ABSTRACT
The Food and Drug Administration, which now has regulatory authority over all tobacco products meeting the statutory definition, is tasked with communicating the risks of these products to the public through health warnings and public education. However, there have been no attempts to summarize what is known about non-cigarette tobacco product (NCTP) health messaging. We conducted a systematic review to examine the existing literature on health communication for NCTPs and identify key research gaps. A total of 45 unique studies were retrieved and coded, with the majority focused on messaging for smokeless tobacco (SLT, k = 32, 71.1%), followed by waterpipe tobacco (WT, k = 9, 20%), electronic nicotine delivery systems (ENDS, k = 2, 4.4%), cigars (k = 2, 4.4%), and a potentially reduced exposure product (k = 1, 2.2%). Studies most commonly examined tobacco product warnings (k = 26, 57.8%) and public education (k = 19, 42.2%), which included mass media campaigns. Most studies examined knowledge, attitudes, and beliefs as outcomes (k = 27, 60%), while behavior was an outcome in the minority of studies (k = 8, 17.8%). Pictorial warnings and public education about NCTPs demonstrated positive impact in some studies, although the literature is nascent. Given the increasing use of NCTPs such as ENDS, WT, and cigars, particularly among adolescents and young adults, more research is needed on effective ways to communicate product risk to those audiences most at risk.
There has been a substantial increase in availability and use of non-cigarette tobacco products (NCTPs), including several types of electronic nicotine delivery systems (ENDS), smokeless tobacco (SLT), waterpipe tobacco (WT), and little cigars and cigarillos. Recently, NCTP use has substantially increased among adolescents and young adults (Johnston, O’Malley, Bachman, Schulenberg, & Miech, Citation2016). High rates of use may be partially attributed to users’ lack of knowledge about the products, including their health risks (e.g., Sutfin et al., Citation2011). Generally, people perceive NCTPs to be less risky compared to cigarettes because they are used infrequently, are perceived to have “filtering” features (waterpipe), and are viewed as less addictive (e.g., Cornacchione et al., Citation2016; Wagoner et al., Citation2016). However, NCTPs are a threat to public health for several reasons: (1) the smoke/aerosol contains constituents that are harmful to human health (Koszowski et al., Citation2015; Shihadeh et al., Citation2015), (2) they contain nicotine that can lead to and maintain addiction (Aboaziza & Eissenberg, 2014), and (3) they have known health risks, such as decreased lung function, cancer, and heart disease (e.g., Chang, Corey, Rostron, & Apelberg, Citation2015; Waziry, Jawad, Ballout, Al Akel, & Akl, Citation2016). Noncombustible tobacco products (ENDS, SLT) are believed to be less harmful than combusted tobacco products because the tobacco is not burned (e.g., Wagoner et al., Citation2016). Although they may be less harmful than cigarettes, these products still present some health risks, yet long-term health effects of ENDS are still unknown.
Across diverse health arenas, there is a substantial body of evidence demonstrating how health communication impacts individuals’ knowledge, beliefs, attitudes, and behavior (e.g., Atkin & Rice, 2013; Snyder & LaCroix, Citation2013). In particular, there is evidence that messages in the form of health warnings (Brewer et al., 2016; Noar, Francis et al., Citation2016; Noar, Hall et al., Citation2016) and communication campaigns (Durkin, Brennan, & Wakefield, Citation2012) are effective in reducing tobacco use. For instance, exposure to anti-smoking campaigns has been shown to reduce smoking initiation (Farrelly, Nonnemaker, Davis, & Hussin, Citation2009), increase anti-tobacco attitudes and beliefs (Farrelly et al., Citation2002), and increase calls to cessation quitlines (Farrelly, Hussin, & Bauer, Citation2007). In a meta-analysis of experiments, Noar, Hall et al. (Citation2016) found pictorial warnings on cigarette packs to be more effective than text-only warnings on several outcomes, including negative pack/brand attitudes, and intention to not start smoking. Observational studies suggest that pictorial warnings on cigarette packs increase knowledge of smoking health risks and calls to quitlines (Noar, Francis et al., Citation2016), and a recent trial found pictorial warnings on cigarette packs increased quit attempts (Brewer et al., 2016). Although messaging is effective, the majority of this research has focused on cigarettes.
