif plays a critical role in catalysis. Interestingly, this residue is replaced by a glutamine residue in human and murine zDHHC13. Whereas this replacement is isosteric and it is not expected to introduce major structural perturbations at the active site, it is unclear whether this glutamine could play a role in the nucleophilic attack or an alternative mechanism needs to be evoked (Mitchell et al. Citation2010). Finally, Rana, Kumar et al. speculate that the acyl-CoA inserts into the hydrophobic cavity directly from within the membrane (Rana et al. Citation2018). Whether this is actually the case remains to be addressed. It would require lateral opening of the teepee-like structure to allow membrane-anchored acyl-CoA to diffuse into the cavity. Moreover, considering that DHHC PATs are low abundance proteins () and at risk of being outcompeted by more abundant enzymes involved in lipid biosynthesis and degradation, they might rely on acyl-CoA binding proteins to deliver acyl-CoA to them.
The observed teepee-like structure is probably common to all members of the DHHC S-acyltransferase family and will be invaluable in guiding the rational design of inhibitors, a major unmet need in the field.
Acyl chain selectivity of DHHC PATs
As alluded to earlier, S-acylation is not restricted to S-palmitoylation. Chains of different lengths and saturation may also be transferred. Indeed, a study in human platelets revealed that 74% of the pool of S-acylated proteins was bound to C16:0 while the remaining 26% was bound to C18:0 or C18:1 acyl chains (Muszbek et al. Citation1999). While these proportions might partly be due to the relative abundances of the corresponding acyl-CoA, DHHC PATs may have an acyl chain preference as first reported by the Linder group (Jennings and Linder Citation2012). Using purified DHHC PATs in micelles, zDHHC2 was found to incorporate and transfer a broad range of acyl-CoA chain lengths, while zDHHC3 showed a clear preference for C14 (myristoyl-CoA) and C16 (palmitoyl-CoA and palmitoleoyl-CoA) chains over longer fatty acids. More recently, the Chamberlain group obtained comparable results using clickable acyl-CoA analogs in cell culture (Greaves et al. Citation2017). zDHHC2 and zDHHC4 did not show any fatty acid preference; zDHHC3, 5, 7, 11, and 15 preferred C14 and C16 fatty acids over C18 chains; zDHHC17 preferred C16 and C18 over C14; and finally zDHHC23 displayed a marked preference for C18. The acyl chain preference appears to be determined at the formation of the acyl-enzyme intermediate and is thus dictated by the geometry of the hydrophobic cavity revealed by the X-ray structures (Jennings and Linder Citation2012; Greaves et al. Citation2017; Rana et al. Citation2018). This is consistent, for instance, with the observation that coimmunoprecipitation of zDHHC3 with its substrate NCAM180 was only observed for the wild-type protein and not for the catalytic-defective Cys157Ser mutant, suggesting that in this case the formation of the acyl-enzyme intermediate is required for the interaction with the protein substrate (Lievens et al. Citation2016).
Circumstantial evidence, however, indicates that protein substrates might also dictate acyl length preference. Influenza A virus hemagglutinin has three “S-acylatable” cysteine residues, two of which were found to be modified by palmitate (C16:0) and one by stearate (C18:0) (Kordyukova et al. Citation2008). Also, zDHHC6 was found to catalyze the preferential addition of C16:0 to the ER chaperone calnexin (Lakkaraju et al. Citation2012) but of both C16:0 and C18:0 to transferrin receptor (Senyilmaz et al. Citation2015).
Protein substrate recognition by DHHC PATs
One of the remaining enigmas in the field is: what makes a cytosolic cysteine an S-acylation site? Is it protein structure? Is it sequence? Is it its proximity to membranes? What controls the enzyme-protein substrate specificity?
A first layer of specificity is provided by the subcellular localization, since the presence of the enzymes in a specific membrane-bound compartment confines their action to transmembrane protein substrates that pass by or reside in this compartment. For soluble proteins, it is generally believed that for S-acylation to occur, the protein substrate needs to weakly bind to membranes through either another lipidation event (e.g. myristoylation or prenylation) or some intrinsic weak membrane affinity (electrostatic or hydrophobic) (Shahinian and Silvius Citation1995; Greaves et al. Citation2008, Citation2009; Weber et al. Citation2017). While some DHHC PATs have been shown to preferentially acylate soluble, transmembrane, or lipidated protein substrates (Roth et al. Citation2006; González Montoro et al. Citation2011; Ohno et al. Citation2012), a firm conclusion on this classification awaits a comprehensive view of enzyme-substrate pairs.
Protein substrate recognition can also be mediated by protein-protein interaction motifs/domains in the N- or C-termini of DHHC PATs. zDHHC3, 5, and 8 contain potential PDZ-binding motifs; zDHHC13 and 17 contain several N-terminal ankyrin repeats; and zDHHC6 contains a C-terminal SH3_2 domain (Singaraja et al. Citation2002; Tsutsumi et al. Citation2008; Gottlieb and Linder Citation2017). The ankyrin repeats of zDHHC13 and 17 mediate their interaction with proteins such as huntingtin (HTT), SNAP23, SNAP25, and cysteine string protein (CSP). Moreover, an ankyrin repeat-binding short linear motif was identified in these and other protein substrates (Huang et al. Citation2009; Lemonidis et al. Citation2014, Citation2015). The ankyrin repeat domain of zDHHC17 (ANK17) was co-crystalized with the SNAP25b recognition motif revealing key residues involved in binding that were also present in another substrate, HTT (Gao et al. Citation2009; Verardi et al. Citation2017). When an ankyrin repeat domain was added to zDHHC3, the enzyme acquired the ability to S-acylate HTT (Huang et al. Citation2009). Similarly, the PDZ-binding domains of zDHHC5 and 8 are required to recruit their respective substrates Grip1 and Pick1 (Thomas et al. Citation2012, Citation2013). The presence of a classical protein-protein interaction domain does not exclude binding to some protein substrates through other domains. zDHHC5 was indeed found to bind phospholemman through an intrinsically disordered 120 residue-long region after the fourth transmembrane domain (TMD) (Howie et al. Citation2014). Interaction with their protein substrates through disordered regions could be a common theme amongst DHHC PATs () (Howie et al. Citation2014; Gottlieb and Linder Citation2017). It is important to note that interaction of a protein that can undergo S-acylation with a member of the DHHC S-acyltransferase family does not imply that it is a substrate of that enzyme (see section Non-enzymatic functions of DHHC PATs). For instance, zDHHC13 strongly interacts with SNAP25 and CSP, but does not modify them (Lemonidis et al. Citation2014).
On the protein substrate side, specific sequences or regions involved in interaction with their S-acyltransferases have also been reported. For instance, the C-terminal membrane-binding domain of ClipR-59 was shown to mediate the interaction of the protein with its S-acyltransferase zDHHC17 (Ren et al. Citation2013b). In turn, an amphipathic α-helix in the large intracellular loop of the electrogenic sodium/calcium exchanger NCX1 was recently found to critically control the S-acylation of one specific cysteine residue (Plain et al. Citation2017). A potential interplay between an amphipathic helix and the S-acylation of nearby cysteine residues has also been suggested for the interferon-induced transmembrane protein IFITM3 (Chesarino et al. Citation2017).
Strikingly, some residues or regions were shown to dictate the interaction of a protein substrate with only one of its several DHHC PATs. The interaction between SNAP25b and one of its S-acyltransferases, zDHHC17, was shown to be regulated by a proline residue (Pro117) near the S-acylation sites (Greaves et al. Citation2009), which it is proposed to constrain SNAP25b in order to fit into the concave side of the ANK17 domain (Verardi et al. Citation2017). This residue however does not seem to be important for the interaction of SNAP25b with zDHHC3 or 7, also responsible of its S-acylation but not having ankyrin repeat domains (Greaves et al. Citation2009). Similarly, the closely related SNAP25 and SNAP23 SNARE proteins are not S-acylated by the exact same set of DHHC PATs as zDHHC15 is active against SNAP25b but not SNAP23. However, a single Cys-to-Phe mutation in the cysteine-rich domain of SNAP23 renders it susceptible to zDHHC15-mediated S-acylation (Greaves et al. Citation2010). In yeast, it was shown that the vacuolar protein Vac8 is S-acylated exclusively by Pfa3, but the isolated SH4 domain of Vac8 (containing the cysteine residues target of S-acylation) could get S-acylated by all five DHHC PATs tested in the study (Nadolski and Linder Citation2009).
The proximity of cysteine residues to TMDs makes them strong candidates for S-acylation, despite the fact that this is not picked up by the available prediction programs (Blanc et al. Citation2015). In a recent study based on native MS, Rodenburg et al. proposed that S-acylation occurs stochastically whenever a cysteine localizes at the appropriate distance from a membrane (Rodenburg et al. Citation2017). In support of their hypothesis, they observed that prokaryotic proteins (normally not S-acylated (Mitchell et al. Citation2006)) undergo S-acylation when ectopically expressed in human cells. Mutagenesis experiments on the mammalian hepatic asialoglycoprotein receptor (ASGP-R) similarly indicated that the distance of the cysteine residue relative to the TMD is the major determinant for its S-acylation, more so than the identity of surrounding amino acids (Yik and Weigel Citation2002). In contrast with these reports, early studies on viral glycoproteins showed that the spacing between the cysteine residue and the TMD was not sufficient but rather S-acylation was promoted by conformational signals contained in the sequence of the transmembrane and cytoplasmic domains (Ponimaskin and Schmidt Citation1998). Likewise, MS-based thermodynamics studies indicated that a proline residue at the -1 position of the target cysteine increases flexibility and lowers the enthalpic barrier for the S-acylation reaction (Khanal et al. Citation2015). The presence of prolines was similarly found to influence other post-translational modifications, such as phosphorylation and N-glycosylation (Bause Citation1983; Zhu et al. Citation2005; Alexander et al. Citation2011). When analyzing reported S-acylation sites, proline residues at the -1 position are however relatively rare. In contrast, positively charged residues are frequently found after S-acylation sites (Blanc et al. Citation2015) and it has been suggested that this could favor binding of the negatively charged acyl-CoA molecule and/or increase the ionization of the target thiol (Bizzozero et al. Citation2001). Thus, while juxtamembrane cysteines are frequent S-acylation sites, not all proteins having cysteine residues at this location get S-acylated. Moreover, it is unclear that their S-acylation is stochastic since this would indicate that any DHHC PAT that localizes to the same membranes could modify target proteins, which conflicts with reported enzyme–protein substrates specificities. Also, stochasticity is a priori incompatible with our observations that the two contiguous “acylatable” cysteine residues in the ER chaperone calnexin get S-acylated in a preferred sequential manner (Dallavilla et al. Citation2016). It thus remains to be investigated whether the reported stochastic S-acylation corresponds to an enzymatic or non-enzymatic process.
Finally, whether it is important for substrate recognition or not, some DHHC PATs require cofactors to be active. In S. cerevisiae, Erf2 was shown to depend on Erf4 to acylate Ras (Lobo et al. Citation2002). Interestingly, this requirement seems to be evolutionarily conserved as the Erf2 orthologs Sp-Erf2 in S. pombe and zDHHC9 in human rely on Sp-Erf4 and GCP16, respectively, to modify Ras (Swarthout et al. Citation2005; Young et al. Citation2014). Mammalian genomes contain several GCP16 related proteins suggesting that other DHHC PATs might also employ cofactors (Tsutsumi et al. Citation2008). Human zDHHC6 was reported to interact with selenoprotein K (also known as SelK or SelenoK) via its SH3_2 domain and this interaction to be required for activity (Fredericks et al. Citation2014). SelK was first found as necessary for the palmitoylation of CD36 in macrophages (Meiler et al. Citation2013) and later on proposed to be a zDHHC6 cofactor for the S-acylation of the inositol 1,4,5-triphosphate (IP3) receptor type 1 (IP3R1) and of ASAP2 (Fredericks et al. Citation2014; Fredericks and Hoffmann Citation2015; Norton et al. Citation2017).
Non-enzymatic functions of DHHC PATs
It is important to keep in mind that some DHHC PATs might exert functions that are independent of their S-acyltransferase activity. zDHHC17 was found to contribute to acute ischemic brain injury through interaction with c-Jun N terminus kinase (JNK) and recruitment of its mitogen-activated protein kinase kinase MKK7, independently of its S-acyltransferase activity (Harada et al. Citation2003; Yang and Cynader Citation2011). Both zDHHC13 and 17 were reported to mediate Mg2+ transport and proposed to be “chanzymes”, i.e. proteins mediating ion transport and bearing an enzymatic activity (Goytain et al. Citation2008). zDHHC1 was found to be a positive regulator of STING (stimulator of interferon genes), involved in DNA virus-triggered innate immunity (Zhou et al. Citation2014). Intriguingly, STING does undergo S-acylation and this is necessary for its ability to signal (see below) (Mukai et al. Citation2016), yet S-acylation is not mediated by zDHHC1. zDHHC1 appears to act upstream of STING, independently of its DHHC motif, promoting STING multimerization and recruitment of the downstream signaling components TBK1 and IRF3 (Zhou et al. Citation2014). A role for zDHHC1 in STING-dependent innate immune signaling was also reported in teleosts but whether this required acyltransferase activity was not addressed (Xu et al. Citation2017). The S-acyltransferase zDHHC16 was first named Abl-philin 2 (Aph2) given it was identified as a c-Abl-interacting protein (Li et al. Citation2002). Aph2 was proposed to regulate the levels of c-Abl and jointly affect apoptosis irrespective of the presence of its DHHC motif. zDHHC16 has subsequently been linked to DNA damage repair. In the latter case, however, its involvement required the S-acyltransferase activity (Cao et al. Citation2016).
In yeast, Akr1 was shown to be responsible for the suppression of Gβγ-mediated mating signaling pathway in the absence of mating pheromone independently of its S-acyltransferase activity (Hemsley and Grierson Citation2011). Gβγ requires S-acylation for plasma membrane targeting but this modification is mediated by Erf2 and Swf1 (Roth et al. Citation2006). In turn, Swf1 might also carry out non-canonical functions, since both the wild-type and DHHA variants could regulate the actin cytoskeleton and polarized secretion by interacting with the Rho GTPase Cdc42 (Dighe and Kozminski Citation2008). DHHA Swf1 might, however, have partial S-acyltransferase activity since it was shown that a DHHR Swf1 variant as well as DHHA and DHHR variants of Pfa4 retain partial activity (González Montoro et al. Citation2015). Whether canonical and non-canonical functions of these enzymes are coordinated or whether they influence one another is currently unknown.
Acyl protein thioesterases
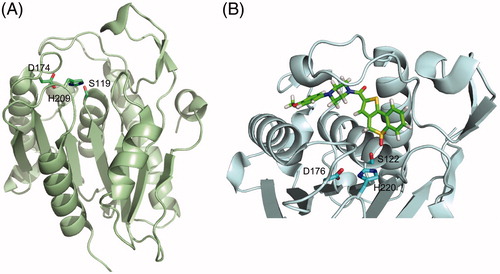
The S-deacylation reaction is catalyzed by a set of enzymes named acyl protein thioesterases (APTs) or deacylases/depalmitoylases, belonging to the metabolic serine hydrolase superfamily (mSH) (Long and Cravatt Citation2011). Members of the mSH family adopt an α/β hydrolase fold, consisting of a central β-sheet surrounded by α-helices, and rely on an active site serine for catalysis. This serine is part of a catalytic dyad or triad, which increases its nucleophilicity and allows for the attack of the substrate (Long and Cravatt Citation2011). The first deacylase identified was palmitoyl-protein thioesterase 1 (PPT1) (Camp and Hofmann Citation1993) but it was later shown to reside inside lysosomes and thus not responsible for deacylation of cytosolic substrates (Verkruyse and Hofmann Citation1996). Its homolog PPT2 appears to be devoid of catalytic activity (Soyombo and Hofmann Citation1997; Calero et al. Citation2003). Two other members of the family, APT1 and APT2 (also called LYPLA1 and LYPLA2, respectively), were first identified as lysophospholipases (Sugimoto et al. Citation1996; Toyoda et al. Citation1999) but later shown to have enhanced activity as protein thioesterases (Duncan and Gilman Citation1998; Rocks et al. Citation2005; Rusch et al. Citation2011). The crystal structures of human APT1 and APT2 confirm they have a classical catalytic triad with the nucleophilic residue in a strained conformation between a β-strand and an α-helix, although they adopt a rather atypical α/β hydrolase fold with a seven-stranded β-sheet () (Devedjiev et al. Citation2000; Won et al. Citation2016). A detailed review on protein depalmitoylases has been recently published (Won et al. Citation2018).
