ABSTRACT
This study was a 3 (Brand: Blu, MarkTen, Vuse) by 3 (Warning Size: 20%, 30%, or 50% of advertisement surface) by 2 (Warning Background: White, Red) experimental investigation of the effects of electronic cigarette (e-cigarette) warning label design features. Young adults aged 18–30 years (n = 544) were recruited online, completed demographic and tobacco use history measures, and randomized to view e-cigarette advertisements with warning labels that varied by the experimental conditions. Participants completed a task assessing self-reported visual attention to advertisements with a-priori regions of interest defined around warning labels. Warning message recall and perceived addictiveness of e-cigarettes were assessed post-exposure. Approximately half of participants reported attending to warning labels and reported attention was greater for warnings on red versus white backgrounds. Recall of the warning message content was also greater among those reporting attention to the warning label. Overall, those who viewed warnings on red backgrounds reported lower perceived addictiveness than those who viewed warnings on white backgrounds, and e-cigarette users reported lower perceived addictiveness than non-users. Among e-cigarette users, viewing warnings on white backgrounds produced perceptions more similar to non-users. Greater recall was significantly correlated with greater perceived addictiveness. This study provides some of the first evidence that e-cigarette warning label design features including size and coloring affect self-reported attention and content recall.
Introduction
Tobacco use, primarily cigarette smoking, causes nearly half a million premature deaths and incurs approximately $300 billion in costs to society each year in the U.S. (Centers for Disease Control and Prevention [CDC], Citation2014). Tobacco companies spend billions on advertising each year to promote their products (CDC, Citation2014), and advertising exposure is causally linked to youth tobacco use (Lovato, Watts, & Stead, Citation2011). Although cigarette advertising remains pervasive, electronic cigarettes (“e-cigarettes”) are the most widely advertised non-cigarette tobacco product, and the majority of advertising expenditures are accounted for by products made by companies that are the leading producers of cigarettes (Truth Initiative, Citation2015). There is evidence that cigarette companies use distinctive marketing strategies for their e-cigarette products, including exclusively marketing nicotine-containing “cigalike” products resembling traditional cigarettes (Seidenberg, Jo, & Ribisl, Citation2016).
In 2016, the U.S. Surgeon General issued a report on the potential public health impact of e-cigarette use among youth and young adults (U.S. Department of Health and Human Services [US DHHS], Citation2016). National data indicate that 20% of young adults aged 18 to 24 have tried e-cigarettes, 6% use them every day or some days, and 14% use them intermittently (Delnevo et al., Citation2016; Hu et al., Citation2016). Although e-cigarette use is most common among cigarette smokers (Delnevo et al., Citation2016), e-cigarette use is also occurring among young adult non-smokers (Coleman et al., Citation2015), and an estimated 1 of 4 young adults who are not e-cigarette users may be susceptible to using them (Mays et al., Citation2016a). Such data have prompted substantial debate about the potential harms and benefits of e-cigarettes to public health. If young adults who do not use tobacco initiate e-cigarettes use, are at an increased risk of potential harm due to nicotine exposure, and at an increased risk of transitioning to more harmful forms of tobacco use (e.g., cigarette smoking), this may create public health harms (US DHHS, Citation2016). If young adult cigarette smokers switch from cigarettes to e-cigarettes or use e-cigarettes to quit tobacco completely, this can create public health benefits (Levy et al., 2017).
Although many factors may contribute to such patterns of e-cigarette use, young adults’ e-cigarette use is likely affected by advertising. Nearly 90% of U.S. young adults report e-cigarette advertising exposure (Duke et al., Citation2014; Truth Initiative, Citation2015), and advertisements for the most heavily marketed e-cigarette brands have been designed to appeal to young adults by portraying them using the products in an attractive way (Cantrell, Emelle, Ganz, Hair, & Vallone, Citation2016). E-cigarette advertisements have been shown in prior research to affect young adults’ perceptions of e-cigarettes, interest in using them, and e-cigarette trial (Pokhrel et al., Citation2016; Trumbo & Kim, Citation2015; Villanti et al., Citation2016). Health warning labels on tobacco advertisements are a recommended strategy to offset the potential promoting effects of tobacco advertising by communicating the potential risks of tobacco use to consumers (Hammond, Wakefield, Durkin, & Brennan, Citation2013). The Family Smoking Prevention and Tobacco Control Act authorized the U.S. Food and Drug Administration (FDA) to regulate cigarettes, smokeless, and roll-your-own tobacco, including the authority to require health warnings on advertisements and to issue regulations on advertising within the limits of commercial free speech protections of the First Amendment (Husten & Deyton, Citation2013). In May 2016, FDA published a “deeming” rule extending the agency’s tobacco regulatory authority to e-cigarettes and other tobacco products. This rule includes a requirement that e-cigarette advertisements display a warning label comprised of black and white text, occupying 20% of the advertisement surface, and communicating their addictiveness (Food and Drug Administration [FDA], Citation2016). This rule also allows for potential changes to warning label requirements in the future (FDA, Citation2016). Critically, any future warning label regulations must be supported by evidence demonstrating they are appropriate for the protection of public health, including evidence of their effects among populations such as young people, tobacco users, and non-users (Husten & Deyton, Citation2013).