Under the Family Smoking Prevention and Tobacco Control Act, the Food and Drug Administration (FDA) recently “deemed” all tobacco products to be under their regulatory authority (FDA Deeming Regulations, Citation2016). This extends the FDA’s regulatory authority to cover tobacco products that were not originally covered by the Tobacco Control Act, such as ENDS, WT, and cigars. As part of these rules, the FDA will require the display of at least one health warning message (“Warning: This product contains nicotine. Nicotine is an addictive chemical.”) on all newly deemed tobacco product packaging and advertisements for the protection of public health. Additionally, the FDA is required to communicate tobacco product risk to consumers (Food and Drug Administration, Citation2016), which could take the form of campaigns. Given this, the FDA has indicated that research on health communication about NCTPs – including warnings and campaigns – is a significant research priority (e.g., see National Institutes of Health RFA-OD-17-003).
Despite a large literature on health communication to reduce cigarette smoking, including many reviews and meta-analyses, there have been no attempts to synthesize what is known about NCTP health messaging. Because the nature of NCTPs and their corresponding risk perceptions are different than for cigarettes (e.g., Wackowski & Delnevo, Citation2015) health communications that have been effective for cigarettes might not be effective or easily adapted for NCTPs. To gain a comprehensive understanding of what is known about NCTP messaging, we conducted a systematic review to examine studies on NCTP messaging to date. Identifying what is effective for NCTPs is important to inform policy, withstand legal challenges, and guide future research. Thus, the current review aims to determine what health communication message approaches for NCTPs have been developed and tested in the extant literature. In so doing, we illuminate gaps in the literature and highlight needs for future research.
Methods
Search strategy
A comprehensive, systematic detailed strategy was undertaken at two time points to search for all articles related to health communication messaging and NCTPs. First, two research assistants searched several computerized databases in September 2014 and again in May 2016 to locate all relevant articles. A list of search terms was generated by the authors to encompass different types of NCTPs and different forms of messaging. This systematic review was focused on messaging generally, rather than a specific message type (e.g., warning labels), so the list of keywords was broad to capture all such work. Communication- and tobacco-relevant keywords (including variations of these terms, especially for ENDS) were used in combination in the search, such as “waterpipe,” “e-cigarette,” “little cigar,” and “warning,” “campaign,” “media”. The keywords were searched in Communication & Mass Media Complete, PsycInfo, PubMed, Web of Science, and Science Direct. Second, to include grey and unpublished literature, we sent emails asking for conference work and unpublished studies to individual researchers and relevant listservs (Society for Research on Nicotine & Tobacco; Communication Research & Theory Network). We also included any unpublished work that came up in our database searches (e.g., dissertations). Third, once we identified the final sample of articles to include in the review, we searched the references cited in those articles to identify other possible articles to include. Finally, a Google Scholar search was conducted to see if any of the studies in our review were cited by other relevant studies to potentially include.
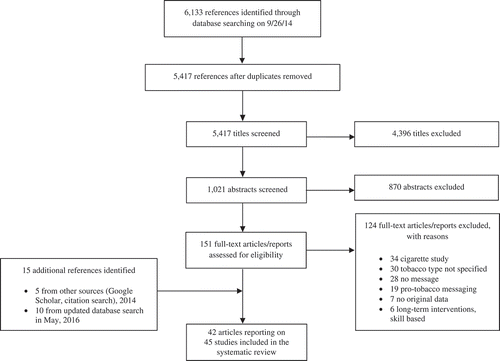
Inclusion criteria were kept broad so that a description of the current state of the research could be provided. Studies included in the review had to: (1) examine at least one NCTP, (2) examine health messaging for NCTPs using any communication channel, and (3) report original data using quantitative or qualitative methods. The two research assistants applied these inclusion criteria throughout the screening process. First, articles were screened based on title, with articles clearly not relevant to the review removed. Next, articles were screened based on abstracts. Finally, the remaining full articles were obtained and screened based on a full text review. displays the PRISMA flow diagram that demonstrates the retrieval and inclusion/exclusion process.