Figure 3. Tridimensional structure of acyl protein thioesterases. (A) Cartoon representation of the X-ray crystal structure of human APT1 (PDB 1FJ2) showing its seven-stranded α/β hydrolase fold and highlighting the position of the catalytic triad (Ser119, Asp174, and His209, shown as sticks). (B) Cartoon and sticks representation of the active site of human APT2 co-crystallized with its selective inhibitor ML349 (PDB 5SYN). The inhibitor is shown in green and the catalytic triad Ser122-Asp176-His220 is highlighted in cyan. Figure created using Pymol v0.99 (DeLano Scientific, San Carlos, CA) (see color version of this figure at www.tandfonline.com/ibmg).
Few targets have so far been reported for these acyl protein thioesterases but some substrate specificity is already apparent. APT2 but not APT1 hydrolyzes prostaglandin glycerol esters (Manna et al. Citation2014) and regulates the dynamic S-acylation of human zDHHC6 (Abrami et al. Citation2017), GAP-43 (Tomatis et al. Citation2010), and Scrib (Hernandez et al. Citation2017). In contrast, BK channel deacylation was reported to be catalyzed by APT1 but not by APT2 (Tian et al. Citation2012).
APT1 and APT2 were reported, and widely accepted, to display a cytosolic localization with preferred membrane association to the Golgi apparatus and the plasma membrane based on overexpression of fusion proteins using myc-FLAG (Kong et al. Citation2013) or mCitrine (Vartak et al. Citation2014) tags. We have, however, recently found that endogenous APT1 or APT1 overexpressed as a fusion to small tags localize primarily (although not exclusively) to mitochondria, whereas overexpression of APT1 using a bulky tag such as mCitrine prevents it from accumulating in mitochondria (Kathayat et al. Citation2018). While Kong et al. (Citation2013) concluded that there was a clear plasma membrane staining, they did not discuss the membrane-associated intracellular staining observed in their microscopy images, which, to our opinion, is not incompatible with a mitochondrial localization of their construct. APT2 does not display this sensitivity to tag size showing a broad distribution including the cytosol, the Golgi apparatus and the plasma membrane (Kong et al. Citation2013; Vartak et al. Citation2014; Kathayat et al. Citation2018). Whereas APT1 has been included in the Mitocarta compendium as a potential mitochondrial protein (Pagliarini et al. Citation2008; Calvo et al. Citation2016), no clear mitochondrion-targeting sequence has been reported. Besides, given the high sequence identity between APT1 and APT2, it is not known which features determine their differential subcellular localization. Mitochondria-targeting sequences are predicted to form amphipathic α-helices and to be enriched in positively charged residues (Bolender et al. Citation2008). A close examination of the N-termini of APT1 and APT2 reveals the presence of three negatively charged residues in APT2, absent in APT1, which we speculate could interfere with a potential weak mitochondrial targeting. So far, no human diseases have been linked to mutations in the genes encoding APT1 or APT2. Still, these proteins are remarkably conserved across mammals and beyond, suggesting that their functions (which may not be restricted to protein S-deacylation) are under strong evolutionary pressure.
An homolog of APT1, LYPLAL1, was reported to mediate deacylation of BK channels (Tian et al. Citation2012). However, the X-ray crystal structure of LYPLAL1 reveals that this enzyme has a shallow, solvent-accessible active site with some charged residues preventing the formation of a hydrophobic cavity of the required size to fit long acyl chains as those bound to S-acylated proteins (Bürger et al. Citation2012). Interestingly, the main residues affecting the shape of the active site are highly conserved in the LYPLAL but less conserved in the LYPLA subfamily. Furthermore, the LYPLAL subfamily seems to be present only in metazoans (Bürger et al. Citation2012). These observations suggest that the LYPLAL subfamily might play an important role hydrolyzing yet unidentified small substrates.
In the search for additional deacylases, the α/β-hydrolase domain-containing protein 17 members A, B, and C (ABHD17A-C) were found to deacylate N-Ras and the neuronal protein PSD-95 (Lin and Conibear Citation2015; Yokoi et al. Citation2016; Lin et al. Citation2017). Also, ABHD17A-C were reported to mediate dynamic S-acylation of microtubule-associated protein 6 (MAP6), controlling its shuttling between membranes and microtubules and its retention in axons (Tortosa et al. Citation2017). ABHD12 and ABHD13 have also been reported to reduce PSD-95 S-acylation upon overexpression (Yokoi et al. Citation2016). Most likely other acyl protein thioesterases remain to be identified. Protein thioesterases (notably APT1 and APT2) are much more abundant than S-acyltransferases (copy numbers per cell up to several orders of magnitude higher, ). A similar quantitative balance (i.e. more genes codifying for the direct enzymes and less but more abundant proteins catalyzing the reverse reaction) has been reported for kinases and phosphatases as well as regulators of histone acetylation and protein ubiquitination, suggesting a general feature of reversible post-translational modifications (Smoly et al. Citation2017).
Interestingly, APT1, APT2, and ABHD17A-C all have been reported to undergo S-acylation near their N-terminus and that this acylation controls their localization (Yang et al. Citation2010; Kong et al. Citation2013; Vartak et al. Citation2014; Won et al. Citation2018). An additional S-acylation site has been identified by MS for APT1 but not yet characterized (Yang et al. Citation2010). APT1 was reported to catalyze its own deacylation and that of APT2, and knockdown of APT1 was shown to promote APT2 plasma membrane association, but not vice versa (Kong et al. Citation2013). Whether S-acylation (by which DHHC PATs?) regulates APT function remains to be addressed.
Deacylase substrate specificity
Very little is known about substrate recognition by acyl protein thioesterases. While deacylases other than APT1/2 and ABHD17 are likely to exist, the lower number of different deacylating enzymes compared to S-acyltransferases suggests that the former are more promiscuous. Several reports, however, indicate that enzymatic deacylation also relies on protein substrate recognition mechanisms (Tomatis et al. Citation2010; Lin and Conibear Citation2015; Abrami et al. Citation2017; Hernandez et al. Citation2017; Tortosa et al. Citation2017). Besides, as for DHHC PATs, differential subcellular localization of deacylases would already restrict their set of substrates. We have recently shown that APT1, but not APT2, localizes primarily to mitochondria, which immediately opens a new spectrum of substrates for APT1 inaccessible to APT2 (Kathayat et al. Citation2018).
Interestingly, recognition by APTs can also be controlled by conformational changes in the protein substrate. S-deacylation of H-Ras has indeed been shown to be dependent on the cis-trans isomerization of a peptidyl-prolyl bond nearby the S-acylated cysteine residues (Ahearn et al. Citation2011). The prolyl-isomerase FKBP12 was shown to bind to H-Ras in an acylation-dependent manner and promote protein S-deacylation. The use of peptidyl-prolyl isomerization as a molecular timer to trigger protein S-deacylation could extend beyond this particular example of H-Ras, as it has been reported that proline residues are enriched close to S-acylation sites (Khanal et al. Citation2015).
Inhibitors and probes
The S-acylation field has suffered from the lack of good probes (there are no commercially available antibodies against acylated cysteines) and specific inhibitors for both S-acylation and deacylation. In the past two decades, 2-bromopalmitate (2-BP) () has been extensively used as a general inhibitor of protein S-acylation. Inhibition of DHHC PATs is based on the formation of an irreversible covalent bond between the thiolate group of the catalytic cysteine (from the DHHC motif) and the second carbon of the 2-BP molecule, with the bromide acting as an excellent leaving group in the nucleophilic substitution reaction. In 2013, the Martin group published an MS-based profiling study where they identified about 450 protein targets of the click-chemistry analog of 2-BP, 2BPN3; among them several (but not all) DHHC S-acyltransferases (Davda et al. Citation2013). Alarmingly, most of the identified 2BPN3 targets were proteins unrelated to S-acylation including carriers, transporters, and channels, as well as many proteins known to be susceptible of S-acylation themselves (Davda et al. Citation2013). Similar results were obtained by Zheng et al. (Citation2013) using other 2-BP-based probes. Furthermore, 2-BP was long-ago shown to inhibit the activity of carnitine palmitoyl transferase (Chase and Tubbs Citation1972), thus disrupting cellular lipid metabolism, and even responsible of the in vitro inhibition of enzymes that are not related to lipid metabolism (Coleman et al. Citation1992). Whereas 2-BP treatment has been considered a useful tool to study protein S-acylation, the available evidence on its off-target effects and cellular toxicity prevents firm conclusions to be drawn (Lanyon-Hogg et al. Citation2017).
Figure 4. Chemical structures of the S-(de)acylation inhibitors and probes mentioned in this review (see color version of this figure at www.tandfonline.com/ibmg).
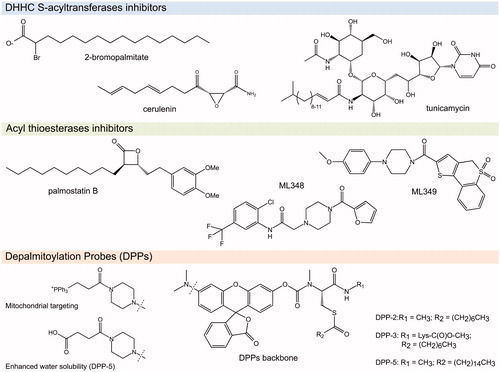
Likewise, the natural antifungal and antibacterial agent cerulenin () was reported to inhibit protein S-acylation (Lawrence et al. Citation1999) but it is also known to bind and inhibit β-keto-acyl-ACP synthase, HMG-CoA synthetase and other lipid metabolic enzymes (Resh Citation2006). The natural compound tunicamycin was also shown to inhibit protein S-acylation (Patterson and Skene Citation1994) but its major application in cell biology relies on its inhibitory effects on protein glycosylation (Kuo and Lampen Citation1974). Fortunately, the effects of these two compounds beyond protein S-acylation are better described and thus their use as exclusive protein S-acylation inhibitors is rare.
A rather similar situation held true, until recently, for the inhibition of the S-deacylation reaction. The acyl-β-lactone Palmostatin B () was originally reported to be an APT1-specific inhibitor (Dekker et al. Citation2010) and used as such in several studies but later on found to act on APT2 as well (Rusch et al. Citation2011). Moreover, activity-based protein profiling studies indicate that this compound can also inactivate several other lipid-processing serine hydrolases, including members of the ABHD17 family, and even enzymes unrelated to protein S-deacylation such as PNPLA6 and FASN (Lin and Conibear Citation2015). Other serine hydrolases inhibitors with varied activity on APTs (including other β-lactones, piperazine amides, benzodiazepinediones, boronic acids, triazole ureas, and chloroisocoumarins) have been reported and are reviewed elsewhere (Davda and Martin Citation2014). Especially in the case of deacylases, where it is probable that other S-deacylating enzymes exist, the use of so-called specific inhibitors demands special caution. As a promising alternative, Adibekian et al. have reported the design of two reversible selective inhibitors: ML348 acting on APT1, and ML349 acting on APT2 (Adibekian et al. Citation2010a, Citation2010b). Evidence on their selectivity in cellulo is starting to become available (Vujic et al. Citation2016; Hernandez et al. Citation2017). These results also bring optimism to the S-acylation field in the quest for DHHC PAT-selective inhibitors. It has been long thought that the high sequence conservation around the catalytic site of DHHC PATs would prevent the design of selective inhibitors. APT1 and APT2 share more than 60% sequence identity. The crystal structures of the APT1-ML348 and APT2-ML349 complexes reveal that despite the remarkable structural similarity of both polypeptides, each compound adopts a distinct conformation within the active site leading to selective inhibition () (Won et al. Citation2016).
Finally, promising fluorescent in vivo depalmitoylation activity probes, named DPPs, were recently developed by the Dickinson group (Kathayat et al. Citation2017; Qiu et al. Citation2018; Kathayat et al. Citation2018). DPPs feature a pro-fluorescent molecule tethered through a carbamate linkage to an S-acylated peptide substrate and have been tuned to localize to specific compartments (DPP-2 and DPP-3 in cytosol, and mito-DPP-2 and mito-DPP-3 in mitochondria), to react better against APT1 than APT2 (DPP-3 versus DPP-2 variants), or to have increased water solubility (DPP-5) ().
Non-enzymatic protein S-acylation and deacylation
While in cells S-acylation is generally thought to be enzymatic, this reaction can occur spontaneously in vitro, as was shown for several proteins when mixed with palmitoyl-CoA in absence of any enzymes (Bizzozero et al. Citation2001; Yang et al. Citation2005; Kümmel et al. Citation2006; Jenkins et al. Citation2011; Chan et al. Citation2016). Besides, due to the intrinsic lability of the thioester linkage, it is assumed that steady state non-enzymatic hydrolysis may occur leading to deacylation (Davda and Martin Citation2014). Still, to what extent spontaneous S-(de)acylation occurs in vivo and what its physiological relevance is remains unknown. In vivo, acyl-CoA binding proteins (ACBPs) bind medium and long-chain acyl-CoA with high affinity and act as intracellular carriers thus regulating acyl-CoA availability (Knudsen et al. Citation2000; Burton et al. Citation2005). Early work has shown that addition of ACBP at physiological molar ratios compared with long-chain acyl-CoA strongly inhibited spontaneous protein S-acylation in vitro (Leventis et al. Citation1997). In turn, the addition of ACBP was shown to have only a minor inhibitory effect on in vitro PAT-mediated S-acylation (Dunphy et al. Citation2000). Calculations based on reported cytosolic concentrations of acyl-CoA and ACBP in mammalian cells suggest that physiological non-enzymatic protein S-acylation would be strongly suppressed and thus enzyme-mediated S-acylation is likely the predominant mechanism in vivo (Leventis et al. Citation1997; Dunphy et al. Citation2000; Knudsen et al. Citation2000). It remains unknown whether DHHC PATs use ACBP-acyl-CoA complexes as substrates or they are able to efficiently function at the very low concentrations of free acyl-CoA available in the cytosolic environment (Faergeman and Knudsen Citation1997).
Interestingly, the transport protein particle component Bet3 and the TEAD transcription factors were reported to undergo non-enzymatic “autoacylation”, which was shown to be non-dynamic and to play a role in protein structure and/or stability (Kümmel et al. Citation2006; Chan et al. Citation2016; Noland et al. Citation2016). Proteins having high affinity acyl-binding pockets might represent a special case in this respect.
A different situation could take place at subcellular compartments with different physiological concentrations of acyl-CoA and other players, such as cytosolic sites of acyl-CoA generation or inside mitochondria (Faergeman and Knudsen Citation1997). Whether local high concentrations of acyl-CoA in the cytosol are linked to non-enzymatic or compartmentalized DHHC PAT-mediated protein S-acylation remains to be explored. A variety of proteins in the mitochondrial matrix, including >methylmalonate semialdehyde dehydrogenase, aldehyde dehydrogenase, glutamate dehydrogenase, nicotinamide nucleotide transhydrogenase, adenine nucleotide transferase, carbamoyl-phosphate synthetase 1, and several mitochondrial carriers have been found to be S-acylated. In some cases, S-acylation was shown to occur at the active site and resulted in enzyme inhibition (Stucki et al. Citation1989; Berthiaume et al. Citation1994; Corvi et al. Citation2001; Kostiuk et al. Citation2008). So far, no DHHC PATs have been reported in mitochondria, even though, as discussed above, protein S-deacylation was found to occur in mitochondria (Kathayat et al. Citation2018). Together these observations raise the possibility that enzymatic S-deacylation could play a critical role in mitochondria homeostasis by “repairing” uncontrolled, spontaneous S-acylation of mitochondrial proteins, as proposed for sirtuin-3 in reverting toxic lysine acetylation (Wagner and Hirschey Citation2014; Weinert et al. Citation2015).