Research on how to optimally design e-cigarette warning labels to promote positive public health outcomes remains limited. Studies to date have primarily examined variations on the content of e-cigarette warning message text (Pesko, Kenkel, Wang, & Hughes, Citation2016; Sanders-Jackson, Schleicher, Fortmann, & Henriksen, Citation2015), as opposed to evaluating other design features (e.g., size, coloring) that may affect their impact. Few studies have focused on young adults, a priority population for research on e-cigarette use and public health communication interventions such as warning labels (FDA, Citation2012; US DHHS, Citation2016).
To address these research gaps, this study aimed to investigate the effects of e-cigarette warning message design features that affect their visual prominence within advertisements, including their size and coloring. The study design and hypotheses were guided by previous theoretical and empirical research on advertising, tobacco control communication, and tobacco product perceptions. Product advertisements affect consumers through a series of steps from message exposure, to formulating product perceptions, and ultimately product use behavior (Lavidge & Steiner, Citation1961). Initial visual attention to advertisements is positively associated with message content recall in this process (Wedel & Peters, Citation2000). Tobacco control communication models similarly specify cognitive responses to advertisements and warning labels therein to characterize their effects, including attention and message content recall (Fong et al., Citation2006; Noar et al., Citation2016). Attention to warning messages and recall of messages content can in turn shape e-cigarette perceptions (Fong et al., Citation2006; Noar et al., Citation2016), which are associated with e-cigarette use (Pearson, Richardson, Niaura, Vallone, & Abrams, Citation2012; Richardson, Pearson, Xiao, Stalgaitis, & Vallone, Citation2014; Trumbo & Kim, Citation2015).
Consistent with these theoretical models and research on warnings for cigarettes (Hammond et al., Citation2013; Meernik et al., Citation2016; Strasser, Tang, Romer, Jepson, & Cappella, Citation2012), we hypothesized that making warnings more visually prominent on e-cigarette advertisements by 1) increasing the amount of the advertisement surface they occupy (i.e., their size); and 2) changing their background color from white to red would increase attention to the warning message and recall of the message content and enhance effects on e-cigarette perceptions. Given the need for evidence of the effects of warning message regulations among tobacco users and non-users to support FDA tobacco regulations (Husten & Deyton, Citation2013), we also explored whether the effects of these e-cigarette warning label design features varied by cigarette smoking status and e-cigarette use. We assessed these effects on self-reported visual attention to warnings, recall of warning message content, and perceptions of e-cigarettes in a convenience sample of U.S. young adults ages 18 to 30 years.
Methods
Setting and sample
Participants were recruited in January 2016 through Amazon Mechanical Turk, a crowdsourcing platform used in similar tobacco-related studies (Hall, Ribisl, & Brewer, Citation2014; Magnan & Cameron, Citation2015; Mays, Smith, Johnson, Tercyak, & Niaura, Citation2016b). After reviewing a description of the study, individuals who were interested in participating proceeded to a consent form and eligibility screener. Young adults ages 18 to 30 were eligible to participate and participation was limited to Mechanical Turk members in the U.S. There were no other restrictions on study inclusion or exclusion. Eligible, consenting individuals proceeded to the online experiment. Participants completing all study procedures were given a monetary credit through Mechanical Turk.
Procedures
The study was a cross-sectional experiment where participants viewed an e-cigarette advertisement and completed a series of measures in a single session. Participants completed demographic and tobacco use history measures and then, using an algorithm embedded in the online survey, were randomized to view one of 18 e-cigarette advertisements in a 3 (Brand: Blu, MarkTen, Vuse) by 3 (Warning Size: 20%, 30%, or 50% of advertisement) by 2 (Warning Background Color: White or Red) experiment. These e-cigarette brands were chosen because they are among the most widely advertised brands in the U.S. (Truth Initiative, Citation2015). The size variations were selected based on existing tobacco regulations (FDA, Citation2016) and international recommendations (i.e., the Framework Convention on Tobacco Control) for tobacco product warnings (Hammond et al., Citation2013). The study compared warnings on white and red backgrounds based on consumer research indicating the color red is attention grabbing and evokes emotion (Singh, Citation2006) and research demonstrating that varying color in tobacco product warnings attracts greater visual attention (Meernik et al., Citation2016).