Article coding
The first author read through several of the final articles to develop the coding form, in collaboration with the study team. Operational definitions and examples were included in the codebook. Decisions were made to code characteristics that have been included in similar reviews on cigarette messaging (e.g., Noar, Hall et al., Citation2016) and were of interest given the goals of the current review project. Given the early stage of this area of research, we aimed to characterize the make-up of the current literature. Articles were extracted and coded by two research assistants in three major areas: sample/demographic characteristics, study characteristics, and message characteristics. Each article was coded by two independent coders, and the first author resolved any coding discrepancies.
Within sample/demographic characteristics, data extracted/coded included raw sample size, age cohort of study (adolescents, young adults, and older adults), sex (percent male and female), race/ethnicity (percentage of each race/ethnicity reported in the study), and sexual orientation of study participants (heterosexual, LGBT, or not reported). For study characteristics, data extracted/coded included study type (experiment, non-experimental survey, focus groups, interviews, content analysis, and other), study design (between or within subjects), type of study data (cross-sectional, longitudinal), dependent variables (DV, construct name, definition, and items), and study findings for each dependent variable. To code study findings, we indicated for each DV whether (1) there was an effect (i.e., statistically significant at p < .05 or a percentage >50%), (2) there was no effect, or (3) there was an effect but in the unintended/undesired direction. This was done for each sub-variable included in . Within message characteristics, data extracted/coded included message type studied (warnings and public education) and message theme (anti-industry, constituents, health effects, cosmetic effects, addiction, relative risk, other, and not reported). For example, a message theme was defined as a health effect message if it referenced the general health effects of using the tobacco product or a specific health effect. Message themes were only coded if they were explicitly stated or presented in the article.
Results
The final sample consisted of 42 articles reporting on 45 distinct studies. Most studies were about SLT (k = 32, 71.1%), including snus (k = 5), chewing tobacco (k = 4), and dissolvable tobacco (k = 4); the remainder of these did not specify SLT type. Nine studies focused on messages for waterpipe (20%), two for ENDS (4.4%), two for all cigar types (4.4%), and one “potentially reduced exposure product”, as defined by the original authors (“heat not burn”, 2.2%).
Study characteristics
The 45 studies were conducted in 8 different countries, with the majority being conducted in the United States (71.1%; see ). Sample sizes ranged from 20 participants to 36,451. Across all studies, males made up over half of the samples (58.9%). The most common participants were white (55.6%), other/mixed (51.1%), or black (42.2%) race. Forty-two percent were Hispanic/Latino. Income was reported in 32.6% of studies, and sexual orientation of participants was not reported in any studies. Fourteen studies included adolescent participants (31.1%), but only 9 of those studies (20%) either focused only on or reported results for adolescents. Young adults were included in the majority of study samples (77.8%) but only 15 studies (33.3%) reported young adult results, including college student samples. Most studies (k = 43; 95.6%) reported on tobacco use of its participants (both cigarettes and NCTPs), with many (k = 26; 57.8%) having tobacco use a component of the participant inclusion criteria.
Table 1. Characteristics of studies (k = 45).
Most studies used convenience samples (66.7%), and some used probability-based sampling (20%). Four study methodological approaches were used, with most being experiments (68.9%), followed by cross-sectional, non-experimental surveys (22.2%), qualitative focus groups or interviews (11.1%), and one content analysis (2.2%). Most studies were cross-sectional (k = 38; 84.4%), while 15.6% were longitudinal (k = 7). Six studies (13.3%) reported use of theory to guide the research.