Physiological relevance of S-acylation
While it has been known for many years that diverse physiological processes critically depend on protein S-acylation, the true importance of this modification is only starting to be revealed. Recent reviews have summarized the roles of S-acylation in synaptic plasticity, immunological synapses, host–pathogens interactions, and membrane trafficking (Fukata and Fukata Citation2010; Aicart-Ramos et al. Citation2011; Blanc et al. Citation2013; Chamberlain and Shipston Citation2015; Fukata et al. Citation2016; Globa and Bamji Citation2017), therefore, these subjects will not be covered here. Additionally, recent studies highlighted the role of protein S-acylation in modulating membrane curvature in Influenza A virus assembly (Chlanda et al. Citation2017); regulating the shuttling of SCP1 between the nucleus and other subcellular compartments (Liao et al. Citation2017); among others. This section provides selected recent examples illustrating important regulatory roles of protein S-acylation in several physiological processes.
Interplay with ubiquitination and protein homeostasis
Work from several groups, including ours, has highlighted the interplay between protein S-acylation and ubiquitination and the regulation of protein homeostasis. S-acylation of the yeast SNARE protein Tlg1 was shown to prevent its ubiquitination and subsequent degradation in the vacuole by reducing the accessibility of the lysine residues nearby the S-acylation site to the E3 ubiquitin ligase (Valdez-Taubas and Pelham Citation2005). Likewise, S-acylation of the anthrax toxin receptors TEM8 and CMG2 prevent their association with lipid rafts and, thus, their premature ubiquitination and lysosomal degradation (Abrami et al. Citation2006). In the biosynthetic pathway, S-acylation of the yeast chitin synthase Chs3 and the Wnt signaling co-receptor LRP6 was shown to be required for their ER export (Lam et al. Citation2006; Abrami et al. Citation2008). In the latter case, we proposed the existence of a ubiquitin-dependent folding system controlling the proteostasis of poor folders, in which S-acylation would prevent targeting to ER-associated degradation (ERAD) allowing proteins more time to fold and thus increase the flux of proteins exiting the ER (Abrami et al. Citation2008; Perrody et al. Citation2016).
The interferon-induced transmembrane protein 3 (IFITM3, see below) was also shown to be regulated by the opposing effects of S-acylation and ubiquitination (Yount et al. Citation2012). Along the same line, S-acylation of the related protein IFITM1 and of the chemokine receptor CCR5 regulates protein turnover by preventing their proteasomal or lysosomal degradation, respectively (Percherancier et al. Citation2001; Hach et al. Citation2013). Interplay between S-acylation and ubiquitination was also reported for sortilin, a sorting receptor involved in targeting proteins to the secretory or endocytic compartments. S-acylation enables sortilin to be recycled back from endosomes to the Golgi apparatus whereas non-S-acylated sortilin gets mono-ubiquitinated and trafficked to lysosomes for degradation (McCormick et al. Citation2008; Dumaresq-Doiron et al. Citation2013). Notably, dimerization of sortilin through a disulfide bond involving the same cysteine residue identified as S-acylation site leads to trafficking of sortilin to extracellular vesicles (Itoh et al. Citation2018). Thus, the amount and localization of sortilin appears to depend on the coordinated regulation of S-acylation, ubiquitination and disulfide bond formation.
The counteracting effects of S-acylation and ubiquitination are likely to be widespread. Systems analysis, aided by the S-palmitoylation database SwissPalm, has indeed revealed that whereas ubiquitination sites are annotated for about one third of the total human proteome, this number almost doubles when considering the human candidate S-acylproteome (Blanc et al. Citation2015). Individual knock-out of zDHHC2, 5, or 6 in HAP1 cells was associated with a general increase in protein ubiquitination (Blanc et al. Citation2015).
Moreover, several proteins involved in protein quality control are S-acylation substrates, including the ER chaperone calnexin, the transitional ER ATPase (VCP), several components of the CCT micro complex (TRiC), and the E3 ligase gp78 (Fairbank et al. Citation2012; Lakkaraju et al. Citation2012; Lynes et al. Citation2012; Blanc et al. Citation2015; Fang et al. Citation2016). Thus, the impact of S-acylation on protein turnover is not limited to its direct effect on a particular protein but it can include indirect effects such as regulation of members of the cellular protein homeostasis machinery. Additional indirect effects on turnover have been observed, such as S-acylation-mediated membrane targeting of inhibitory Smads (I-Smads), which negatively regulate TGF-β/BMP (bone morphogenetic protein) signaling by targeting type I receptors for ubiquitination and turnover (Li et al. Citation2017).
Cell death
S-Acylation was found to regulate components of both the extrinsic and the intrinsic apoptosis pathways. The cell death receptor Fas (CD95) and its ligand FasL were shown to get S-acylated (Chakrabandhu et al. Citation2007; Feig et al. Citation2007; Guardiola-Serrano et al. Citation2010). zDHHC7-mediated S-acylation of Fas facilitates a series of events required for cell death signaling including (i) formation of supramolecular receptor aggregates, (ii) targeting to specific membrane nanodomains, and (iii) recruitment of ezrin and the actin cytoskeleton leading to FasL-induced Fas internalization. Additionally, Fas S-acylation has been reported to prevent its degradation through the lysosomal pathway thus guaranteeing a proper expression level (Rossin et al. Citation2015). Moreover, Fas-FasL binding leads to rapid and transient S-acylation of the tyrosine kinase Lck and activation of downstream proteins in T-cells (Akimzhanov and Boehning Citation2015). Inhibition of Lck S-acylation by downregulation of the corresponding enzyme, zDHHC21, prevents Lck activation and Fas signaling and renders cells resistant to Fas-mediated apoptosis (Akimzhanov and Boehning Citation2015).
The intrinsic apoptosis pathway relies on the proapoptotic BCL-2-associated X protein (BAX), which resides primarily in the cytosol of non-apoptotic cells but stably associate with mitochondria to trigger the permeabilization of the outer mitochondrial membrane (OMM) and the release of cytochrome c into the cytosol (Youle and Strasser Citation2008). A constitutive shuttling between cytosol and mitochondria (Schellenberg et al. Citation2013) and a targeting to mitochondria modulated by N-terminal acetylation (Alves et al. Citation2018) have been postulated for BAX. Notably, it has been shown that association of BAX with the OMM and its subsequent proapoptotic action is regulated by S-acylation at a single cysteine residue, and that a lack of BAX S-acylation reduces BAX mitochondrial translocation and oligomerization as well as caspase activation and apoptosis (Fröhlich et al. Citation2014). Significant reduction of BAX S-acylation levels was observed in malignant B cells from patients with Hodgkin disease, suggesting this could be a mechanism by which tumor cells resist apoptosis (Fröhlich et al. Citation2014).
Caspase-6 (CASP6), another important player in apoptosis, has also been shown to be S-acylated, in this case by zDHHC17 (Skotte et al. Citation2017). Reduced CASP6 S-acylation resulted in increased CASP6 activity, and vice versa (Skotte et al. Citation2017). Importantly, CASP6 was shown to play a critical role in neurodegenerative diseases such as Alzheimer disease (AD) and Huntington disease (HD) (Graham et al. Citation2011) and zDHHC17 is known to also play a direct role in HD by mediating the S-acylation of huntingtin (Singaraja et al. Citation2002; Huang et al. Citation2004).
Cell polarity and migration
The apical-basolateral polarity is a key property of epithelia to fulfill their barrier and communication roles. The scaffolding protein Scribble (Scrib), which localizes to cell-cell junctions, is involved in establishing and maintaining this polarity (Dow et al. Citation2003), and mislocalizes to the cytosol in many human cancers (Vaira et al. Citation2011). Its plasma membrane localization and tumor suppression capacity depend on the S-acylation of two conserved cysteine residues, mostly mediated by zDHHC7 (Chen et al. Citation2016). Scrib S-acylation appears to be highly dynamic (Martin et al. Citation2012). Expression of the epithelial-to-mesenchymal transcription factor Snail in benign epithelial cells resulted in a mislocalization of Scrib to the cytosol and also led to repression of select DHHC PATs and increased expression of APT2. Inhibition of APT2 by the selective inhibitor ML349 efficiently drove membrane re-localization of Scrib in this system (Hernandez et al. Citation2017).
In addition to modifying Scrib, zDHHC7 was found to S-acylate junctional adhesion molecule C (JAM-C), promoting its localization to tight junctions and thereby inhibiting cell migration (Aramsangtienchai et al. Citation2017). Cell migration in turn was shown to depend on the S-acylation of the adhesion protein CD44 (Babina et al. Citation2014). Interestingly, non-canonical Wnt signaling was reported to induce APT1-dependent S-deacylation of CD44 and melanoma cell adhesion molecule (MCAM) in melanoma cells thus promoting polarized protein localization and cell invasion (Wang et al. Citation2015).
Finally, Rho GTPases, key modulators of cell polarity, adhesion and migration, have also been reported to undergo S-acylation. Prenylated Rac1 is targeted to actin cytoskeleton-linked ordered membrane regions through S-acylation and when this fails to occur it leads to defects in cell spreading and migration (Navarro-Lérida et al. Citation2012). S-Acylation of prenylated bCdc42 similarly leads to stable membrane association (Nishimura and Linder Citation2013). Interestingly, S-acylation of the atypical non-prenylated Rho GTPases, RhoU and RhoV, leads to transient and dynamic membrane association (Berzat et al. Citation2005; Chenette et al. Citation2005). Finally, S-acylation of isoprenylated RhoB leads to its targeting to multivesicular bodies and degradation, and thereby to its inactivation (Pérez-Sala et al. Citation2009).
Innate immunity
The innate immune system represents the first line of defense against infections by activating a variety of mechanisms, including the production of interferons (IFN). In this pathway, the ER receptor protein STING (stimulator of interferon genes) is essential to initiate effective IFN production and trigger the expression of IFN-stimulated genes (Ishikawa et al. Citation2009). S-Acylation of STING is required for its activation and for the subsequent type I IFN response. STING S-acylation appears to occur in the late Golgi, primarily on two nearby cysteine residues (Mukai et al. Citation2016). Remarkably, mutation of these two cysteine residues prevented downstream signaling not only for wild-type STING but also for the STING variants found in SAVI (STING-associated vasculopathy with onset in infancy) patients, where a gain-of-function mutation allows them to trigger the production of interferon independently of a stimulus thus causing an autoinflammatory disease (Mukai et al. Citation2016). Thus, targeting S-acylation of STING represents an interesting therapeutic strategy to treat auto-immune or inflammatory diseases. The Ablasser group recently performed a small-molecule searching for inhibitors of STING and identified a molecule that indeed binds covalently to a transmembrane cysteine residue and blocks its S-acylation maintaining the protein in a signaling-incompetent state. These STING antagonists are able to attenuate pathological features of autoinflammatory disease in mice highlighting the potential of this therapy (Haag et al. Citation2018).
Among the interferon-induced proteins, IFITM proteins play critical roles in blocking the entry of a variety of viruses, notably influenza A, dengue and hepatitis C viruses (Brass et al. Citation2009; Bailey et al. Citation2014; Narayana et al. Citation2015). IFITM3 inhibits viral infection by preventing virus fusion with endolysosomal membranes, presumably by disrupting intracellular cholesterol homeostasis and affecting membrane curvature (Amini-Bavil-Olyaee et al. Citation2013; Chesarino et al. Citation2014b, Citation2017). S-acylation of IFITM3 at three conserved cysteine residues promotes antiviral activity, most probably by mediating efficient membrane anchoring (Chesarino et al. Citation2014b, Citation2017). Whereas multiple DHHC PATs were shown to be able to S-acylate IFITM3 in human cells, zDHHC3, 7 and 20 had the most striking effect (McMichael et al. Citation2017). S-Acylation of IFITM proteins represents an interesting case as it was found to be conserved in all tested IFITM proteins but shown to have different effects on different members of the family. As mentioned earlier, murine IFITM1 S-acylation also regulates protein stability by preventing proteasomal degradation (Hach et al. Citation2013); and S-acylation of IFITM5 was reported to promote its function as a bone formation factor (Tsukamoto et al. Citation2013).
Metabolism
Numerous recent articles support a link between protein S-acylation and cellular metabolism. In 2013, an S-acylproteomics study on murine adipocytes revealed more than 600 S-acylation candidates (Ren et al. Citation2013a). In a follow-up study, the authors reported that S-acylation of glucose transporter 4 (Glut4), mainly mediated by zDHHC7, is critical for its insulin-dependent translocation to the plasma membrane (Ren et al. Citation2015; Du et al. Citation2017). Moreover, zDHHC7 knock-out mice developed hyperglycemia and glucose intolerance. Strikingly, the authors observed that zDHHC7 activity (estimated from autoacylation levels) was increased upon insulin treatment, illustrating that activity of DHHC S-acyltransferases can be regulated by extracellular signaling (Du et al. Citation2017). The plasma membrane-associated protein ClipR-59, involved in the regulation of Glut4 translocation, was also reported to depend on S-acylation, in this case primarily mediated by zDHHC17 (Ren et al. Citation2013b). Importantly, zDHHC17 has been found as a candidate disease gene in Type 1 diabetes and shown to regulate glutamate-stimulated insulin secretion and beta-cells survival (Berchtold et al. Citation2011). How the reported indirect and direct effects of protein S-acylation on the localization and function of the Glut4 transporter are coordinated remains to be explored.
zDHHC13 has also been linked to metabolism and mitochondrial function. In one study, zDHHC13-deficient mice were found to exhibit hypermetabolism and defective lipid metabolism (Shen et al. Citation2017). Another study focused on the zDHHC13-dependent S-acylation of dynamin-related protein 1 (Drp1) and reported that loss of zDHHC13 in cortex and cerebellum resulted in altered mitochondrial dynamics accompanied by increased glycolysis, glutaminolysis, and lactic acidosis (Napoli et al. Citation2017).
A variety of studies revealed roles for protein S-acylation in lipid metabolism. The long-chain fatty acid receptor CD36 plays important roles in lipid homeostasis and, not surprisingly, its expression is tightly regulated at the transcriptional, translational, and post-translational levels, amongst which by S-acylation (Tao et al. Citation1996). S-Acylation of CD36 was shown to affect its half-life and regulate its trafficking through the secretory pathway (Thorne et al. Citation2010). S-Acylation of ELMOD2 is required for its targeting to lipid droplets and subsequent regulation of adipocyte triglyceride lipase (ATGL) recruitment and triglycerides biosynthesis (Suzuki et al. Citation2015). S-acylation of the ATP-binding cassette transporter (ABC) A1 (mostly mediated by zDHHC8) is essential for its trafficking and function as mutation of the target cysteine residues resulted in reduced plasma membrane localization and reduced ABCA1-mediated lipid efflux (Singaraja et al. Citation2009). A general connection between S-acylation and metabolism has also been suggested after curation and combined analysis of published S-acylproteomics studies (Blanc et al. Citation2015; Sanders et al. Citation2015).
Finally, Berthiaume et al. have proposed that S-acylation of mitochondrial enzymes, which, as mentioned, might be non-enzymatic, could play a role in metabolic regulation and energy homeostasis. In particular, they observed enzyme inhibition by acylation of a cysteine residue at the active site and proposed a possible metabolic crosstalk between fatty acids metabolism and other pathways, such as amino acids catabolism or ketogenesis (Stucki et al. Citation1989; Berthiaume et al. Citation1994; Corvi et al. Citation2001; Kostiuk et al. Citation2008). In line with aforementioned observations, Kathayat et al. (Citation2018) reported that mitochondrial protein S-deacylation (as monitored by the fluorescent probe mito-DPP2) is sensitive to the levels of acyl-CoA thioesterase 11 (ACOT11). This suggests a connection between mitochondrial S-deacylase activity (primarily mediated by APT1) and the metabolic state of the cell, although no direct link with the S-acylation status of any mitochondrial enzyme has been so far established (Kathayat et al. Citation2018). As mentioned before, it is still unknown whether APT1 in mitochondria serves as part of a regulated enzymatic S-acylation/deacylation cycle or it has a rather “repairing” function removing non-enzymatically attached acyl chains, for example at cysteine residues in the active site of matrix mitochondrial enzymes. In the case of a metabolic crosstalk, the regulation of APT1 activity in a coordinate manner would be expected, although there is currently no evidence to support this.