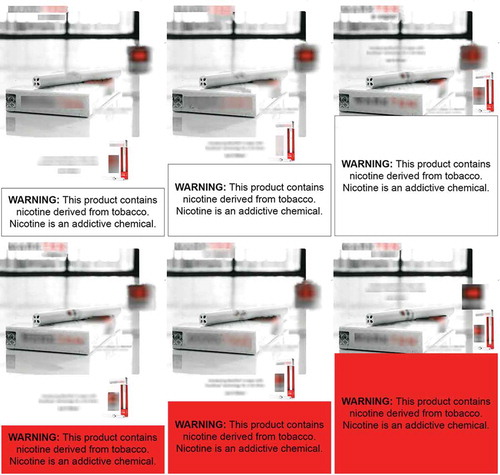
The warning label displayed for all conditions was the warning from FDA’s proposed deeming rule stating “Warning: This product contains nicotine derived from tobacco. Nicotine is an addictive chemical.” This statement is similar to the warning required in the final deeming regulations published in 2016 (FDA, Citation2016). Print advertisements were obtained from a publicly available tobacco advertising database (www.trinketsandtrash.org). Advertisements were sized to identical dimensions and edited to remove minor content differences for consistency across conditions (e.g., presence of web site addresses). Warnings were displayed with consistent black font and placement across conditions but varied in size and color based on the experimental factors. Participants viewed the e-cigarette advertisement for as long as they wished and then completed post-exposure measures. Example stimuli are shown in , and complete stimuli are available from the corresponding author.
Figure 1. Example study stimuli.
Note: Example stimuli from a single brand shown. Branding content visually occluded for publication purposes.
The study employed recommended data quality assurance procedures for Mechanical Turk, including measures to prohibit duplicate responses and a “captcha” verification item required for completion (Mayson & Suri, Citation2012). The university Institutional Review Board approved the research protocol.
Measures
Demographics and tobacco use history
Prior to the experimental exposure, participants completed measures of demographics (age, sex, race/ethnicity, education, and household income), cigarette smoking, and e-cigarette use. Current cigarette smokers were those who reported smoking 100 or more lifetime cigarettes and now smoking every day or some days (Hu et al., Citation2016). Current e-cigarette users were those who reported now using e-cigarettes all days or some days with no minimum threshold for lifetime use (Hu et al., Citation2016). Past 30-day use of other tobacco products including waterpipe, smokeless tobacco, large cigars, and little cigars/cigarillos was also assessed to characterize the sample (Mays et al., Citation2016b).
Visual attention
When presented with the advertisement, participants self-reported visual attention to the stimuli using a task developed in previous studies (Hoek, Gendall, Eckert, Rolls, & Louviere, Citation2016; Mays et al., Citation2016b). Participants were instructed to identify up to five areas in the advertisement that attracted their attention by pointing their cursor and clicking on the areas of the advertisement image. Similar to previous research (Lochbuehler et al., Citation2016; Strasser et al., Citation2012), a priori Regions of Interest (ROIs) were defined around branding content and warning labels. Branding ROIs included brand names or slogans, packaging, and product depictions. The warning label ROI was constructed around the warning label text box at the bottom of each advertisement. This enabled an assessment of the proportion of participants reporting attending to ROIs for any branding content (yes/no) and warning labels (yes/no), and a comparison of self-reported attention to the warning label ROIs across conditions.
Recall of warning message content
Recall of the warning message content was assessed after exposure to the stimuli using a protocol validated in previous research (Strasser et al., Citation2012). An open-ended response item asked participants to describe what the warning label stated. Two reviewers independently coded responses for the following key themes from the warning message: nicotine, tobacco, addictive, chemical. Coding took into account minor variations and spelling errors. The two coders agreed on nearly all (>95%) coding decisions; in the few instances of disagreement, a third reviewer made a final coding determination. This process produced a continuous measure of the total number of key themes recalled and overall recall (yes/no) of all four key themes (Strasser et al., Citation2012).
Perceived addictiveness of e-cigarettes
Since the warning message focused on the addictiveness of e-cigarettes, the study measured perceived addictiveness of e-cigarettes using two items from previous research (Mays et al., Citation2016b). Items captured absolute perceptions that e-cigarettes are addictive on a 1 (not at all) to 4 (very) scale and relative perceived addictiveness compared with cigarettes on a 1 (much less) to 5 (much more) scale. Both were analyzed as continuous variables.