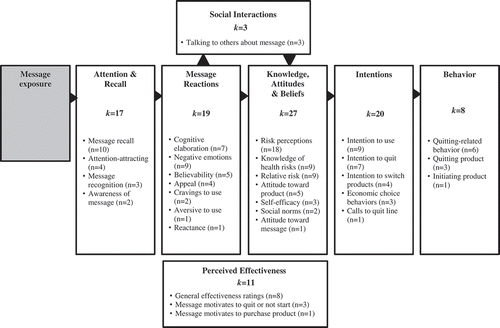
Dependent variables were coded to examine study outcomes and evaluate key study findings. They were coded and organized into the message impact framework (see ; Noar, Hall et al., Citation2016). The most common outcomes studied were knowledge, attitudes, and beliefs (KAB; 60%), followed by behavioral intentions (44.4%). Forty-two percent of studies examined message reactions, while 37.8% assessed attention and recall. Perceived message effectiveness was assessed in 24.4% of studies. The least often assessed category was social interactions (6.7%).
Message characteristics
The message types studied fell into two major categories: warnings (k = 26, 57.8%), such as labels on packaging or advertisements, and public education (k = 19, 42.2%), including mass media campaigns. Warning studies mostly focused on manipulating warning design (e.g., size, colors, text vs. pictorial, and presence of warning), warning text (health effects and relative risk), or asking people to report their awareness, exposure, or recognition of existing warnings. Most public education studies designed or evaluated media campaigns or message-based interventions, or developed or tested campaign messages aimed to educate the public about the health risks of the tobacco product being studied.
The content of the messages was also coded to determine what themes were being communicated. The majority of studies included messages that focused on the health effects of using the NCTPs (k = 37; 82.2%), including causing cancer or gum disease. Several other studies focused on the toxic chemicals (constituents) found in the smoke of the products (k = 13; 28.9%). These messages were mostly focused on nicotine as a constituent, or discussed harmful chemicals vaguely, without specifying constituents. Other messages were focused on addiction to the products (k = 12; 26.7%), relative risk or reduced harm of the NCTPs compared to traditional cigarettes (k = 13; 28.9%), and anti-industry (k = 4; 8.9%) themes. Ten studies (22.2%) focused on an “other” message theme, including social consequences of using the product, and four studies (8.9%) did not report on or provide enough information to code for message content.
Key study findings
Given the range of NCTPs, message types, and study designs, a meta-analysis of study findings was not undertaken. However, whether an effect (significant at p < .05 or a percentage >50%) was found was coded (yes, no). Results are presented by message type: warnings and public education.
For studies on NCTP warnings, attention and recall effects were found in 85.7% (k = 14) of studies that examined this outcome. For example, one study found that the majority of SLT users recalled being exposed to SLT warnings (Agaku, Singh, Rolle, & Ayo-Yusuf, Citation2016). Over half (63.6%, k = 15) found effects on message reactions for warnings studies. In particular, several studies demonstrated the effect of warnings on cognitive elaboration – i.e., thinking about the risks (e.g., Johnson, Wu, Coleman, & Choiniere, Citation2014). Warning messages also had an effect on KAB, with 71.4% (k = 14) of studies reporting a positive effect. For example, warnings resulted in increased negative attitudes toward SLT (Mutti et al., 2015). Intentions were positively influenced by messages in 55.6% (k = 9) of studies. However, this included the influence of warnings aiming to switch cigarette smokers to SLT (Callery, Hammond, O’Connor, & Fong, 2011). Behavior was assessed in two studies, with warnings influencing behavior in both of those studies, including quitting and quit attempts (Agaku et al., Citation2016; Mohammed, Citation2013). Finally, effects for perceived effectiveness were found in all (k = 6) of the studies, including rating the warning as being effective to quit using tobacco (Brubaker & Mitby, 1990).
For public education studies, attention and recall effects were found in all (k = 3) studies that looked at this outcome, such as a sample majority recalling SLT campaign messages (Vogeltanz-Holm, Holm, White Plume, & Poltavski, 2009). The studies that assessed message reactions found positive effects for public education messages in 50% of the studies (k = 8). For example, messages increased worry about the health effects of WT smoking (Lipkus, Eissenberg, Schwartz-Bloom, Prokhorov, & Levy, 2011). In the 13 studies that assessed KAB, 76.9% found effects for public education messages, such as changing relative risk of WT smoking compared to cigarettes in the correct direction (Mays, Tercyak, & Lipkus, 2016). Public education messages increased intentions in 72.7% of studies (k = 11). For example, intention to quit using SLT increased after campaign exposure (Murukutla et al., 2012). Behavior change was seen in 66.7% of studies that looked at this outcome (k = 6), including higher rates of SLT quitting after intervention exposure (Walsh et al., 2003). Public education messages also resulted in increased social interactions about the messages in all studies (k = 3). Finally, messages were rated high in perceived effectiveness in 75% of studies that looked at this outcome (k = 4), such as evaluating an intervention as being effective (Walsh et al., 2003). See the Online Supplement for an overview of each study, including key findings.