Cancer
Over the last decade, the expression and activity of various DHHC PATs have been reported to be altered in numerous cancers and the connection between protein S-acylation and cancer has been extensively reviewed (Ducker et al. Citation2004; Greaves and Chamberlain Citation2014; Yeste-Velasco et al. Citation2014; Tian et al. Citation2015; Sharma et al. Citation2017; Chen et al. Citation2017b; De and Sadhukhan Citation2018). The most classic example of the link between S-acylation and cancer is the small GTPases Ras, and more specifically H-Ras and N-Ras. Ras gain-of-function mutations have been found in about 25% of all human cancers (Hobbs et al. Citation2016). The activity of H- and N-Ras requires S-acylation, and deacylation is essential to downregulate signaling (Swarthout et al. Citation2005; Misaki et al. Citation2010; Eisenberg et al. Citation2013; Lin et al. Citation2017; Pedro et al. Citation2017). Here we have selected two additional, recently reported examples highlighting the potential therapeutic applications of targeting protein S-acylation in cancer treatment.
The epidermal growth factor receptor (EGFR), an oncogenic receptor tyrosine kinase (Normanno et al. Citation2006), was also shown to get S-acylated. Recently, Bollu et al. (Citation2015) and Ali et al. (Citation2018) showed that FASN-mediated S-acylation positively regulates EGFR activity. Runkle et al. (Citation2016), in contrast, reported that zDHHC20-mediated S-acylation suppresses EGFR signaling. Different consequences of S-acylation on EGFR subcellular localization have been reported by the three groups (Bollu et al. Citation2015; Runkle et al. Citation2016; Ali et al. Citation2018), possibly due to the different mutational status of EGFR in each study (Ali et al. Citation2018). Interfering with EGFR S-acylation has been suggested as a clinical approach to treat cancers that are resistant to EGFR-targeted therapy (Runkle et al. Citation2016; Rana et al. Citation2018). Independently of its potential activity on EGFR, zDHHC20 has been reported to play a role in cellular transformation and its expression was shown to be significantly elevated in several types of human tumors including colon, prostate, ovarian, breast and kidney (Draper and Smith Citation2010).
S-Acylation was recently found to protect against skin cancer (Chen et al. Citation2017a). In a preliminary small molecule screen searching for modulators of melanocortin-1 receptor (MC1R) red hair color variants, the authors identified palmitic acid as an interesting candidate. MC1R is a G-protein-coupled receptor expressed in melanocytes, where it mediates the cutaneous responses to solar ultraviolet radiation, including melanin production and DNA repair. Following a series of elegant experiments, Chen et al. found that MC1R undergoes acylation at a C-terminal cysteine residue in a reaction primarily mediated by zDHHC13, and that this modification is essential for activating MC1R signaling. The S-acylation-deficient mutant of MC1R failed to upregulate downstream genes and mice expressing this variant had a similar coat color and skin pheomelanin content to the MC1R loss-of-function mice (Chen et al. Citation2017a). Finally, enhancing MC1R S-acylation by overexpression of zDHHC13 or treatment with Palmostatin B (a deacylases inhibitor) resulted in enhanced DNA damage repair, tumor size reduction and/or extended mice survival after prolonged UVB irradiation. These approaches could thus provide a potential clinical prevention for melanoma in patients with MC1R red hair color variants (Chen et al. Citation2017a).
Regulation and dynamics of S-acylation
An essential feature of protein S-acylation, which distinguishes it from most other types of lipidation, is its reversibility. Thus, S-acylation is not simply a structural static protein modification; it can be a dynamic, tightly regulated post-translational modification (PTM) involved in signaling and protein activation as is phosphorylation. Despite decades of research in the field, our understanding of protein S-acylation regulation and dynamics is still rudimentary.
In-depth understanding of S-acylation dynamics requires considering that proteins in the cell can exist as a mixed population of S-acylated and non-acylated species. Furthermore, many proteins can get S-acylated at two or more cysteine residues (Blanc et al. Citation2015) and the modification of each of these residues can have different consequences. Thus, a protein with two S-acylation sites might exist in four different S-acylation states or species, three S-acylation sites give rise to eight possible species, and so on. The properties and characteristics of the individual species cannot be accessed experimentally. This is partly due to the fact that there are so far no antibodies able to recognize the S-acylation of a specific cysteine. Single cysteine mutants may also be useful but the remaining cysteines will still have two possible states, modified or non-modified. Access to features of the individual species can however be obtained by data-driven mathematical models of the system. Let us assume a protein such as calnexin having two S-acylation sites. The system is modeled as an open system, where the protein is first synthesized and folded, it can then be S-acylated on one or the other site, and it can undergo deacylation at one or the other site. The folded form can thus adopt four S-acylation states. All species can undergo degradation. Each enzymatic reaction has its specific rate constants and the dynamic transitions between states/species of the network can be represented by a general model consisting of ordinary differential equations (ODE) (). The model is then calibrated using data from a subset of experiments, which can include [35S]-cysteine/methionine pulse-chase experiments and/or kinetics of [3H]-palmitate incorporation or loss for the wild-type protein and cysteine mutants, as well as any other quantitative experimental data. Using optimization algorithms, families of parameters that simultaneously fit the entire calibration dataset are defined. These are subsequently used to predict a second set of experiments, which are then compared with the experimental results from these experiments (which were not part of the calibration dataset). If predictions match the experimental results, the model is considered to capture the system. If there is a mismatch, the model needs to be modified with additional knowledge, which could for instance be that the protein can form dimers, or exist in different cellular compartments, etc. Iterative cycles between modeling and experiments lead to an adequate model that can then be interrogated. The modeling allows to deconvolute each experimental kinetic curve into the contribution to this curve of each of the species. This in turn allows to determine for example: the turnover rate of the non-S-acylated versus fully S-acylated species, the rate of S-deacylation of partially S-acylated species versus fully S-acylated species, the relative distribution of species under different cellular conditions, if relevant the activity of the different S-acylation species, etc. ().
Figure 5. Mathematical modeling of S-acylation data. For a protein with two S-acylation sites, an open system with five possible species (U: unfolded; C00: folded, non-acylated; C10 and C01: S-acylated in one or the other site; C11: doubly-acylated) is modeled and the transitions between species can be represented using a set of ordinary differential equations. The model is fed with quantitative experimental data, such as [35S]-cysteine/methionine pulse-chase or [3H]-palmitate incorporation, and iterative cycles of calibration, prediction, and validation are followed until an adequate model is obtained. A model capturing the system allows to deconvolute experimental data into the contribution of each species and thus to study the effect of each S-acylation event on protein stability and function. OE: overexpression, KD: knock-down. See text for more details (see color version of this figure at www.tandfonline.com/ibmg).
![Figure 5. Mathematical modeling of S-acylation data. For a protein with two S-acylation sites, an open system with five possible species (U: unfolded; C00: folded, non-acylated; C10 and C01: S-acylated in one or the other site; C11: doubly-acylated) is modeled and the transitions between species can be represented using a set of ordinary differential equations. The model is fed with quantitative experimental data, such as [35S]-cysteine/methionine pulse-chase or [3H]-palmitate incorporation, and iterative cycles of calibration, prediction, and validation are followed until an adequate model is obtained. A model capturing the system allows to deconvolute experimental data into the contribution of each species and thus to study the effect of each S-acylation event on protein stability and function. OE: overexpression, KD: knock-down. See text for more details (see color version of this figure at www.tandfonline.com/ibmg).](/cms/asset/73a7a048-5ec6-4649-a8e6-7e7fa24c45ff/ibmg_a_1488804_f0005_c.jpg)
By combining experimental determination of protein S-acylation and its dynamics with mathematical modeling by the Hatzimanikatis group, we have increased our understanding of the dynamics of this PTM and the complexity of its regulation (Dallavilla et al. Citation2016; Abrami et al. Citation2017). Notably, it was found that the presence of two contiguous S-acylation sites, frequently seen in S-acylated transmembrane proteins (Collins et al. Citation2017; Blanc et al. Citation2015), could act as an important regulator of S-acylation kinetics and protein stability (Dallavilla et al. Citation2016; Abrami et al. Citation2017). More specifically, we found that the presence of two neighboring juxtamembranous cysteines, when both sites are modified, tends to render S-acylation irreversible, probably by triggering membrane imbedding of the cysteines leading to their inaccessibility to thioesterases. We also found that S-acyltransferase activity can be regulated by S-acylation, i.e. S-acylation can occur in cascades, as phosphorylation or ubiquitination. We found that zDHHC16 catalyzes the S-acylation of zDHHC6 at three cysteine residues in its SH3_2 domain and this S-acylation is required for the correct functioning of the enzyme and subsequent S-acylation of key substrates in the endoplasmic reticulum, such as calnexin and transferrin receptor (Lakkaraju et al. Citation2012; Senyilmaz et al. Citation2015; Abrami et al. Citation2017). While S-palmitoylation of one of the cysteines confers high activity to the enzyme, it also targets it to the ER-associated degradation pathway. Therefore, zDHHC6 must rapidly be depalmitoylated by APT2 (Abrami et al. Citation2017). APT2 itself was found to undergo S-acylation at a conserved cysteine residue in its N-terminus to be active (Kong et al. Citation2013). Thus, the fact that DHHC S-acyltransferases can be regulated by cycles of S-acylation/deacylation, through enzymes that undergo similar cycles, leads to rapid increase in the complexity of the regulatory network (). Many members of the human DHHC S-acyltransferase family appear to undergo regulatory S-acylation (). Thus, it is important to consider their possible regulation through S-acylation cascades.
Figure 6. Schematic representation of zDHHC6 S-acylation cascade. zDHHC6 requires S-acylation for activation and subsequent S-acylation of its different substrates, including transferrin receptor (TfnR), calnexin (CNX), and inositol 1,4,5-triphosphate (IP3) receptor type 1 (IP3R1). zDHHC6 S-acylation is mediated by zDHHC16 and S-deacylation is catalyzed by APT2. The latter is susceptible to S-acylation and deacylation by a yet-to-be-identified zDHHC PAT and APT1, respectively, which, in turn, can potentially require S-acylation for function (see color version of this figure at www.tandfonline.com/ibmg).
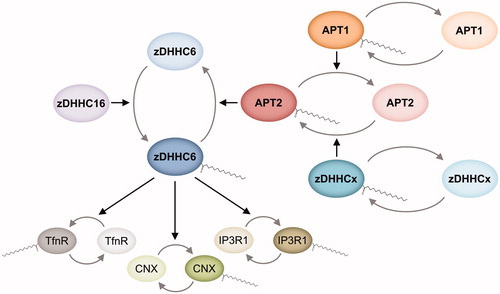
Beyond S-acylation cascades, DHHC S-acyltransferases and acyl protein thioesterases can be subjected to other complex regulatory schemes. In neurons, expression of human zDHHC9 and the subsequent membrane trafficking of its substrate H-Ras have been reported to be regulated by the microRNA-134 (in somatostatin-positive interneurons) (Chai et al. Citation2013), and microRNA-138-dependent expression of the depalmitoylase APT1 has been linked to dendritic spine morphogenesis through, at least partially, membrane localization of its substrate G-protein α subunit α13 (Siegel et al. Citation2009). Interestingly, H-Ras has also been reported to be a substrate of APT1 (Kong et al. Citation2013), and microRNA-134 and microRNA-138 appear to be differentially regulated by neuronal activity (Siegel et al. Citation2009; Chai et al. Citation2013). Yet, whether zDHHC9 and APT1 are regulated in a coordinated fashion remains unexplored. More recently, APT1 and APT2 were shown to be under the control of microRNA-138 and -424, respectively, and regulate cell death through S-deacylation of Fas/CD95 in primary chronic lymphocytic leukemia cells (Berg et al. Citation2015).
Dynamic S-acylation was also shown to be stimulated by extracellular signaling. As mentioned before, zDHHC7 was reported to be activated upon insulin treatment in HEK293 cells (Du et al. Citation2017). The abundance of zDHHC3 and S-acylation of its substrate δ-catenin were increased upon exposure of cultured dorsal root ganglion neurons to the inflammatory cytokine TNF-α (Zhang et al. Citation2018). Additionally, zDHHC3 enzymatic activity was reported to be controlled by tyrosine phosphorylation mediated by fibroblast growth factor receptor 1 (FGFR1) and Src kinases in neuroblastoma N2a cells (Lievens et al. Citation2016). Notably, it was found that Src-mediated phosphorylation of Tyr295 and Tyr297 has an inhibitory effect on zDHHC3 enzymatic activity while FGFR1-mediated phosphorylation of Tyr18 can overcome such inhibition (Lievens et al. Citation2016). This study provides the first evidence of DHHC PAT activity modulated by phosphorylation and highlights, once more, that S-acylation enzymes can be part of complex and dynamic signaling networks. Finally, an additional regulatory role has been proposed for dynamic changes in DHHC PAT localization (Greaves et al. Citation2011; Lai and Linder Citation2013; Chamberlain and Shipston Citation2015; Abrami et al. Citation2017).
S-Acylation of a given protein can be modulated independently of the regulation of S-(de)acylation enzymes. Besides the previously described interplay between S-acylation and ubiquitination (see above), phosphorylation of several proteins can positively or negatively regulate their S-acylation. While phosphorylation of phosphodiesterase 10A (PDE10A) and calnexin was shown to act as a negative modulator of their S-acylation (Charych et al. Citation2010; Dallavilla et al. Citation2016), S-acylation of tissue factor (TF) was reported to negatively regulate its phosphorylation (Dorfleutner and Ruf Citation2003). In turn, the cross-talk between S-acylation and phosphorylation was shown to regulate BK potassium channels activity (Tian et al. Citation2008) and GAP43 plasma membrane targeting (Gauthier-Kemper et al. Citation2014). Other PTMs targeting cysteine residues, and thus acting as potential competitors, have been reported to affect protein S-acylation. S-Nitrosylation of PSD-95 was shown to decrease its S-acylation (Ho et al. Citation2011), and S-glutathionylation of H-Ras was reported to prevent its S-acylation under specific metabolic stress conditions (Burgoyne et al. Citation2012).
Thus, as schematized in , the S-acylation of a given protein can be modulated at multiple levels and have diverse physiological effects. The activity of S-(de)acylation enzymes can be regulated at the translational and post-translational level as well as by extracellular signaling; and the S-acylation of the target protein might respond to the interplay with other PTMs. In turn, S-acylation can impact protein structure, stability, localization, and/or function, and have varied (patho)physiological consequences.
Figure 7. Regulation and physiological relevance of protein S-acylation. S-acylation can be regulated by affecting the activity of the S-(de)acylation enzymes or by direct regulation of the select S-acylation event of a given protein substrate or cysteine residue. In turn, the molecular consequences of this S-acylation can be varied and impact a broad spectrum of physiological processes (see color version of this figure at www.tandfonline.com/ibmg).
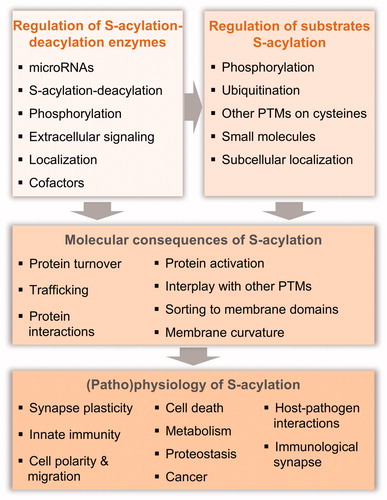
Concluding remarks
The last few years have witnessed major advances in the field of protein S-acylation: technologies to study this modification have drastically improved, many proteins for which S-acylation is key for their function have been identified, structures of the enzymes involved in acyl chain attachment and removal are becoming available, and promising probes and inhibitors are being developed. These advances will allow the field to reach the next level of understanding. Particularly exciting will be the study of this modification in the context of signaling cascades and considering its possible cross-talk with other regulatory events. Obtaining a more systems level of comprehension will allow the identification of feedback loops. Modeling approaches will be particularly useful and the fact that DHHC PATs are present in far lower numbers than kinases or ubiquitinating enzymes might provide a unique opportunity to model the full system and obtain an understanding of the full network of a dynamic post-translational modification. The coming years are also likely to see the development of new drugs to control S-acyltransferases and thioesterases, which will not only expand our basic knowledge of this complex modification but could also be of therapeutic use for a number of diseases with an identified S-acylation component.
Acknowledgements
The authors thank Laurence Abrami, Oksana Sergeeva, and Luciano Abriata for helpful discussions and critical reading of this manuscript.