Statistical analyses
Descriptive statistics were used to characterize the sample, and bivariate tests were used to examine whether participant characteristics differed across experimental factors. No participant characteristics differed significantly by the factors, and therefore multivariable analyses described below did not adjust for covariates. Visual attention ROI data were analyzed with bivariate chi-square tests to examine whether reported visual attention differed based on the experimental factors. Logistic regression was then used to examine the main effects of the experimental factors, their two- and three-way interactions, and potential interactions between the experimental factors, cigarette smoking, and e-cigarette use on the binary dependent variable indicating visual attention to the warning label ROI (yes/no). Analysis of variance (ANOVA) was used to test for differences in total number of key themes recalled and perceived addictiveness of e-cigarettes across experimental factors, to test for interactions among experimental factors, and to test for interactions between the experimental factors, cigarette smoking, and e-cigarette use. Pair-wise least squares mean differences were inspected, and Bonferroni correction was applied to account for the experiment-wise error rate for multiple comparisons in interaction effects.
Results
Sample characteristics
In total 1,281 individuals responded to eligibility screening questions, and 544 (42.5%) were eligible and completed study procedures. All who screened ineligible were older than 30 years of age. Participants averaged 25.4 (Standard Deviation [SD] 3.1) years of age, 50.6% were female, 80.3% self-identified as white race, and 10.7% as Hispanic ethnicity (). Overall, 47.2% of the sample was current cigarette smokers, 19.7% reported current e-cigarette use, and 22.4% reported past 30-day use of other tobacco products ().
Table 1. Sample characteristics (n = 544).
Visual attention
Approximately half (50.9%) of participants reported attending to warning label ROIs, while 70.0% reported attending to any ROIs involving branding (e.g., slogans, packaging). In bivariate analyses, attention to the warning label ROIs did not differ significantly by e-cigarette brand (p = .695). Attention to the warning label ROIs was greater for warnings appearing on red versus white (55.2% vs. 46.7%, p = .048) backgrounds but did not differ significantly for warnings covering 20% (52.5%), 30% (44.9%), or 50% (55.6%) of the advertisement (p = .107).
In the logistic regression model examining attention to the warning label ROIs (yes/no), there were no statistically significant main effects or interaction effects for the experimental factors, current cigarette smoking, or e-cigarette use (all p’s ≥ .05). When the non-significant interactions were removed, the main effect for warning label color was statistically significant (p = .048). Participants were more likely to report attention to warnings appearing on red backgrounds versus those on white backgrounds (Odds Ratio = 1.41, 95% Confidence Interval = 1.00, 1.97).
Warning message recall
Participants recalled on average 1.1 (SD 1.2) of the four warning message themes coded. The proportion of participants recalling individual themes coded was 45.6% for nicotine, 35.5% for addictive, 16.4% for tobacco, and 13.2% for chemical. Only 4.2% of participants recalled all four key themes. In bivariate analyses, the average number of themes recalled did not differ significantly by e-cigarette brand (p = .486), warning label size (p = .163), or color (p = .077). In the ANOVA, there were no statistically significant main or interaction effects for the experimental factors, and there were no statistically significant interactions between experimental factors, current cigarette smoking, and e-cigarette use ().
Table 2. Analysis of variance results for warning message recall and perceived addictiveness of electronic cigarettes (e-cigarettes).
When a factor indicating whether or not participants reported attending to the warning label ROI was introduced into the ANOVA, there was a statistically significant main effect for reported attention to the warning label ROI (F = 22.7, η2 = .042, p < .001). Those reporting attending to the warning label ROI recalled more key themes on average (Mean [M] 1.35, Standard Error [SE] 0.07) than those who did not (M 0.86, SE 0.07, p < .001).
Perceived addictiveness of e-cigarettes
There were statistically significant main effects for warning color and e-cigarette use for absolute perceived addictiveness of e-cigarettes (). Participants who viewed warnings on red backgrounds reported lower perceived addictiveness (M 2.79, SE 0.06) than those who viewed warnings on white backgrounds (M 2.99, SE 0.07, p = .033), and e-cigarette users reported lower perceived addictiveness (M 2.74, SE 0.08) than non-users (M 3.04, SE 0.04, p = .002). There were statistically significant interactions between warning label size and color and e-cigarette use (both p’s = .013). Non-users reported higher absolute perceived addictiveness than e-cigarette users when they viewed warnings on a red background (M non-users 3.06, SE 0.06, M users 2.52 SE 0.11, p < .001, ), but there were no significant differences between these groups when they viewed warnings on a white background (M non-users 3.02, SE 0.06, M users 2.96, SE 0.12, p = 1.00, ). Non-users also reported significantly higher absolute perceived addictiveness than e-cigarette users when they viewed warnings covering 20% of the advertisement (M non-users 3.17, SE 0.07, M users 2.48, SE 0.14, p < .001), but there were no significant differences between these groups when they viewed warnings covering 30% (M non-users 2.99, SE 0.07, M users 2.91 SE 0.14, p = 1.0) or 50% (M non-users 2.95, SE 0.07, M users 2.83 SE 0.15, p = 1.0) of the advertisement. The three-way interaction between warning label size, color, and e-cigarette use tested in a separate model was not significant (p = .149).