Discussion
This systematic review is the first examination of the health communication literature about NCTPs. Our comprehensive literature search identified 42 articles that tested NCTP messaging, with 45 individual studies examined. Most were conducted in the US, focused on adults, and examined SLT. The majority were short-term and cross-sectional, with experiments as the most common method. Additionally, the majority of messages focused on the health effects of using the tobacco product and examined NCTP warnings.
This review demonstrates the dearth of research on health communication about diverse NCTPs, with very few studies for any NCTPs other than SLT. Thus, research needs to expand to other products. Research is urgently needed to inform FDA’s communication efforts for both warning labels and public education campaigns for NCTPs. At least one specific text-only warning will be required on all tobacco products (“Warning: This product contains nicotine. Nicotine is an addictive chemical”) beginning in May 2018. This warning will be the only warning for both ENDS and WT. All cigar products will require the display of six different text-only warnings. To our knowledge, there is currently no published research assessing the effectiveness of these warnings for the newly deemed products. It is particularly important to provide evidence demonstrating the effectiveness of these policies for the protection of public health, and to help withstand legal challenges that may claim that these policies do not advance the government’s interest in increasing knowledge and decreasing tobacco use. The FDA has been challenged twice in regard to cigarette graphic warnings (Discount Tobacco vs. Food and Drug Administration in 2009 (6th Circuit), and RJ Reynolds Tobacco Co. vs. Food and Drug Administration in 2011 (DC Circuit)). In the RJ Reynolds case, which was the challenge to the cigarette warning images and text selected by the FDA in its rulemaking, the plaintiffs claimed that the warning images violated their first amendment rights. The DC circuit ruled that the graphic warnings did not directly advance the government’s interest in decreasing smoking rates, and found specific warnings to be “neither factual nor accurate” because the images did not necessarily represent the text warning and they were designed to evoke emotions, rather than educate consumers about risks. Lessons learned from these court cases should be considered as researchers develop and implement studies on NCTP warnings. For example, it is important for studies to assess appropriate images for text warnings and to demonstrate the effects that emotional reactions have on message processing and knowledge to counteract arguments that pictorial warnings only evoke emotions without increasing knowledge, which is not the case (see Popova, Owusu, Jensen, & Neilands, 2017). Although research has grown in this area for cigarette warnings, relatively little published work has been conducted with NCTP warnings that FDA is preparing to implement.
Research is needed not only on NCTP warning content, but also on placement, such as location and size, and ways to improve the ability of warnings to have impact (e.g., inclusion of images). Substantial evidence exists for the superiority of pictorial over text-only warnings for cigarettes (e.g., Brennan, Maloney, Ophir, & Cappella, 2016; Noar, Francis et al., Citation2016; Noar, Hall et al., Citation2016), but there is not yet a large enough literature for other tobacco products, making drawing conclusions about their effectiveness for NCTPs difficult.
The review also identified an area of research that merits future studies to provide scientific evidence for the FDA’s ability to communicate NCTP risk through public education efforts, such as mass media campaigns. Many studies in this review included messages/campaigns that contained multiple themes, such as health risks, constituents, and addiction. It is possible that including a variety of message themes may be a promising approach for effective messaging, as it may reduce message staleness by providing a variety of novel information that is not typically communicated to the public, especially youth and young adults, who typically underestimate the health risks of NCTPs (e.g., Cornacchione et al., Citation2016). The FDA has recently implemented a specific NCTP campaign that includes multiple message themes; a portion of “The Real Cost” campaign is aimed at decreasing SLT use among rural adolescent males. This campaign communicates the message that “smokeless doesn’t mean harmless,” with message themes including health effects, cosmetic effects, and constituents. Future research should test the relative effectiveness of a single message theme vs. multiple themes within a given campaign.