Disclosure statement
The authors declare no potential conflicts of interest.
Additional information
Funding
References
- Abrami L, Dallavilla T, Sandoz PA, Demir M, Kunz B, Savoglidis G, Hatzimanikatis V, van der Goot FG. 2017. Identification and dynamics of the human ZDHHC16-ZDHHC6 palmitoylation cascade. eLife. 6:e27826.
- Abrami L, Kunz B, Iacovache I, van der Goot FG. 2008. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 105:5384–5389.
- Abrami L, Leppla SH, van der Goot FG. 2006. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 172:309–320.
- Adibekian A, Martin BR, Chang JW, Hsu K-L, Tsuboi K, Bachovchin DA, Speers AE, Brown SJ, Spicer T, Fernandez-Vega V, et al. 2010a. Characterization of a selective, reversible inhibitor of lysophospholipase 1 (LYPLA1). In: Probe reports from the NIH molecular libraries program. Bethesda (MD): National Center for Biotechnology Information (US).
- Adibekian A, Martin BR, Chang JW, Hsu K-L, Tsuboi K, Bachovchin DA, Speers AE, Brown SJ, Spicer T, Fernandez-Vega V, et al. 2010b. Characterization of a selective, reversible inhibitor of lysophospholipase 2 (LYPLA2). In: Probe reports from the NIH molecular libraries program. Bethesda (MD): National Center for Biotechnology Information (US).
- Ahearn IM, Tsai FD, Court H, Zhou M, Jennings BC, Ahmed M, Fehrenbacher N, Linder ME, Philips MR. 2011. FKBP12 binds to acylated H-ras and promotes depalmitoylation. Mol Cell. 41:173–185.
- Aicart-Ramos C, Valero RA, Rodriguez-Crespo I. 2011. Protein palmitoylation and subcellular trafficking. Biochim Biophys Acta. 1808:2981–2994.
- Akimzhanov AM, Boehning D. 2015. Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling. Proc Natl Acad Sci U S A. 112:11876–11880.
- Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JRA, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, et al. 2011. Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci Signal. 4:ra42.
- Ali A, Levantini E, Teo JT, Goggi J, Clohessy JG, Wu CS, Chen L, Yang H, Krishnan I, Kocher O, et al. 2018. Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated non‐small cell lung cancer. EMBO Mol Med. 10:e8313.
- Alves S, Neiri L, Chaves SR, Vieira S, Trindade D, Manon S, Dominguez V, Pintado B, Jonckheere V, Van Damme P, et al. 2018. N-terminal acetylation modulates Bax targeting to mitochondria. Int J Biochem Cell Biol. 95:35–42.
- Amini-Bavil-Olyaee S, Choi YJ, Lee JH, Shi M, Huang I-C, Farzan M, Jung JU. 2013. The antiviral effector IFITM3 disrupts intracellular cholesterol homeostasis to block viral entry. Cell Host Microbe. 13:452–464.
- Aramsangtienchai P, Spiegelman NA, Cao J, Lin H. 2017. S-palmitoylation of junctional adhesion molecule C regulates its tight junction localization and cell migration. J Biol Chem. 292:5325–5334.
- Babina IS, McSherry EA, Donatello S, Hill ADK, Hopkins AM. 2014. A novel mechanism of regulating breast cancer cell migration via palmitoylation-dependent alterations in the lipid raft affiliation of CD44. Breast Cancer Res. 16:R19.
- Bailey CC, Zhong G, Huang I-C, Farzan M. 2014. IFITM-family proteins: the cell’s first line of antiviral defense. Annu Rev Virol. 1:261–283.
- Bannan BA, Van Etten J, Kohler JA, Tsoi Y, Hansen NM, Sigmon S, Fowler E, Buff H, Williams TS, Ault JG, et al. 2008. The Drosophila protein palmitoylome: characterizing palmitoyl-thioesterases and DHHC palmitoyl-transferases. Fly (Austin). 2:198–214.
- Bartels DJ, Mitchell DA, Dong X, Deschenes RJ. 1999. Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol Cell Biol. 19:6775–6787.
- Bause E. 1983. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 209:331–336.
- Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Rinner O, Ellenberg J, Aebersold R. 2011. The quantitative proteome of a human cell line. Mol Syst Biol. 7:549.
- Berchtold LA, Størling ZM, Ortis F, Lage K, Bang-Berthelsen C, Bergholdt R, Hald J, Brorsson CA, Eizirik DL, Pociot F, et al. 2011. Huntingtin-interacting protein 14 is a type 1 diabetes candidate protein regulating insulin secretion and β-cell apoptosis. Proc Natl Acad Sci. 108:E681–E688.
- Berg V, Rusch M, Vartak N, Jüngst C, Schauss A, Waldmann H, Hedberg C, Pallasch CP, Bastiaens PIH, Hallek M, et al. 2015. miRs-138 and -424 control palmitoylation-dependent CD95-mediated cell death by targeting acyl protein thioesterases 1 and 2 in CLL. Blood. 125:2948–2957.
- Berthiaume L, Deichaite I, Peseckis S, Resh MD. 1994. Regulation of enzymatic activity by active site fatty acylation. A new role for long chain fatty acid acylation of proteins. J Biol Chem. 269:6498–6505.
- Berzat AC, Buss JE, Chenette EJ, Weinbaum CA, Shutes A, Der CJ, Minden A, Cox AD. 2005. Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J Biol Chem. 280:33055–33065.
- Bheda P, Jing H, Wolberger C, Lin H. 2016. The substrate specificity of sirtuins. Annu Rev Biochem. 85:405–429.
- Bizzozero OA, Bixler HA, Pastuszyn A. 2001. Structural determinants influencing the reaction of cysteine-containing peptides with palmitoyl-coenzyme A and other thioesters. Biochim Biophys Acta. 1545:278–288.
- Blanc M, Blaskovic S, van der Goot FG. 2013. Palmitoylation, pathogens and their host. Biochm Soc Trans. 41:84–88.
- Blanc M, David F, Abrami L, Migliozzi D, Armand F, Bürgi J, van der Goot FG. 2015. SwissPalm: protein palmitoylation database. F1000Res. 4:261.
- Blaskovic S, Blanc M, van der Goot FG. 2013. What does S-palmitoylation do to membrane proteins? FEBS J. 280:2766–2774.
- Bolender N, Sickmann A, Wagner R, Meisinger C, Pfanner N. 2008. Multiple pathways for sorting mitochondrial precursor proteins. EMBO Rep. 9:42–49.
- Bollu LR, Katreddy RR, Blessing AM, Pham N, Zheng B, Wu X, Weihua Z. 2015. Intracellular activation of EGFR by fatty acid synthase dependent palmitoylation. Oncotarget. 6:34992–35003.
- Brass AL, Huang I-C, Benita Y, John SP, Krishnan MN, Feeley EM, Ryan BJ, Weyer JL, van der Weyden L, Fikrig E, et al. 2009. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 139:1243–1254.
- Brigidi GS, Santyr B, Shimell J, Jovellar B, Bamji SX. 2015. Activity-regulated trafficking of the palmitoyl-acyl transferase DHHC5. Nat Commun. 6:8200.
- Brown RWB, Sharma AI, Engman DM. 2017. Dynamic protein S-palmitoylation mediates parasite life cycle progression and diverse mechanisms of virulence. Crit Rev Biochem Mol Biol. 52:145–162.
- Bürger M, Zimmermann TJ, Kondoh Y, Stege P, Watanabe N, Osada H, Waldmann H, Vetter IR. 2012. Crystal structure of the predicted phospholipase LYPLAL1 reveals unexpected functional plasticity despite close relationship to acyl protein thioesterases. J Lipid Res. 53:43–50.
- Bürgi J, Xue B, Uversky VN, van der Goot FG. 2016. Intrinsic disorder in transmembrane proteins: roles in signaling and topology prediction. PLoS One. 11:e0158594.
- Burgoyne JR, Haeussler DJ, Kumar V, Ji Y, Pimental DR, Zee RS, Costello CE, Lin C, McComb ME, Cohen RA, et al. 2012. Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis. FASEB J Off Publ Fed Am Soc Exp Biol. 26:832–841.
- Burton M, Rose TM, Faergeman NJ, Knudsen J. 2005. Evolution of the acyl-CoA binding protein (ACBP). Biochem J. 392:299–307.
- Caballero MC, Alonso AM, Deng B, Attias M, de Souza W, Corvi MM. 2016. Identification of new palmitoylated proteins in Toxoplasma gondii. Biochim Biophys Acta. 1864:400–408.
- Calero G, Gupta P, Nonato MC, Tandel S, Biehl ER, Hofmann SL, Clardy J. 2003. The crystal structure of palmitoyl protein thioesterase-2 (PPT2) reveals the basis for divergent substrate specificities of the two lysosomal thioesterases, PPT1 and PPT2. J Biol Chem. 278:37957–37964.
- Calvo SE, Clauser KR, Mootha VK. 2016. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44:D1251–D1257.
- Camp LA, Hofmann SL. 1993. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 268:22566–22574.
- Cao N, Li J-K, Rao Y-Q, Liu H, Wu J, Li B, Zhao P, Zeng L, Li J. 2016. A potential role for protein palmitoylation and zDHHC16 in DNA damage response. BMC Mol Biol. 17:12.
- Castro EA. 1999. Kinetics and mechanisms of reactions of thiol, thiono, and dithio analogues of carboxylic esters with nucleophiles. Chem Rev. 99:3505–3524.
- Chai S, Cambronne XA, Eichhorn SW, Goodman RH. 2013. MicroRNA-134 activity in somatostatin interneurons regulates H-Ras localization by repressing the palmitoylation enzyme, DHHC9. Proc Natl Acad Sci U S A. 110:17898–17903.
- Chakrabandhu K, Hérincs Z, Huault S, Dost B, Peng L, Conchonaud F, Marguet D, He H-T, Hueber A-O. 2007. Palmitoylation is required for efficient Fas cell death signaling. EMBO J. 26:209–220.
- Chamberlain LH, Shipston MJ. 2015. The physiology of protein S-acylation. Physiol Rev. 95:341–376.
- Chan P, Han X, Zheng B, DeRan M, Yu J, Jarugumilli GK, Deng H, Pan D, Luo X, Wu X. 2016. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat Chem Biol. 12:282–289.
- Charron G, Zhang MM, Yount JS, Wilson J, Raghavan AS, Shamir E, Hang HC. 2009. Robust fluorescent detection of protein fatty-acylation with chemical reporters. J Am Chem Soc. 131:4967–4975.
- Charych EI, Jiang L-X, Lo F, Sullivan K, Brandon NJ. 2010. Interplay of palmitoylation and phosphorylation in the trafficking and localization of phosphodiesterase 10A: implications for the treatment of schizophrenia. J Neurosci Off J Soc Neurosci. 30:9027–9037.
- Chase JF, Tubbs PK. 1972. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme A and carnitine esters. Biochem J. 129:55–65.
- Chen S, Zhu B, Yin C, Liu W, Han C, Chen B, Liu T, Li X, Chen X, Li C, et al. 2017a. Palmitoylation-dependent activation of MC1R prevents melanomagenesis. Nature. 549:399–403.
- Chen X, Ma H, Wang Z, Zhang S, Yang H, Fang Z. 2017b. EZH2 palmitoylation mediated by ZDHHC5 in p53-mutant glioma drives malignant development and progression. Cancer Res. 77:4998–5010.
- Chenette EJ, Abo A, Der CJ. 2005. Critical and distinct roles of amino- and carboxyl-terminal sequences in regulation of the biological activity of the Chp atypical Rho GTPase. J Biol Chem. 280:13784–13792.
- Chesarino NM, Compton AA, McMichael TM, Kenney AD, Zhang L, Soewarna V, Davis M, Schwartz O, Yount JS. 2017. IFITM3 requires an amphipathic helix for antiviral activity. EMBO Rep. 18:1740–1751.
- Chesarino NM, Hach JC, Chen JL, Zaro BW, Rajaram MV, Turner J, Schlesinger LS, Pratt MR, Hang HC, Yount JS. 2014a. Chemoproteomics reveals Toll-like receptor fatty acylation. BMC Biol. 12:91.
- Chesarino NM, McMichael TM, Yount JS. 2014b. Regulation of the trafficking and antiviral activity of IFITM3 by post-translational modifications. Future Microbiol. 9:1151–1163.
- Chlanda P, Mekhedov E, Waters H, Sodt A, Schwartz C, Nair V, Blank PS, Zimmerberg J. 2017. Palmitoylation contributes to membrane curvature in influenza A virus assembly and hemagglutinin-mediated membrane fusion. J Virol. 91:*e00947-17.
- Chen B, Zheng B, DeRan M, Jarugumilli GK, Fu J, Brooks YS, Wu X. 2016. ZDHHC7-mediated S-palmitoylation of Scribble regulates cell polarity. Nat Chem Biol. 12:686–693.
- Cho E, Park M. 2016. Palmitoylation in Alzheimer’s disease and other neurodegenerative diseases. Pharmacol Res. 111:133–151.
- Coleman DT, Gray AL, Kridel SJ, Cardelli JA. 2016. Palmitoylation regulates the intracellular trafficking and stability of c-Met. Oncotarget. 7:32664–32677.
- Coleman RA, Rao P, Fogelsong RJ, Bardes ES-G. 1992. 2-Bromopalmitoyl-CoA and 2-bromopalmitate: promiscuous inhibitors of membrane-bound enzymes. Biochim Biophys Acta BBA – Lipids Lipid Metab. 1125:203–209.
- Collins MO, Woodley KT, Choudhary JS. 2017. Global, site-specific analysis of neuronal protein S-acylation. Sci Rep. 7:4683.
- Colquhoun DR, Lyashkov AE, Ubaida Mohien C, Aquino VN, Bullock BT, Dinglasan RR, Agnew BJ, Graham DRM. 2015. Bioorthogonal mimetics of palmitoyl-CoA and myristoyl-CoA and their subsequent isolation by click chemistry and characterization by mass spectrometry reveal novel acylated host-proteins modified by HIV-1 infection. Proteomics. 15:2066–2077.
- Corvi MM, Soltys CL, Berthiaume LG. 2001. Regulation of mitochondrial carbamoyl-phosphate synthetase 1 activity by active site fatty acylation. J Biol Chem. 276:45704–45712.
- Dallavilla T, Abrami L, Sandoz PA, Savoglidis G, Hatzimanikatis V, van der Goot FG. 2016. Model-driven understanding of palmitoylation dynamics: regulated acylation of the endoplasmic reticulum chaperone calnexin. PLoS Comput Biol. 12:e1004774.
- Davda D, El Azzouny MA, Tom CTMB, Hernandez JL, Majmudar JD, Kennedy RT, Martin BR. 2013. Profiling targets of the irreversible palmitoylation inhibitor 2-bromopalmitate. ACS Chem Biol. 8:1912–1917.
- Davda D, Martin BR. 2014. Acyl protein thioesterase inhibitors as probes of dynamic S-palmitoylation. MedChemComm. 5:268–276.
- de Castro E, Sigrist CJA, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N. 2006. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 34:W362–W365.
- De I, Sadhukhan S. 2018. Emerging roles of DHHC-mediated protein S-palmitoylation in physiological and pathophysiological context. Eur J Cell Biol. 97:319–338.
- Dekker FJ, Rocks O, Vartak N, Menninger S, Hedberg C, Balamurugan R, Wetzel S, Renner S, Gerauer M, Schölermann B, et al. 2010. Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat Chem Biol. 6:449–456.
- Devedjiev Y, Dauter Z, Kuznetsov SR, Jones TL, Derewenda ZS. 2000. Crystal structure of the human acyl protein thioesterase I from a single X-ray data set to 1.5 A. Structure. 8:1137–1146.
- Dighe SA, Kozminski KG. 2008. Swf1p, a member of the DHHC-CRD family of palmitoyltransferases, regulates the actin cytoskeleton and polarized secretion independently of its DHHC motif. Mboc. 19:4454–4468.
- Doll S, Dreszen M, Geyer PE, Itzhak DN, Braun C, Doppler SA, Meier F, Deutsch M-A, Lahm H, Lange R, et al. 2017. Region and cell-type resolved quantitative proteomic map of the human heart. Nat Commun. 8:1469.