Figure 2. Least squares means for perceived addictiveness of electronic cigarettes (e-cigarettes) based on interactions between warning label color and current e-cigarette use.
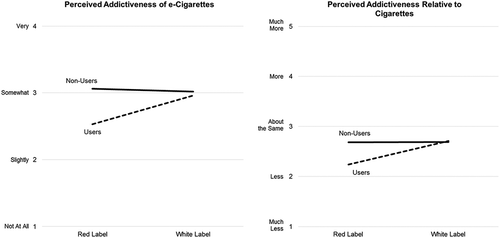
For perceived addictiveness of e-cigarettes relative to cigarettes, there were statistically significant main effects for warning color and e-cigarette use (). Participants who viewed warnings on red backgrounds reported lower perceived relative addictiveness (M 2.46, SE 0.06) than those who viewed warnings on white backgrounds (M 2.70, SE 0.07, p = .009), and e-cigarette users reported lower perceived relative addictiveness (M 2.47, SE 0.08) than non-users (M 2.68, SE 0.04, p = .026). There was a statistically significant interaction between e-cigarette use and warning label color (p = .015). E-cigarette non-users reported higher perceived relative addictiveness of e-cigarettes than e-cigarette users when they viewed warnings on red backgrounds (M non-users 2.68, SE 0.06, M users 2.24 SE 0.11 p = .005, ). These groups did not differ significantly when viewing warnings on white backgrounds (M non-users 2.69, SE 0.06, M users 2.71 SE 0.12, p = 1.00, ). Among e-cigarette users, perceived relative addictiveness was greater among those viewing warnings on white (M 2.69, SE 0.06) versus red (M 2.24, SE 0.11) backgrounds (p = .032, ).
Finally, in the full sample, we examined whether attention to the warning label ROIs and recall of the warning message content were associated with perceptions of e-cigarettes. There were no significant differences (p’s ≥ .05) in either measure of perceived addictiveness based on reported attention to the warning label ROIs. Greater recall of warning label message content was correlated with greater absolute perceived addictiveness (r = 0.15, p < .001).
Discussion
FDA’s deeming rule provides an initial requirement for warnings conveying the addictiveness of e-cigarettes through black and white text occupying 20% of the advertisement surface (FDA, Citation2016). FDA has the authority to issue additional warning label requirements in the future if such requirements are determined to be appropriate for the protection of public health and are within the commercial free speech protections of the First Amendment (Husten & Deyton, Citation2013; Lindblom, Citation2015). This study is among the first to investigate the effects of making e-cigarette warning labels more visually prominent by altering their size and background color on outcomes including reported visual attention to the warnings, warning content recall, and e-cigarette perceptions among young adults to inform future regulatory considerations.
The study revealed a complex pattern of findings that lend mixed support to our hypotheses. Overall participants were more likely to report visually attending to warning labels on red versus white backgrounds, but reported attention did not differ significantly by warning label size. Recall of the warning content did not differ significantly based on the warning background color or size; however, those who reported attending to the warning label endorsed greater recall of the warning message content. Greater recall was associated with greater perceived addictiveness of e-cigarettes overall. The findings are consistent with our hypothesis that self-reported attention was greater for warnings appearing on red versus white background, and attention was positively associated with recall. This is also consistent with studies using eye tracking to demonstrate that greater visual attention to tobacco warning labels is associated with greater content recall (Meernik et al., Citation2016; Strasser et al., Citation2012).
The effects of the warning label design features tested on perceived addictiveness of e-cigarettes also varied based on e-cigarette use behavior. Overall, e-cigarette non-users perceived e-cigarettes to be more addictive than e-cigarette users; however, e-cigarette users exposed to warnings on white backgrounds endorsed perceptions more similar to those of non-users. There was a similar pattern with respect to warning label size: for larger warning labels perceived addictiveness among e-cigarette users becomes more similar to the perceptions of non-users. To determine the potential public health impact of such warning label features, it will be critical in future studies to assess if such shifts in e-cigarette perceptions in response to warning design features are associated with e-cigarette and other tobacco use behaviors over time.