Interestingly, no studies in this review looked at public education or campaign evaluation for ENDS, despite several existing state- and county-level anti-ENDS campaigns, such as in Alaska, California (stillblowingsmoke.org), and Orange County, CA (notsosafe.org), as well as a public service announcement released alongside the 2016 Surgeon’s General Report on ENDS. Given that ENDS present risks, but are likely to be less harmful than combusted tobacco products, optimal messaging for this product is urgently needed. A major challenge is how to most effectively communicate ENDS product risk to the public, which was recently identified as a significant priority by the US Surgeon General (US Department of Health & Human Services [USDHHS], Citation2016). The public health community generally believes that youth should not use any tobacco product, including ENDS, given the potential for addiction and the adverse effects of nicotine on the adolescent brain (USDHHS, Citation2016). Caution should be taken when developing these messages, and evaluations should examine whether these messages result in unintended consequences such as increased use of combustible products. Similarly, there is a lack of consensus about the best way to message on SLT because relative risk messages may not encourage people to quit SLT use, and health warnings could drive people to use combustible tobacco products. Research is needed to understand the most effective ways to communicate a continuum of tobacco product risk to the public. Specifically, research is needed on how different populations might be affected differently by these messages (e.g., youth vs. adult smokers), unintended consequences of anti- or pro- ENDS messages (e.g., driving people to smoke cigarettes because they believe ENDS are no safer), and the best delivery channels.
This systematic review also highlighted other research gaps. Priority populations for tobacco use have not been well-studied, for example. Most studies in this review focused on adults, while very few targeted youth and young adults. Youth and young adults use NCTPs at higher rates than older adults (Johnston et al., Citation2016) and tobacco initiation starts most often during the teenage years, so it is imperative to understand how to most effectively communicate product risk to these younger age groups. Additionally, no studies reported the sexual orientation of its participants – i.e., there were no studies examining impact of messages on lesbian, gay, bisexual, and transgender (LGBT) populations. Disparities in tobacco use exist for LGBT people, who use tobacco at higher rates than their heterosexual counterparts (Lee, Griffin, & Melvin, Citation2009). Studying message effects for specific priority populations such as LGBT is important because it will inform prevention efforts for tobacco product initiation and cessation targeted to the highest risk groups, ultimately reducing health disparities. Indeed, the FDA views the LGBT population as a priority and itself recently launched a campaign targeting tobacco use among this population – the “This Free Life” campaign.
This systematic review is not without limitations. Given the heterogeneity of studies in this review, we were not able to conduct a meta-analysis of the effects of NCTP messaging on various outcomes. However, this review provides an overview of published studies that currently exist, describing their make-up and findings. This area of research is young and growing quickly, and as more studies are published, topic specific meta-analyses can be undertaken. Another limitation is that there is likely much in-progress research in this area not captured in this review, and there are campaigns ongoing that will fill some gaps once their evaluations are complete. Thus, there will be much knowledge generation in this area in the coming years.
Conclusion
This is the first systematic examination of health communication studies about NCTPs. Most notably, this investigation demonstrates a significant need for more research in this area, particularly for recently deemed tobacco products, and especially for priority populations such as youth and LGBT. It also demonstrates a need for longitudinal studies with larger samples examining behavioral outcomes. New research will provide a better understanding of effective (and ineffective) NCTP messages and will facilitate effective implementation of such messages to communicate product risk, ultimately improving public health. Research on product messaging such as warnings will help the FDA withstand the inevitable legal challenges that seek to halt the implementation of effective risk communications. The public health potential of the new deeming rule will ultimately be realized when such research is undertaken and the FDA is able to implement effective communications for all NCTPs, reducing death and disease from tobacco use and thereby improving public health.