- Dorfleutner A, Ruf W. 2003. Regulation of tissue factor cytoplasmic domain phosphorylation by palmitoylation. Blood. 102:3998–4005.
- Dosztányi Z, Csizmok V, Tompa P, Simon I. 2005. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinforma Oxf Engl. 21:3433–3434.
- Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, Trapani JA, Russell SM, Richardson HE, Humbert PO. 2003. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene. 22:9225–9230.
- Dowal L, Yang W, Freeman MR, Steen H, Flaumenhaft R. 2011. Proteomic analysis of palmitoylated platelet proteins. Blood. 118:e62–e73.
- Draper JM, Smith CD. 2010. DHHC20: a human palmitoyl acyltransferase that causes cellular transformation. Mol Membr Biol. 27:123–136.
- Drisdel RC, Green WN. 2004. Labeling and quantifying sites of protein palmitoylation. BioTechniques. 36:276–285.
- Du K, Murakami S, Sun Y, Kilpatrick CL, Luscher B. 2017. DHHC7 palmitoylates glucose transporter 4 (Glut4) and regulates Glut4 membrane translocation. J Biol Chem. 292:2979–2991.
- Ducker CE, Stettler EM, French KJ, Upson JJ, Smith CD. 2004. Huntingtin interacting protein 14 is an oncogenic human protein: palmitoyl acyltransferase. Oncogene. 23:9230–9237.
- Dumaresq-Doiron K, Jules F, Lefrancois S. 2013. Sortilin turnover is mediated by ubiquitination. Biochem Biophys Res Commun. 433:90–95.
- Duncan JA, Gilman AG. 1998. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS). J Biol Chem. 273:15830–15837.
- Dunphy JT, Schroeder H, Leventis R, Greentree WK, Knudsen JK, Silvius JR, Linder ME. 2000. Differential effects of acyl-CoA binding protein on enzymatic and non-enzymatic thioacylation of protein and peptide substrates. Biochim Biophys Acta. 1485:185–198.
- Edmonds MJ, Geary B, Doherty MK, Morgan A. 2017. Analysis of the brain palmitoyl-proteome using both acyl-biotin exchange and acyl-resin-assisted capture methods. Sci Rep. 7:3299.
- Edmonds MJ, Morgan A. 2014. A systematic analysis of protein palmitoylation in Caenorhabditis elegans. BMC Genomics. 15:841.
- Eisenberg S, Laude AJ, Beckett AJ, Mageean CJ, Aran V, Hernandez-Valladares M, Henis YI, Prior IA. 2013. The role of palmitoylation in regulating Ras localization and function. Biochm Soc Trans. 41:79–83.
- Emmer BT, Nakayasu ES, Souther C, Choi H, Sobreira TJP, Epting CL, Nesvizhskii AI, Almeida IC, Engman DM. 2011. Global analysis of protein palmitoylation in African trypanosomes. Eukaryot Cell. 10:455–463.
- Faergeman NJ, Knudsen J. 1997. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J. 323:1–12.
- Fairbank M, Huang K, El-Husseini A, Nabi IR. 2012. RING finger palmitoylation of the endoplasmic reticulum Gp78 E3 ubiquitin ligase. FEBS Lett. 586:2488–2493.
- Fang C, Zhang X, Zhang L, Gao X, Yang P, Lu H. 2016. Identification of palmitoylated transitional endoplasmic reticulum ATPase by proteomic technique and pan antipalmitoylation antibody. J Proteome Res. 15:956–962.
- Feig C, Tchikov V, Schütze S, Peter ME. 2007. Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling. EMBO J.26:221–231.
- Feldman JL, Baeza J, Denu JM. 2013. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. 288:31350–31356.
- Fernández-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. 2006. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 174:369–377.
- Foe IT, Child MA, Majmudar JD, Krishnamurthy S, van der Linden WA, Ward GE, Martin BR, Bogyo M. 2015. Global analysis of palmitoylated proteins in toxoplasma gondii. Cell Host Microbe. 18:501–511.
- Forrester MT, Hess DT, Thompson JW, Hultman R, Moseley MA, Stamler JS, Casey PJ. 2011. Site-specific analysis of protein S-acylation by resin-assisted capture. J Lipid Res. 52:393–398.
- Fredericks GJ, Hoffmann FW, Rose AH, Osterheld HJ, Hess FM, Mercier F, Hoffmann PR. 2014. Stable expression and function of the inositol 1,4,5-triphosphate receptor requires palmitoylation by a DHHC6/selenoprotein K complex. Proc Natl Acad Sci U S A. 111:16478–16483.
- Fredericks GJ, Hoffmann PR. 2015. Selenoprotein K and protein palmitoylation. Antioxid Redox Signal. 23:854–862.
- Fröhlich M, Dejanovic B, Kashkar H, Schwarz G, Nussberger S. 2014. S-palmitoylation represents a novel mechanism regulating the mitochondrial targeting of BAX and initiation of apoptosis. Cell Death Dis. 5:e1057.
- Fukata Y, Fukata M. 2010. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 11:161–175.
- Fukata Y, Iwanaga T, Fukata M. 2006. Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods San Diego Calif. 40:177–182.
- Fukata Y, Murakami T, Yokoi N, Fukata M. 2016. Local palmitoylation cycles and specialized membrane domain organization. Curr Top Membr. 77:97–141.
- Gao T, Collins RE, Horton JR, Zhang X, Zhang R, Dhayalan A, Tamas R, Jeltsch A, Cheng X. 2009. The ankyrin repeat domain of Huntingtin interacting protein 14 contains a surface aromatic cage, a potential site for methyl-lysine binding. The ankyrin repeat domain of Huntingtin interacting protein 14 contains a surface aromatic cage, a potential site for methyl-lysine binding. Proteins. 76:772–777.
- Gao X, Hannoush RN. 2014. Method for cellular imaging of palmitoylated proteins with clickable probes and proximity ligation applied to Hedgehog, tubulin, and Ras. J Am Chem Soc. 136:4544–4550.
- Gao X, Hannoush RN. 2018. A decade of click chemistry in protein palmitoylation: impact on discovery and new biology. Cell Chem Biol. 25:236–246.
- Gauthier-Kemper A, Igaev M, Sündermann F, Janning D, Brühmann J, Moschner K, Reyher H-J, Junge W, Glebov K, Walter J, et al. 2014. Interplay between phosphorylation and palmitoylation mediates plasma membrane targeting and sorting of GAP43. Mboc. 25:3284–3299.
- Globa AK, Bamji SX. 2017. Protein palmitoylation in the development and plasticity of neuronal connections. Curr Opin Neurobiol. 45:210–220.
- González Montoro A, Chumpen Ramirez S, Quiroga R, Valdez Taubas J. 2011. Specificity of transmembrane protein palmitoylation in yeast. PLoS One. 6:e16969.
- González Montoro A, Chumpen Ramirez S, Valdez Taubas J. 2015. The canonical DHHC motif is not absolutely required for the activity of the yeast S-acyltransferases Swf1 and Pfa4. J Biol Chem. 290:22448–22459.
- González Montoro A, Quiroga R, Maccioni HJF, Valdez Taubas J. 2009. A novel motif at the C-terminus of palmitoyltransferases is essential for Swf1 and Pfa3 function in vivo. Biochem J. 419:301–308.
- Gorleku OA, Barns A-M, Prescott GR, Greaves J, Chamberlain LH. 2011. Endoplasmic reticulum localization of DHHC palmitoyltransferases mediated by lysine-based sorting signals. J Biol Chem. 286:39573–39584.
- Gottlieb CD, Linder ME. 2017. Structure and function of DHHC protein S-acyltransferases. Biochem Soc Trans. 45:923–928.
- Gould NS, Evans P, Martínez-Acedo P, Marino SM, Gladyshev VN, Carroll KS, Ischiropoulos H. 2015. Site-specific proteomic mapping identifies selectively modified regulatory cysteine residues in functionally distinct protein networks. Chem Biol. 22:965–975.
- Goytain A, Hines RM, Quamme GA. 2008. Huntingtin-interacting proteins, HIP14 and HIP14L, mediate dual functions, palmitoyl acyltransferase and Mg2+ transport. J Biol Chem. 283:33365–33374.
- Graham RK, Ehrnhoefer DE, Hayden MR. 2011. Caspase-6 and neurodegeneration. Trends Neurosci. 34:646–656.
- Greaves J, Carmichael JA, Chamberlain LH. 2011. The palmitoyl transferase DHHC2 targets a dynamic membrane cycling pathway: regulation by a C-terminal domain. Mboc. 22:1887–1895.
- Greaves J, Chamberlain LH. 2014. New links between S-acylation and cancer. J Pathol. 233:4–6.
- Greaves J, Gorleku OA, Salaun C, Chamberlain LH. 2010. Palmitoylation of the SNAP25 protein family: specificity and regulation by DHHC palmitoyl transferases. J Biol Chem. 285:24629–24638.
- Greaves J, Munro KR, Davidson SC, Riviere M, Wojno J, Smith TK, Tomkinson NCO, Chamberlain LH. 2017. Molecular basis of fatty acid selectivity in the zDHHC family of S-acyltransferases revealed by click chemistry. Proc Natl Acad Sci U S A. 114:E1365–E1374.
- Greaves J, Prescott GR, Fukata Y, Fukata M, Salaun C, Chamberlain LH. 2009. The hydrophobic cysteine-rich domain of SNAP25 couples with downstream residues to mediate membrane interactions and recognition by DHHC palmitoyl transferases. Mboc. 20:1845–1854.
- Greaves J, Salaun C, Fukata Y, Fukata M, Chamberlain LH. 2008. Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein. J Biol Chem. 283:25014–25026.
- Guardiola-Serrano F, Rossin A, Cahuzac N, Lückerath K, Melzer I, Mailfert S, Marguet D, Zörnig M, Hueber A-O. 2010. Palmitoylation of human FasL modulates its cell death-inducing function. Cell Death Dis. 1:e88.
- Haag SM, Gulen MF, Reymond L, Gibelin A, Abrami L, Decout A, Heymann M, van der Goot FG, Turcatti G, Behrendt R, et al. 2018. Identification of covalent small-molecule inhibitors of STING. Nature (in Press).
- Hach JC, McMichael T, Chesarino NM, Yount JS. 2013. Palmitoylation on conserved and nonconserved cysteines of murine IFITM1 regulates its stability and anti-influenza A virus activity. J Virol. 87:9923–9927.
- Hancock JF. 1995. Prenylation and palmitoylation analysis. Meth Enzymol. 255:237–245.
- Hannoush RN, Arenas-Ramirez N. 2009. Imaging the lipidome: omega-alkynyl fatty acids for detection and cellular visualization of lipid-modified proteins. ACS Chem Biol. 4:581–587.
- Harada T, Matsuzaki O, Hayashi H, Sugano S, Matsuda A, Nishida E. 2003. AKRL1 and AKRL2 activate the JNK pathway. Genes Cells. 8:493–500.
- Hein MY, Hubner NC, Poser I, Cox J, Nagaraj N, Toyoda Y, Gak IA, Weisswange I, Mansfeld J, Buchholz F, et al. 2015. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell. 163:712–723.
- Hemsley PA, Grierson CS. 2011. The ankyrin repeats and DHHC S-acyl transferase domain of AKR1 act independently to regulate switching from vegetative to mating states in yeast. PLoS One. 6:e28799.
- Hemsley PA. 2009. Protein S-acylation in plants (Review). Mol Membr Biol. 26:114–125.
- Hemsley PA. 2013. Assaying protein S-acylation in plants. Methods Mol Biol. 1043:141–146.
- Hernandez JL, Davda D, Kit MCS, Majmudar JD, Won SJ, Gang M, Pasupuleti SC, Choi AI, Bartkowiak CM, Martin BR. 2017. APT2 inhibition restores scribble localization and S-palmitoylation in snail-transformed cells. Cell Chem Biol. 24:87–97.
- Hernandez JL, Davda D, Majmudar JD, Won SJ, Prakash A, Choi AI, Martin BR. 2016. Correlated S-palmitoylation profiling of Snail-induced epithelial to mesenchymal transition. Mol Biosyst. 12:1799–1808.
- Ho GPH, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA, Snyder SH. 2011. S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron. 71:131–141.
- Hobbs GA, Der CJ, Rossman KL. 2016. RAS isoforms and mutations in cancer at a glance. J Cell Sci. 129:1287–1292.
- Howie J, Reilly L, Fraser NJ, Vlachaki Walker JM, Wypijewski KJ, Ashford MLJ, Calaghan SC, McClafferty H, Tian L, Shipston MJ, et al. 2014. Substrate recognition by the cell surface palmitoyl transferase DHHC5. Proc Natl Acad Sci U S A. 111:17534–17539.
- Howie J, Wypijewski KJ, Plain F, Tulloch LB, Fraser NJ, Fuller W. 2018. Greasing the wheels or a spanner in the works? Regulation of the cardiac sodium pump by palmitoylation. Crit Rev Biochem Mol Biol. 53:175–191.
- Huang K, Sanders S, Singaraja R, Orban P, Cijsouw T, Arstikaitis P, Yanai A, Hayden MR, El-Husseini A. 2009. Neuronal palmitoyl acyl transferases exhibit distinct substrate specificity. FASEB J Off Publ Fed Am Soc Exp Biol. 23:2605–2615.
- Huang K, Yanai A, Kang R, Arstikaitis P, Singaraja RR, Metzler M, Mullard A, Haigh B, Gauthier-Campbell C, Gutekunst C-A, et al. 2004. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron. 44:977–986.
- Hurst CH, Hemsley PA. 2015. Current perspective on protein S-acylation in plants: more than just a fatty anchor? J Exp Bot. 66:1599–1606.
- Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 461:788–792.
- Itoh S, Mizuno K, Aikawa M, Aikawa E. 2018. Dimerization of sortilin regulates its trafficking to extracellular vesicles. J Biol Chem. 293:4532–4544.
- Ivaldi C, Martin BR, Kieffer-Jaquinod S, Chapel A, Levade T, Garin J, Journet A. 2012. Proteomic analysis of S-acylated proteins in human B cells reveals palmitoylation of the immune regulators CD20 and CD23. PLoS One. 7:e37187.
- Jenkins CM, Yang J, Sims HF, Gross RW. 2011. Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J Biol Chem. 286:11937–11950.
- Jennings BC, Linder ME. 2012. DHHC protein S-acyltransferases use similar ping-pong kinetic mechanisms but display different acyl-CoA specificities. J Biol Chem. 287:7236–7245.
- Jiang H, Zhang X, Chen X, Aramsangtienchai P, Tong Z, Lin H. 2018. Protein lipidation: occurrence, mechanisms, biological functions, and enabling technologies. Chem Rev. 118:919–988.
- Jing H, Zhang X, Wisner SA, Chen X, Spiegelman NA, Linder ME, Lin H. 2017. SIRT2 and lysine fatty acylation regulate the transforming activity of K-Ras4a. eLife. 6:e32436.
- Jones ML, Tay CL, Rayner JC. 2012. Getting stuck in: protein palmitoylation in Plasmodium. Trends Parasitol. 28:496–503.
- Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, Thompson JX, Roth AF, Drisdel RC, Mastro R, et al. 2008. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 456:904–909.
- Kathayat RS, Cao Y, Elvira PD, Sandoz PA, Zaballa M-E, Springer MZ, Drake LE, Macleod KF, van der Goot FG, Dickinson BC. 2018. Active and dynamic mitochondrial S-depalmitoylation revealed by targeted fluorescent probes. Nat Commun. 9:334.
- Kathayat RS, Elvira PD, Dickinson BC. 2017. A fluorescent probe for cysteine depalmitoylation reveals dynamic APT signaling. Nat Chem Biol. 13:150–152.
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoè PM, Lüscher B. 2004. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci Off J Soc Neurosci. 24:5881–5891.
- Khanal N, Pejaver V, Li Z, Radivojac P, Clemmer DE, Mukhopadhyay S. 2015. Position of proline mediates the reactivity of S-palmitoylation. ACS Chem Biol. 10:2529–2536.
- Knudsen J, Neergaard TBF, Gaigg B, Jensen MV, Hansen JK. 2000. Role of Acyl-CoA binding protein in Acyl-CoA metabolism and Acyl-CoA–mediated cell signaling. J Nutr. 130:294S–298S.