Previous studies of e-cigarette warning labels have primarily examined how variations on the warning message content (e.g., addiction, health risks) affect consumers (Pesko et al., Citation2016; Sanders-Jackson et al., Citation2015). The primary implication of our findings is that variations to the design of e-cigarette warnings including background color and size of the warnings can also affect outcomes that are relevant to understanding their ultimate public health impact, including drawing greater attention, promoting recall, and affecting product perceptions. Our results show these effects are complex across population subgroups (e.g., e-cigarette users and non-users) even when testing only a limited set of warning design features. Importantly, we did not examine variation in the effects of such e-cigarette warning design features by other population subgroups associated with tobacco use behavior (Hu et al., Citation2016), such as by gender, race/ethnicity, or other demographic characteristics. While this study provides initial insights into the design of e-cigarette warnings, it also shows the optimal combination of design features to ensure consumers view e-cigarette warnings, cognitively process their content, and are informed about potential product risks remains to be developed. As the evidence on the potential public health impact of e-cigarettes continues to evolve (Levy et al., 2017; US DHHS, Citation2016), research is needed to understand how to design warnings in such a way that optimizes their public health impact in priority populations for tobacco control by not only adjusting the message content but also adapting other design features that are within the limits of FDA’s regulatory authority.
A number of other factors could be examined in the future to continue to understand how to design e-cigarette warning labels to optimize their potential effects for benefiting public health. Although we chose to compare warnings appearing on white versus red backgrounds based on previous research, there is evidence to suggest that exploring additional warning label background color variations may be important. For example, findings of a recent analysis of tobacco industry research on package design suggest warning labels featuring dark lettering on a contrasting yellow background may attract greater attention (Lempert & Glantz, Citation2016). Another recent study also suggests that embedding health messaging such as the content conveyed in warnings within the body of tobacco advertisements, as opposed to within a stand-alone warning text box, may also be an effective strategy to convey the potential risks of e-cigarettes to consumers (Lochbuehler et al., Citation2016). Investigating the effects of these e-cigarette warning design features may be especially useful to inform future regulatory actions because, unlike graphic images or substantive text changes, they may be less likely to prompt legal challenges by the tobacco industry on the basis of commercial free speech protections of the First Amendment (Lindblom, Citation2015).
Our findings should be interpreted in light of important study limitations. The study involved a cross-sectional convenience sample of young adults recruited through an online crowdsourcing data collection platform, limiting the potential generalizability of the findings to broader populations. Our methods relied on self-report measures, including assessment of visual attention. Although this approach is based on previous work (Mays et al., Citation2016b), future studies can apply assessment methods such as eye tracking to capture visual attention more objectively and with greater resolution (Lochbuehler et al., Citation2016; Meernik et al., Citation2016; Strasser et al., Citation2012). Finally, our findings are based on a single brief exposure to warning labels on e-cigarette advertisements and the observed effects were modest as a result. In future studies, it will be important to determine how such effects unfold after repeated, prospective exposures.
Despite these limitations, this study is among the first to experimentally investigate the effects of altering design features for e-cigarette warning labels among young adults, including the warning label size and background color. Our findings generally suggest making e-cigarette warning labels more prominent and noticeable can enhance young adults’ attention to the warnings and recall of the health messages and affect product perceptions. However, our results also show the patterns of these effects are complex across population subgroups, including e-cigarette users and non-users. Future research can further inform potential FDA regulations surrounding e-cigarette warning labels by investigating whether other design features, such as coloring and placement within e-cigarette advertisements, may affect their public health impact.
Funding
This study was supported by the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) Center for Tobacco Products under NIH grant number K07CA172217. This work was also supported by the Georgetown University Center of Excellence in Regulatory Science and Innovation (CERSI; U01FD004319), a collaborative effort between the university and the FDA to promote regulatory science through innovative research and education, and the Georgetown Lombardi Comprehensive Cancer Center Support Grant under NIH grant number P30CA051008. Dr. Villanti was supported by the NIH and FDA under award P50DA036114. Dr. Niaura was supported by Truth Initiative. Dr. Strasser was supported by P50CA179546. The study sponsors had no role in the study design; in the collection, analysis and interpretation data; in the writing of the report; and in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Additional information
Funding
References
- Cantrell, J., Emelle, B., Ganz, O., Hair, E. C., & Vallone, D. (2016). Rapid increase in e-cigarette advertising spending as altria’s MarkTen enters the marketplace. Tobacco Control, 25(e1), e16–8. doi:10.1136/tobaccocontrol-2015-052532
- Centers for Disease Control and Prevention. (2014). The health consequences of smoking—50 years of progress: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services.