Online_Supplement_table_4.27.17.docx
Download MS Word (50.1 KB)Funding
Research reported in this publication was supported by grant number P50CA180907 from the National Cancer Institute and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/10410236.2017.1407274.
Additional information
Funding
References
- Aboaziza, E., & Eissenberg, T. (2014). Waterpipe tobacco smoking: What is the evidence that it supports nicotine/tobacco dependence? Tobacco Control, 24, i44–i53. doi:10.1136/tobaccocontrol-2014-051910
- Agaku, I. T., Singh, T., Rolle, I. V., & Ayo-Yusuf, O. A. (2016). Exposure and response to current text-only smokeless tobacco health warnings among smokeless tobacco users aged ≥18years, United States, 2012-2013. Preventive Medicine, 87, 200–206. doi:10.1016/j.ypmed.2016.02.014
- Atkin, C. K. & Rice, R. E. (2013). Advances in public communication campaigns. In E. Scharrer (Ed.), The international encyclopedia of media studies: Vol. 5: Media effects/Media psychology (pp. 526–551). London, UK: Wiley-Blackwell.
- Brennan, E., Maloney, E. K., Ophir, Y., & Cappella, J. N. (2016). Potential effectiveness of pictorial warning labels that feature the images and personal details of real people. Nicotine & Tobacco Research. online first. doi:10.1093/ntr/ntw319
- Brewer, N. T., Hall, M. G., Noar, S. M., Parada, H., Stein-Seroussi, A., Bach, L. E., … Ribisl, K. M. (2016). Effect of pictorial cigarette pack warnings on changes in smoking behavior: A randomized clinical trial. JAMA Internal Medicine, 176, 905–912. doi:10.1001/jamainternmed.2016.2621
- Brubaker, R. G., & Mitby, S. K. (1990). Health-risk warning labels on smokeless tobacco products: Are they effective? Addictive Behaviors, 15, 115–118. doi:10.1016/0306-4603(09)90014-O
- Callery, W. E., Hammond, D., O’Connor, R. J., & Fong, G. T. (2011). The appeal of smokeless tobacco products among young Canadian smokers: The impact of pictorial health warnings and relative risk messages. Nicotine & Tobacco Research, 13, 373–383. doi:10.1093/ntr/ntr013
- Chang, C. M., Corey, C. G., Rostron, B. L., & Apelberg, B. J. (2015). Systematic review of cigar smoking and all cause and smoking related mortality. BMC Public Health, 15, 390. doi:10.1186/s12889-015-1617-5
- Cornacchione, J., Wagoner, K., Wiseman, K., Kelley, D., Noar, S. M., Smith, M. H., & Sutfin, E. L. (2016). Adolescent and young adult perceptions of hookah and little cigars/cigarillos: Implications for risk messages. Journal of Health Communication, 21, 818–825. doi:10.1080/10810730.2016.1177141
- Durkin, S., Brennan, E., & Wakefield, E. (2012). Mass media campaigns to promote smoking cessation among adults: An integrative review. Tobacco Control, 21, 127–138. doi:10.1136/tobaccocontrol-2011-050345
- Farrelly M. C., Hussin, A., & Bauer, U. E. (2007). Effectiveness and cost effectiveness of television, radio and print advertisements in promoting the New York smokers’ quitline. Tobacco Control, 16, i21–i23. doi: 10.1136-tc.2007.019984
- Farrelly, M. C., Healton, C. G., Davis, K. C., Messeri, P., Hersey, J. C., & Haviland, M. L. (2002). Getting to the Truth: Evaluating national tobacco countermarketing campaigns. American Journal of Public Health, 92, 901–907. doi:10.2105/AJPH.92.6.901
- Farrelly, M. C., Nonnemaker, J., Davis, K. C., & Hussin, A. (2009). The influence of the national truth® campaign on smoking initiation. American Journal of Preventive Medicine, 36, 379–384. doi:10.1016/j.amepre.2009.01.019
- Food and Drug Administration. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products; 81 F.R., C.F.R. 21 parts 1100, 1140, 1143 (2016).