- Kong E, Peng S, Chandra G, Sarkar C, Zhang Z, Bagh MB, Mukherjee AB. 2013. Dynamic palmitoylation links cytosol-membrane shuttling of acyl-protein thioesterase-1 and acyl-protein thioesterase-2 with that of proto-oncogene H-ras product and growth-associated protein-43. J Biol Chem. 288:9112–9125.
- Kordyukova LV, Serebryakova MV, Baratova LA, Veit M. 2008. S acylation of the hemagglutinin of influenza viruses: mass spectrometry reveals site-specific attachment of stearic acid to a transmembrane cysteine. J Virol. 82:9288–9292.
- Kostiuk MA, Corvi MM, Keller BO, Plummer G, Prescher JA, Hangauer MJ, Bertozzi CR, Rajaiah G, Falck JR, Berthiaume LG. 2008. Identification of palmitoylated mitochondrial proteins using a bio-orthogonal azido-palmitate analogue. FASEB J Off Publ Fed Am Soc Exp Biol. 22:721–732.
- Kümmel D, Heinemann U, Veit M. 2006. Unique self-palmitoylation activity of the transport protein particle component Bet3: a mechanism required for protein stability. Proc Natl Acad Sci. 103:12701–12706.
- Kuo S-C, Lampen JO. 1974. Tunicamycin—an inhibitor of yeast glycoprotein synthesis. Biochem Biophys Res Commun. 58:287–295.
- Lai J, Linder ME. 2013. Oligomerization of DHHC protein S-acyltransferases. J Biol Chem. 288:22862–22870.
- Lakkaraju AK, Abrami L, Lemmin T, Blaskovic S, Kunz B, Kihara A, Dal Peraro M, van der Goot FG. 2012. Palmitoylated calnexin is a key component of the ribosome-translocon complex. EMBO J. 31:1823–1835.
- Lam KKY, Davey M, Sun B, Roth AF, Davis NG, Conibear E. 2006. Palmitoylation by the DHHC protein Pfa4 regulates the ER exit of Chs3. J Cell Biol. 174:19–25.
- Lanyon-Hogg T, Faronato M, Serwa RA, Tate EW. 2017. Dynamic protein acylation: new substrates, mechanisms, and drug targets. Trends Biochem Sci. 42:566–581.
- Lawrence DS, Zilfou JT, Smith CD. 1999. Structure-activity studies of cerulenin analogues as protein palmitoylation inhibitors. J Med Chem. 42:4932–4941.
- Lemonidis K, Gorleku OA, Sanchez-Perez MC, Grefen C, Chamberlain LH. 2014. The Golgi S-acylation machinery comprises zDHHC enzymes with major differences in substrate affinity and S-acylation activity. Mboc. 25:3870–3883.
- Lemonidis K, Sanchez-Perez MC, Chamberlain LH. 2015. Identification of a novel sequence motif recognized by the ankyrin repeat domain of zDHHC17/13 S-acyltransferases. J Biol Chem. 290:21939–21950.
- Leventis R, Juel G, Knudsen JK, Silvius JR. 1997. Acyl-CoA binding proteins inhibit the nonenzymic S-acylation of cysteinyl-containing peptide sequences by long-chain acyl-CoAs. Biochemistry. 36:5546–5553.
- Li B, Cong F, Tan CP, Wang SX, Goff SP. 2002. Aph2, a protein with a zf-DHHC motif, interacts with c-Abl and Has pro-apoptotic activity. J Biol Chem. 277:28870–28876.
- Li W, Li W, Zou L, Ji S, Li C, Liu K, Zhang G, Sun Q, Xiao F, Chen D. 2017. Membrane targeting of inhibitory Smads through palmitoylation controls TGF-β/BMP signaling. Proc Natl Acad Sci U S A. 114:13206–13211.
- Li Y, Martin BR, Cravatt BF, Hofmann SL. 2012. DHHC5 protein palmitoylates flotillin-2 and is rapidly degraded on induction of neuronal differentiation in cultured cells. J Biol Chem. 287:523–530.
- Liao P, Wang W, Li Y, Wang R, Jin J, Pang W, Chen Y, Shen M, Wang X, Jiang D, et al. 2017. Palmitoylated SCP1 is targeted to the plasma membrane and negatively regulates angiogenesis. eLife. 6:e22058.
- Lievens PM-J, Kuznetsova T, Kochlamazashvili G, Cesca F, Gorinski N, Galil DA, Cherkas V, Ronkina N, Lafera J, Gaestel M, et al. 2016. ZDHHC3 tyrosine phosphorylation regulates neural cell adhesion molecule palmitoylation. Mol Cell Biol. 36:2208–2225.
- Lin DTS, Conibear E. 2015. ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. eLife. 4:e11306.
- Lin DTS, Davis NG, Conibear E. 2017. Targeting the Ras palmitoylation/depalmitoylation cycle in cancer. Biochem Soc Trans. 45:913–921.
- Lin H, Su X, He B. 2012. Protein lysine acylation and cysteine succination by intermediates of energy metabolism. ACS Chem Biol. 7:947–960.
- Linder ME, Deschenes RJ. 2007. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 8:74–84.
- Lobo S, Greentree WK, Linder ME, Deschenes RJ. 2002. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 277:41268–41273.
- Long JZ, Cravatt BF. 2011. The metabolic serine hydrolases and their functions in mammalian physiology and disease. Chem Rev. 111:6022–6063.
- Lynes EM, Bui M, Yap MC, Benson MD, Schneider B, Ellgaard L, Berthiaume LG, Simmen T. 2012. Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J. 31:457–470.
- Manna JD, Wepy JA, Hsu K-L, Chang JW, Cravatt BF, Marnett LJ. 2014. Identification of the major prostaglandin glycerol ester hydrolase in human cancer cells. J Biol Chem. 289:33741–33753.
- Marin EP, Derakhshan B, Lam TT, Davalos A, Sessa WC. 2012. Endothelial cell palmitoylproteomic identifies novel lipid-modified targets and potential substrates for protein acyl transferases. Circ Res. 110:1336–1344.
- Martin BR, Cravatt BF. 2009. Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods. 6:135–138.
- Martin BR, Wang C, Adibekian A, Tully SE, Cravatt BF. 2012. Global profiling of dynamic protein palmitoylation. Nat Methods. 9:84–89.
- Maynard TM, Meechan DW, Dudevoir ML, Gopalakrishna D, Peters AZ, Heindel CC, Sugimoto TJ, Wu Y, Lieberman JA, LaMantia A-S. 2008. Mitochondrial localization and function of a subset of 22q11 deletion syndrome candidate genes. Mol Cell Neurosci. 39:439–451.
- McCormick PJ, Dumaresq-Doiron K, Pluviose A-S, Pichette V, Tosato G, Lefrancois S. 2008. Palmitoylation controls recycling in lysosomal sorting and trafficking. Traffic Cph Den. 9:1984–1997.
- McMichael TM, Zhang L, Chemudupati M, Hach JC, Kenney AD, Hang HC, Yount JS. 2017. The palmitoyltransferase ZDHHC20 enhances interferon-induced transmembrane protein 3 (IFITM3) palmitoylation and antiviral activity. J Biol Chem. 292:21517–21526.
- Meiler S, Baumer Y, Huang Z, Hoffmann FW, Fredericks GJ, Rose AH, Norton RL, Hoffmann PR, Boisvert WA. 2013. Selenoprotein K is required for palmitoylation of CD36 in macrophages: implications in foam cell formation and atherogenesis. J Leukoc Biol. 93:771–780.
- Merrick BA, Dhungana S, Williams JG, Aloor JJ, Peddada S, Tomer KB, Fessler MB. 2011. Proteomic profiling of S-acylated macrophage proteins identifies a role for palmitoylation in mitochondrial targeting of phospholipid scramblase 3. Mol Cell Proteomics. 10:M110.006007.
- Misaki R, Morimatsu M, Uemura T, Waguri S, Miyoshi E, Taniguchi N, Matsuda M, Taguchi T. 2010. Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis. J Cell Biol. 191:23–29.
- Mitchell DA, Mitchell G, Ling Y, Budde C, Deschenes RJ. 2010. Mutational analysis of Saccharomyces cerevisiae Erf2 reveals a two-step reaction mechanism for protein palmitoylation by DHHC enzymes. J Biol Chem. 285:38104–38114.
- Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. 2006. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 47:1118–1127.
- Morrison E, Kuropka B, Kliche S, Brügger B, Krause E, Freund C. 2015. Quantitative analysis of the human T cell palmitome. Sci Rep. 5:11598.
- Mukai K, Konno H, Akiba T, Uemura T, Waguri S, Kobayashi T, Barber GN, Arai H, Taguchi T. 2016. Activation of STING requires palmitoylation at the Golgi. Nat Commun. 7:11932.
- Muszbek L, Haramura G, Cluette-Brown JE, Van Cott EM, Laposata M. 1999. The pool of fatty acids covalently bound to platelet proteins by thioester linkages can be altered by exogenously supplied fatty acids. Lipids. 34:S331–S337.
- Nadolski MJ, Linder ME. 2009. Molecular recognition of the palmitoylation substrate Vac8 by its palmitoyltransferase Pfa3. J Biol Chem. 284:17720–17730.
- Nagaraj N, Wisniewski JR, Geiger T, Cox J, Kircher M, Kelso J, Pääbo S, Mann M. 2011. Deep proteome and transcriptome mapping of a human cancer cell line. Mol Syst Biol. 7:548.
- Napoli E, Song G, Liu S, Espejo A, Perez CJ, Benavides F, Giulivi C. 2017. Zdhhc13-dependent Drp1 S-palmitoylation impacts brain bioenergetics, anxiety, coordination and motor skills. Sci Rep. 7:12796.
- Narayana SK, Helbig KJ, McCartney EM, Eyre NS, Bull RA, Eltahla A, Lloyd AR, Beard MR. 2015. The interferon-induced transmembrane proteins, IFITM1, IFITM2, and IFITM3 inhibit hepatitis C virus entry. J Biol Chem. 290:25946–25959.
- Navarro-Lérida I, Sánchez-Perales S, Calvo M, Rentero C, Zheng Y, Enrich C, Del Pozo MA. 2012. A palmitoylation switch mechanism regulates Rac1 function and membrane organization. EMBO J. 31:534–551.
- Nishimura A, Linder ME. 2013. Identification of a novel prenyl and palmitoyl modification at the CaaX motif of Cdc42 that regulates RhoGDI binding. Mol Cell Biol. 33:1417–1429.
- Noland CL, Gierke S, Schnier PD, Murray J, Sandoval WN, Sagolla M, Dey A, Hannoush RN, Fairbrother WJ, Cunningham CN. 2016. Palmitoylation of TEAD transcription factors is required for their stability and function in hippo pathway signaling. Structure. 1993 24:179–186.
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. 2006. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 366:2–16.
- Norton RL, Fredericks GJ, Huang Z, Fay JD, Hoffmann FW, Hoffmann PR. 2017. Selenoprotein K regulation of palmitoylation and calpain cleavage of ASAP2 is required for efficient FcγR-mediated phagocytosis. J Leukoc Biol. 101:439–448.
- O'Brien PJ, Zatz M. 1984. Acylation of bovine rhodopsin by [3H]palmitic acid. J Biol Chem. 259:5054–5057.
- Ohno Y, Kashio A, Ogata R, Ishitomi A, Yamazaki Y, Kihara A. 2012. Analysis of substrate specificity of human DHHC protein acyltransferases using a yeast expression system. Mboc. 23:4543–4551.
- Ohno Y, Kihara A, Sano T, Igarashi Y. 2006. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 1761:474–483.
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong S-E, Walford GA, Sugiana C, Boneh A, Chen WK, et al. 2008. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 134:112–123.
- Patterson SI, Skene JH. 1994. Novel inhibitory action of tunicamycin homologues suggests a role for dynamic protein fatty acylation in growth cone-mediated neurite extension. J Cell Biol. 124:521–536.
- Pedro MP, Vilcaes AA, Gomez GA, Daniotti JL. 2017. Individual S-acylated cysteines differentially contribute to H-Ras endomembrane trafficking and acylation/deacylation cycles. Mboc. 28:962–974.
- Peng T, Hang HC. 2015. Bifunctional fatty acid chemical reporter for analyzing S-palmitoylated membrane protein-protein interactions in mammalian cells. J Am Chem Soc. 137:556–559.
- Percher A, Ramakrishnan S, Thinon E, Yuan X, Yount JS, Hang HC. 2016. Mass-tag labeling reveals site-specific and endogenous levels of protein S-fatty acylation. Proc Natl Acad Sci U S A. 113:4302–4307.
- Percherancier Y, Planchenault T, Valenzuela-Fernandez A, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. 2001. Palmitoylation-dependent control of degradation, life span, and membrane expression of the CCR5 receptor. J Biol Chem. 276:31936–31944.
- Pérez-Sala D, Boya P, Ramos I, Herrera M, Stamatakis K. 2009. The C-terminal sequence of RhoB directs protein degradation through an endo-lysosomal pathway. PLoS One. 4:e8117.
- Perrody E, Abrami L, Feldman M, Kunz B, Urbé S, van der Goot FG. 2016. Ubiquitin-dependent folding of the Wnt signaling coreceptor LRP6. eLife. 5:e19083.
- Pinner AL, Tucholski J, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. 2016. Decreased protein S-palmitoylation in dorsolateral prefrontal cortex in schizophrenia. Schizophr Res. 177:78–87.
- Plain F, Congreve SD, Yee RSZ, Kennedy J, Howie J, Kuo C-W, Fraser NJ, Fuller W. 2017. An amphipathic α-helix directs palmitoylation of the large intracellular loop of the sodium/calcium exchanger. J Biol Chem. 292:10745–10752.
- Ponimaskin E, Schmidt MF. 1998. Domain-structure of cytoplasmic border region is main determinant for palmitoylation of influenza virus hemagglutinin (H7). Virology. 249:325–335.
- Qiu T, Kathayat RS, Cao Y, Beck MW, Dickinson BC. 2018. A fluorescent probe with improved water solubility permits the analysis of protein S-depalmitoylation activity in live cells. Biochemistry. 57:221–225.
- Rana MS, Kumar P, Lee C-J, Verardi R, Rajashankar KR, Banerjee A. 2018. Fatty acyl recognition and transfer by an integral membrane S -acyltransferase. Science. 359:eaao6326.
- Ren W, Jhala US, Du K. 2013a. Proteomic analysis of protein palmitoylation in adipocytes. Adipocyte. 2:17–28.
- Ren W, Sun Y, Du K. 2013b. DHHC17 palmitoylates ClipR-59 and modulates ClipR-59 association with the plasma membrane. Mol Cell Biol. 33:4255–4265.
- Ren W, Sun Y, Du K. 2015. Glut4 palmitoylation at Cys223 plays a critical role in Glut4 membrane trafficking. Biochem Biophys Res Commun. 460:709–714.
- Resh MD. 2006. Use of analogs and inhibitors to study the functional significance of protein palmitoylation. Methods San Diego Calif. 40:191–197.
- Ritzefeld M, Wright MH, Tate EW. 2017. New developments in probing and targeting protein acylation in malaria, leishmaniasis and African sleeping sickness. Parasitology. 1: 18.
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PIH. 2005. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 307:1746–1752.
- Rodenburg RNP, Snijder J, van de Waterbeemd M, Schouten A, Granneman J, Heck AJR, Gros P. 2017. Stochastic palmitoylation of accessible cysteines in membrane proteins revealed by native mass spectrometry. Nat Commun. 8:1280.
- Rossin A, Durivault J, Chakhtoura-Feghali T, Lounnas N, Gagnoux-Palacios L, Hueber A-O. 2015. Fas palmitoylation by the palmitoyl acyltransferase DHHC7 regulates Fas stability. Cell Death Differ. 22:643–653.
- Roth AF, Feng Y, Chen L, Davis NG. 2002. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol. 159:23–28.
- Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR, Davis NG. 2006. Global analysis of protein palmitoylation in yeast. Cell. 125:1003–1013.
- Runkle KB, Kharbanda A, Stypulkowski E, Cao X-J, Wang W, Garcia BA, Witze ES. 2016. Inhibition of DHHC20-mediated EGFR palmitoylation creates a dependence on EGFR signaling. Mol Cell. 62:385–396.