- Coleman, B. N., Apelberg, B. J., Ambrose, B. K., Green, K. M., Choiniere, C. J., Bunnell, R., & King, B. A. (2015). Association between electronic cigarette use and openness to cigarette smoking among US young adults. Nicotine & Tobacco Research, 17, 212–218. doi:10.1093/ntr/ntu211
- Delnevo, C. D., Giovenco, D. P., Steinberg, M. B., Villanti, A. C., Pearson, J. L., Niaura, R. S., & Abrams, D. B. (2016). Patterns of electronic cigarette use among adults in the United States. Nicotine & Tobacco Research, 18, 715–719. doi:10.1093/ntr/ntv237
- Duke, J. C., Lee, Y. O., Kim, A. E., Watson, K. A., Arnold, K. Y., Nonnemaker, J. M., & Porter, L. (2014). Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics, 134(1), e29–36. doi:10.1542/peds.2014-0269
- Fong, G. T., Cummings, K. M., Borland, R., Hastings, G., Hyland, A., Giovino, G. A., … Thompson, M. E. (2006). The conceptual framework of the international tobacco control (ITC) policy evaluation project. Tobacco Control, 15(Suppl 3), iii3–11. doi:10.1136/tc.2005.015438
- Food and Drug Administration. (2012). Center for Tobacco Products Research priorities. Retrieved from http://www.fda.gov/downloads/TobaccoProducts/NewsEvents/UCM293998.pdf
- Food and Drug Administration. (2016). Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register, 81, 28973–29106.
- Hall, M. G., Ribisl, K. M., & Brewer, N. T. (2014). Smokers’ and nonsmokers’ beliefs about harmful tobacco constituents: Implications for FDA communication efforts. Nicotine & Tobacco Research, 16, 343–350. doi:10.1093/ntr/ntt158
- Hammond, D., Wakefield, M., Durkin, S., & Brennan, E. (2013). Tobacco packaging and mass media campaigns: Research needs for articles 11 and 12 of the WHO Framework Convention on Tobacco Control. Nicotine & Tobacco Research, 15, 817–831. doi:10.1093/ntr/nts202
- Hoek, J., Gendall, P., Eckert, C., Rolls, K., & Louviere, J. (2016). A comparison of on-pack quitline information formats. Tobacco Control, 25, 211–217. doi:10.1136/tobaccocontrol-2014-051820
- Hu, S. S., Neff, L., Agaku, I. T., Cox, S., Day, H. R., Holder-Hayes, E., & King, B. A. (2016). Tobacco product use among adults - United States, 2013-2014. Morbidity and Mortality Weekly Report, 65, 685–691. doi:10.15585/mmwr.mm6527a1
- Husten, C. G., & Deyton, L. R. (2013). Understanding the tobacco control act: Efforts by the US Food and Drug Administration to make tobacco-related morbidity and mortality part of the USA’s past, not its future. Lancet, 381, 1570–1580. doi:10.1016/S0140-6736(13)60735-7
- Lavidge, R. J., & Steiner, G. A. (1961). A model for predictive measurements of advertising effectiveness. Journal of Marketing, 25, 59–62. doi:10.2307/1248516
- Lempert, L. K., & Glantz, S. A. (2016). Implications of tobacco industry research on packaging colors for designing health warning labels. Nicotine & Tobacco Research, 18, 1910–1914. doi:10.1093/ntr/ntw127
- Levy, D. T., Cummings, K. M., Villanti, A. C., Niaura, R., Abrams, D. B., Fong, G. T., & Borland, R. (2017). A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction, 112, 8–17. doi:10.1111/add.13394
- Lindblom, E. N. (2015). Effectively regulating e-cigarettes and their advertising–And the First Amendment. Food and Drug Law Journal, 70, 55–92.
- Lochbuehler, K., Tang, K. Z., Souprountchouk, V., Campetti, D., Cappella, J. N., Kozlowski, L. T., & Strasser, A. A. (2016). Using eye-tracking to examine how embedding risk corrective statements improves cigarette risk beliefs: Implications for tobacco regulatory policy. Drug and Alcohol Dependence, 164, 97–105. doi:10.1016/j.drugalcdep.2016.04.031
- Lovato, C., Watts, A., & Stead, L. F. (2011). Impact of tobacco advertising and promotion on increasing adolescent smoking behaviours. The Cochrane Database of Systematic Reviews, 10, CD003439. doi:10.1002/14651858.CD003439.pub2
- Magnan, R. E., & Cameron, L. D. (2015). Do young adults perceive that cigarette graphic warnings provide new knowledge about the harms of smoking? Annals of Behavioral Medicine, 49, 594–604. doi:10.1007/s12160-015-9691-6
- Mays, D., Arrazola, R. A., Tworek, C., Rolle, I. V., Neff, L. J., & Portnoy, D. B. (2016a). Openness to using non-cigarette tobacco products among U.S. young adults. American Journal of Preventive Medicine, 50, 528–534. doi:10.1016/j.amepre.2015.08.015
- Mays, D., Smith, C., Johnson, A. C., Tercyak, K. P., & Niaura, R. S. (2016b). An experimental study of the effects of electronic cigarette warnings on young adult nonsmokers’ perceptions and behavioral intentions. Tobacco Induced Diseases, 14, 17-016-0083-x. doi:10.1186/s12971-016-0083-x
- Mayson, W. M., & Suri, S. (2012). Conducting behavioral research on Amazon’s Mechanical Turk. Behavioral Research, 44, 1–23. doi:10.3758/s13428-011-0124-6
- Meernik, C., Jarman, K., Wright, S. T., Klein, E. G., Goldstein, A. O., & Ranney, L. (2016). Eye tracking outcomes in tobacco control regulation and communication: A systematic review. Tobacco Regulatory Science, 2, 377–403. doi:10.18001/TRS.2.4.9
- Noar, S. M., Hall, M. G., Francis, D. B., Ribisl, K. M., Pepper, J. K., & Brewer, N. T. (2016). Pictorial cigarette pack warnings: A meta-analysis of experimental studies. Tobacco Control, 25, 341–354. doi:10.1136/tobaccocontrol-2014-051978
- Pearson, J. L., Richardson, A., Niaura, R. S., Vallone, D. M., & Abrams, D. B. (2012). E-cigarette awareness, use, and harm perceptions in US adults. American Journal of Public Health, 102, 1758–1766. doi:10.2105/AJPH.2011.300526
- Pesko, M. F., Kenkel, D. S., Wang, H., & Hughes, J. M. (2016). The effect of potential electronic nicotine delivery system regulations on nicotine product selection. Addiction, 111, 734–744. doi:10.1111/add.13257
- Pokhrel, P., Fagan, P., Herzog, T. A., Chen, Q., Muranaka, N., Kehl, L., & Unger, J. B. (2016). E-cigarette advertising exposure and implicit attitudes among young adult non-smokers. Drug and Alcohol Dependence, 163, 134–140. PMID: 27125661. doi:10.1016/j.drugalcdep.2016.04.008
- Richardson, A., Pearson, J., Xiao, H., Stalgaitis, C., & Vallone, D. (2014). Prevalence, harm perceptions, and reasons for using noncombustible tobacco products among current and former smokers. American Journal of Public Health, 8, 1437–1444. doi:10.2105/AJPH.2013.301804
- Sanders-Jackson, A., Schleicher, N. C., Fortmann, S. P., & Henriksen, L. (2015). Effect of warning statements in e-cigarette advertisements: An experiment with young adults in the United States. Addiction, 110, 2015–2024. doi:10.1111/add.12838
- Seidenberg, A. B., Jo, C. L., & Ribisl, K. M. (2016). Differences in the design and sale of e-cigarettes by cigarette manufacturers and non-cigarette manufacturers in the USA. Tobacco Control, 25(e1), e3–5. doi:10.1136/tobaccocontrol-2015-052375
- Singh, S. (2006). Impact of color on marketing. Management Decision, 44, 783–789. doi:10.1108/00251740610673332
- Strasser, A. A., Tang, K. Z., Romer, D., Jepson, C., & Cappella, J. N. (2012). Graphic warning labels in cigarette advertisements: Recall and viewing patterns. American Journal of Preventive Medicine, 43, 41–47. doi:10.1016/j.amepre.2012.02.026
- Trumbo, C. W., & Kim, S. J. (2015). The effect of electronic cigarette advertising on intended use among college students. Addictive Behaviors, 46, 77–81. doi:10.1016/j.addbeh.2015.03.005
- Truth Initiative. (2015). Vaporized: Youth and young adult exposure to e-cigarette marketing. Retrieved from http://truthinitiative.org/sites/default/files/Vaporized-Youth_and_young_adult_exposure_to_e-cigarette_marketing.pdf
- U.S. Department of Health and Human Services. (2016). E-cigarette use among youth and young adults: A report of the Surgeon General. Retrieved from https://e-cigarettes.surgeongeneral.gov/.
- Villanti, A. C., Rath, J. M., Williams, V. F., Pearson, J. L., Richardson, A., Abrams, D. B., … Vallone, D. M. (2016). Impact of exposure to electronic cigarette advertising on susceptibility and trial of electronic cigarettes and cigarettes in US young adults: A randomized controlled trial. Nicotine & Tobacco Research, 18, 1331–1339. doi:10.1093/ntr/ntv235
- Wedel, M., & Peters, R. (2000). Eye fixations on advertisements and memory for brands: A model and findings. Marketing Science, 19, 297–312. doi:10.1287/mksc.19.4.297.11794