- Rusch M, Zimmermann TJ, Bürger M, Dekker FJ, Görmer K, Triola G, Brockmeyer A, Janning P, Böttcher T, Sieber SA, et al. 2011. Identification of Acyl protein thioesterases 1 and 2 as the cellular targets of the Ras-signaling modulators palmostatin B and M. Angew Chem Int Ed. 50:9838–9842.
- Sanders SS, Martin DDO, Butland SL, Lavallée-Adam M, Calzolari D, Kay C, Yates JR, Hayden MR. 2015. Curation of the mammalian palmitoylome indicates a pivotal role for palmitoylation in diseases and disorders of the nervous system and cancers. PLoS Comput Biol. 11:e1004405.
- Santiago-Tirado FH, Doering TL. 2016. All about that fat: lipid modification of proteins in Cryptococcus neoformans. J Microbiol. 54:212–222.
- Santiago-Tirado FH, Peng T, Yang M, Hang HC, Doering TL. 2015. A single protein S-acyl transferase acts through diverse substrates to determine cryptococcal morphology, stress tolerance, and pathogenic outcome. PLoS Pathog. 11:e1004908.
- Schellenberg B, Wang P, Keeble JA, Rodriguez-Enriquez R, Walker S, Owens TW, Foster F, Tanianis-Hughes J, Brennan K, Streuli CH, et al. 2013. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol Cell. 49:959–971.
- Schmidt MFG, Schlesinger MJ. 1979. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 17:813–819.
- Senyilmaz D, Virtue S, Xu X, Tan CY, Griffin JL, Miller AK, Vidal-Puig A, Teleman AA. 2015. Regulation of mitochondrial morphology and function by stearoylation of TFR1. Nature. 525:124–128.
- Serwa RA, Abaitua F, Krause E, Tate EW, O’Hare P. 2015. Systems analysis of protein fatty acylation in Herpes simplex virus-infected cells using chemical proteomics. Chem Biol. 22:1008–1017.
- Shahinian S, Silvius JR. 1995. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry. 34:3813–3822.
- Sharma C, Wang H-X, Li Q, Knoblich K, Reisenbichler ES, Richardson AL, Hemler ME. 2017. Protein acyltransferase DHHC3 regulates breast tumor growth, oxidative stress, and senescence. Cancer Res. 77:6880–6890.
- Shen L-F, Chen Y-J, Liu K-M, Haddad ANS, Song I-W, Roan H-Y, Chen L-Y, Yen JJY, Chen Y-J, Wu J-Y, et al. 2017. Role of S-palmitoylation by ZDHHC13 in mitochondrial function and metabolism in liver. Sci Rep. 7:2182.
- Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, Khudayberdiev S, Leuschner PF, Busch CJL, Kane C, et al. 2009. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 11:705–716.
- Singaraja RR, Hadano S, Metzler M, Givan S, Wellington CL, Warby S, Yanai A, Gutekunst C-A, Leavitt BR, Yi H, et al. 2002. HIP14, a novel ankyrin domain-containing protein, links huntingtin to intracellular trafficking and endocytosis. Hum Mol Genet. 11:2815–2828.
- Singaraja RR, Kang MH, Vaid K, Sanders SS, Vilas GL, Arstikaitis P, Coutinho J, Drisdel RC, El-Husseini AED, Green WN, et al. 2009. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ Res. 105:138–147.
- Skotte NH, Sanders SS, Singaraja RR, Ehrnhoefer DE, Vaid K, Qiu X, Kannan S, Verma C, Hayden MR. 2017. Palmitoylation of caspase-6 by HIP14 regulates its activation. Cell Death Differ. 24:433–444.
- Smoly I, Shemesh N, Ziv-Ukelson M, Ben-Zvi A, Yeger-Lotem E. 2017. An asymmetrically balanced organization of kinases versus phosphatases across eukaryotes determines their distinct impacts. PLoS Comput Biol. 13:e1005221.
- Sobocińska J, Roszczenko-Jasińska P, Zaręba-Kozioł M, Hromada-Judycka A, Matveichuk OV, Traczyk G, Łukasiuk K, Kwiatkowska K. 2018. LPS upregulates palmitoylated enzymes of the phosphatidylinositol cycle. An insight from proteomic studies. Mol Cell Proteomics. 17:233.
- Soyombo AA, Hofmann SL. 1997. Molecular cloning and expression of palmitoyl-protein thioesterase 2 (PPT2), a homolog of lysosomal palmitoyl-protein thioesterase with a distinct substrate specificity. J Biol Chem. 272:27456–27463.
- Stucki JW, Lehmann LH, Siegel E. 1989. Acylation of proteins by myristic acid in isolated mitochondria. J Biol Chem. 264:6376–6380.
- Sugimoto H, Hayashi H, Yamashita S. 1996. Purification, cDNA cloning, and regulation of lysophospholipase from rat liver. J Biol Chem. 271:7705–7711.
- Suzuki M, Murakami T, Cheng J, Kano H, Fukata M, Fujimoto T. 2015. ELMOD2 is anchored to lipid droplets by palmitoylation and regulates adipocyte triglyceride lipase recruitment. Mboc. 26:2333–2342.
- Swarthout JT, Lobo S, Farh L, Croke MR, Greentree WK, Deschenes RJ, Linder ME. 2005. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J Biol Chem. 280:31141–31148.
- Tao N, Wagner SJ, Lublin DM. 1996. CD36 is palmitoylated on both N- and C-terminal cytoplasmic tails. J Biol Chem. 271:22315–22320.
- Thinon E, Percher A, Hang HC. 2016. Bioorthogonal chemical reporters for monitoring unsaturated fatty-acylated proteins. Chembiochem Eur J Chem Biol. 17:1800–1803.
- Thomas GM, Hayashi T, Chiu S-L, Chen C-M, Huganir RL. 2012. Palmitoylation by DHHC5/8 targets GRIP1 to dendritic endosomes to regulate AMPA-R trafficking. Neuron. 73:482–496.
- Thomas GM, Hayashi T, Huganir RL, Linden DJ. 2013. DHHC8-dependent PICK1 palmitoylation is required for induction of cerebellar long-term synaptic depression. J Neurosci Off J Soc Neurosci. 33:15401–15407.
- Thorne RF, Ralston KJ, de Bock CE, Mhaidat NM, Zhang XD, Boyd AW, Burns GF. 2010. Palmitoylation of CD36/FAT regulates the rate of its post-transcriptional processing in the endoplasmic reticulum. Biochim Biophys Acta. 1803:1298–1307.
- Tian H, Lu J-Y, Shao C, Huffman KE, Carstens RM, Larsen JE, Girard L, Liu H, Rodriguez-Canales J, Frenkel EP, et al. 2015. Systematic siRNA screen unmasks NSCLC growth dependence by palmitoyltransferase DHHC5. Mol Cancer Res. 13:784–794.
- Tian L, Jeffries O, McClafferty H, Molyvdas A, Rowe ICM, Saleem F, Chen L, Greaves J, Chamberlain LH, Knaus H-G, et al. 2008. Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proc Natl Acad Sci U S A. 105:21006–21011.
- Tian L, McClafferty H, Knaus H-G, Ruth P, Shipston MJ. 2012. Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels. J Biol Chem. 287:14718–14725.
- Tomatis VM, Trenchi A, Gomez GA, Daniotti JL. 2010. Acyl-protein thioesterase 2 catalyzes the deacylation of peripheral membrane-associated GAP-43. PLoS One. 5:e15045.
- Tortosa E, Adolfs Y, Fukata M, Pasterkamp RJ, Kapitein LC, Hoogenraad CC. 2017. Dynamic palmitoylation targets MAP6 to the axon to promote microtubule stabilization during neuronal polarization. Neuron. 94:809–825.e7.
- Toyoda T, Sugimoto H, Yamashita S. 1999. Sequence, expression in Escherichia coli, and characterization of lysophospholipase II. Biochim Biophys Acta. 1437:182–193.
- Tsukamoto T, Li X, Morita H, Minowa T, Aizawa T, Hanagata N, Demura M. 2013. Role of S-palmitoylation on IFITM5 for the interaction with FKBP11 in osteoblast cells. PLoS One. 8:e75831.
- Tsutsumi R, Fukata Y, Fukata M. 2008. Discovery of protein-palmitoylating enzymes. Pflugers Arch – Eur J Physiol. 456:1199–1206.
- Upadhyay S, Xu X, Lin X. 2016. Interactions between melanin enzymes and their atypical recruitment to the secretory pathway by palmitoylation. mBio. 7:e01925-16.
- Vaira V, Faversani A, Dohi T, Maggioni M, Nosotti M, Tosi D, Altieri DC, Bosari S. 2011. Aberrant overexpression of the cell polarity module scribble in human cancer. Am J Pathol. 178:2478–2483.
- Valdez-Taubas J, Pelham H. 2005. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. EMBO J. 24:2524–2532.
- Vartak N, Papke B, Grecco HE, Rossmannek L, Waldmann H, Hedberg C, Bastiaens PIH. 2014. The autodepalmitoylating activity of APT maintains the spatial organization of palmitoylated membrane proteins. Biophys J. 106:93–105.
- Verardi R, Kim J-S, Ghirlando R, Banerjee A. 2017. Structural basis for substrate recognition by the ankyrin repeat domain of human DHHC17 palmitoyltransferase. Structure. 25:1337–1347.e6.
- Verkruyse LA, Hofmann SL. 1996. Lysosomal targeting of palmitoyl-protein thioesterase. J Biol Chem. 271:15831–15836.
- Vujic I, Sanlorenzo M, Esteve-Puig R, Vujic M, Kwong A, Tsumura A, Murphy R, Moy A, Posch C, Monshi B, et al. 2016. Acyl protein thioesterase 1 and 2 (APT-1, APT-2) inhibitors palmostatin B, ML348 and ML349 have different effects on NRAS mutant melanoma cells. Oncotarget. 7:7297–7306.
- Wagner GR, Hirschey MD. 2014. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol Cell. 54:5–16.
- Wan J, Savas JN, Roth AF, Sanders SS, Singaraja RR, Hayden MR, Yates JR, Davis NG. 2013. Tracking brain palmitoylation change: predominance of glial change in a mouse model of Huntington’s disease. Chem Biol. 20:1421–1434.
- Wang W, Runkle KB, Terkowski SM, Ekaireb RI, Witze ES. 2015. Protein depalmitoylation is induced by Wnt5a and promotes polarized cell behavior. J Biol Chem. 290:15707–15716.
- Waterborg JH, Matthews HR. 1994. Fluorography of polyacrylamide gels containing tritium. Methods Mol Biol. 32:163–167.
- Weber P, Batoulis H, Rink KM, Dahlhoff S, Pinkwart K, Söllner TH, Lang T. 2017. Electrostatic anchoring precedes stable membrane attachment of SNAP25/SNAP23 to the plasma membrane. eLife. 6:e19394.
- Wei X, Song H, Semenkovich CF. 2014. Insulin-regulated protein palmitoylation impacts endothelial cell function. Arterioscler Thromb Vasc Biol. 34:346–354.
- Weinert BT, Moustafa T, Iesmantavicius V, Zechner R, Choudhary C. 2015. Analysis of acetylation stoichiometry suggests that SIRT3 repairs nonenzymatic acetylation lesions. EMBO J. 34:2620–2632.
- Wilson JP, Raghavan AS, Yang Y-Y, Charron G, Hang HC. 2011. Proteomic analysis of fatty-acylated proteins in mammalian cells with chemical reporters reveals S-acylation of histone H3 variants. Mol Cell Proteomics. 10:M110.001198.
- Won SJ, Cheung See Kit M, Martin BR. 2018. Protein depalmitoylases. Crit Rev Biochem Mol Biol. 53:83–98.
- Won SJ, Davda D, Labby KJ, Hwang SY, Pricer R, Majmudar JD, Armacost KA, Rodriguez LA, Rodriguez CL, Chong FS, et al. 2016. Molecular mechanism for isoform-selective inhibition of Acyl protein thioesterases 1 and 2 (APT1 and APT2). ACS Chem Biol. 11:3374–3382.
- Wyckoff TJO, Lin S, Cotter RJ, Dotson GD, Raetz CRH. 1998. Hydrocarbon rulers in UDP-N-acetylglucosamine acyltransferases. J Biol Chem. 273:32369–32372.
- Xu X, Li M, Wu Z, Wang H, Wang L, Huang K, Liu X, Hou Q, Lin G, Hu C. 2017. Endoplasmic reticulum transmembrane proteins ZDHHC1 and STING both act as direct adaptors for IRF3 activation in teleost. J Immunol. 199:3623–3633.
- Yang G, Cynader MS. 2011. Palmitoyl acyltransferase zD17 mediates neuronal responses in acute ischemic brain injury by regulating JNK activation in a signaling module. J Neurosci Off J Soc Neurosci. 31:11980–11991.
- Yang W, Di Vizio D, Kirchner M, Steen H, Freeman MR. 2010. Proteome scale characterization of human S-acylated proteins in lipid raft-enriched and non-raft membranes. Mol Cell Proteomics. 9:54–70.
- Yang J, Gibson B, Snider J, Jenkins CM, Han X, Gross RW. 2005. Submicromolar concentrations of palmitoyl-CoA specifically thioesterify cysteine 244 in glyceraldehyde-3-phosphate dehydrogenase inhibiting enzyme activity: a novel mechanism potentially underlying fatty acid induced insulin resistance. Biochemistry. 44:11903–11912.
- Yeste-Velasco M, Mao X, Grose R, Kudahetti SC, Lin D, Marzec J, Vasiljević N, Chaplin T, Xue L, Xu M, et al. 2014. Identification of ZDHHC14 as a novel human tumour suppressor gene. J Pathol. 232:566–577.
- Yik JHN, Weigel PH. 2002. The position of cysteine relative to the transmembrane domain is critical for palmitoylation of H1, the major subunit of the human asialoglycoprotein receptor. J Biol Chem. 277:47305–47312.
- Yokoi N, Fukata Y, Sekiya A, Murakami T, Kobayashi K, Fukata M. 2016. Identification of PSD-95 depalmitoylating enzymes. J Neurosci. 36:6431–6444.
- Youle RJ, Strasser A. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 9:47–59.
- Young E, Zheng Z-Y, Wilkins AD, Jeong H-T, Li M, Lichtarge O, Chang EC. 2014. Regulation of Ras localization and cell transformation by evolutionarily conserved palmitoyltransferases. Mol Cell Biol. 34:374–385.
- Yount JS, Karssemeijer RA, Hang HC. 2012. S-palmitoylation and ubiquitination differentially regulate interferon-induced transmembrane protein 3 (IFITM3)-mediated resistance to influenza virus. J Biol Chem. 287:19631–19641.
- Yount JS, Moltedo B, Yang Y-Y, Charron G, Moran TM, López CB, Hang HC. 2010. Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nat Chem Biol. 6:610–614.
- Zhang MM, Wu P-YJ, Kelly FD, Nurse P, Hang HC. 2013. Quantitative control of protein S-palmitoylation regulates meiotic entry in fission yeast. PLoS Biol. 11:e1001597.
- Zhang X-L, Ding H-H, Xu T, Liu M, Ma C, Wu S-L, Wei J-Y, Liu C-C, Zhang S-B, Xin W-J. 2018. Palmitoylation of δ-catenin promotes kinesin-mediated membrane trafficking of Nav1.6 in sensory neurons to promote neuropathic pain. Sci Signal. 11:eaar4394.
- Zheng B, DeRan M, Li X, Liao X, Fukata M, Wu X. 2013. 2-Bromopalmitate analogues as activity-based probes to explore palmitoyl acyltransferases. J Am Chem Soc. 135:7082–7085.
- Zhou Q, Lin H, Wang S, Wang S, Ran Y, Liu Y, Ye W, Xiong X, Zhong B, Shu H-B, et al. 2014. The ER-associated protein ZDHHC1 is a positive regulator of DNA virus-triggered, MITA/STING-dependent innate immune signaling. Cell Host Microbe. 16:450–461.
- Zhu G, Fujii K, Belkina N, Liu Y, James M, Herrero J, Shaw S. 2005. Exceptional disfavor for proline at the P + 1 position among AGC and CAMK kinases establishes reciprocal specificity between them and the proline-directed kinases. J Biol Chem. 280:10743–10748.