- Johnson, S. E., Wu, C. C., Coleman, B. N., & Choiniere, C. J. (2014). Self-reported exposure to tobacco warning labels among U.S. middle and high school students. American Journal of Preventive Medicine, 47, S69–75. doi:10.1016/j.amepre.2014.05.005
- Johnston, L. D., O’Malley, P. M., Bachman, J. G., Schulenberg, J. E., & Miech, R. A. (2016). Monitoring the Future national survey results on drug use, 1975-2015: Volume II, college students and adults ages 19-55. Ann Arbor, MI: Institute for Social Research, The University of Michigan.
- Koszowski, B., Rosenberry, Z. R., Kanu, A., Viray, L. C., Potts, J. L., & Pickworth, W. B. (2015). Nicotine and carbon monoxide exposure from inhalation of cigarillo smoke. Pharmacology, Biochemistry, and Behavior, 139, 7–14. doi:10.1016/j.pbb.2015.10.007
- Lee, J. G. L., Griffin, G. K., & Melvin, C. L. (2009). Tobacco use among sexual minorities in the USA, 1987-May 2007: A systematic review. Tobacco Control, 18, 275–282. doi:10.1136/tc.2008.028241
- Lipkus, I. M., Eissenberg, T., Schwartz-Bloom, R. D., Prokhorov, A.V., & Levy, J. (2011). Affecting perceptions of harm and addiction among college waterpipe tobacco smokers. Nicotine & Tobacco Research, 13, 599–610. doi:10.1093/ntr/ntr049
- Mays, D., Tercyak, K. P., & Lipkus, I. M. (2016). The effects of brief waterpipe tobacco use harm and addiction education messages among young adult waterpipe tobacco users. Nicotine & Tobacco Research, 18, 777–784. doi:10.1093/ntr/ntv223
- Mohammed, H. T. (2013). The efficacy of viewing health warnings on shisha smoking among shisha smokers. Retrieved from https://uwspace.uwaterloo.ca/handle/10012/7419
- Murukutla, N., Turk, T., Prasad, C. V. S., Saradhi, R., Kaur, J., Gupta, S., … Wakefield, M. (2012). Results of a national mass media campaign in India to warn against the dangers of smokeless tobacco consumption. Tobacco Control: An International Journal, 21, 12–17. doi:10.1136/tc.2010.039438
- Mutti, S., Reid, J. L., Gupta, P. C., Pednekar, M. S., Dhumal, G., Nargis, N., … Hammond, D. (2015). Perceived effectiveness of text and pictorial health warnings for smokeless tobacco packages in Navi Mumbai, India, and Dhaka, Bangladesh: findings from an experimental study. Tobacco Control, 25, 437–443. doi:10.1136/tobaccocontrol-2015-052315
- Noar, S. M., Francis, D. B., Bridges, C., Sontag, J. M., Ribisl, K. M., & Brewer, N. T. (2016). The impact of strengthening cigarette pack warnings: Systematic review of longitudinal observational studies. Social Science & Medicine, 164, 118–129. doi:10.1016/j.socscimed.2016.06.011
- Noar, S. M., Hall, M. G., Francis, D., Ribisl, K. M., Pepper, J. K., & Brewer, N. T. (2016). Pictorial cigarette pack warnings: A meta-analysis of experimental studies. Tobacco Control, 25, 341–354. doi:10.1136/tobaccocontrol-2014-051978
- Popova, L., Owusu, D., Jensen, D., & Neilands, T. B. (2017). Factual text and emotional pictures: Overcoming a false dichotomy of cigarette warning labels. Tobacco Control. Published online first. doi:10.1136/tobaccocontrol-2016-053563
- Shihadeh, A., Schubert, J., Klaiany, J., El Sabban, M., Luch, A., & Saliba, N. A. (2015). Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tobacco Control, 24, i1–i9. doi:10.1136/tobaccocontrol-2014-051907
- Snyder, L. B., & LaCroix, J. M. (2013). How effective are mediated health campaigns? A synthesis of meta-analyses. In R. E. Rice & C. K. Atkin (Eds.), Public communication campaigns (4th ed.) (113–129). Thousand Oaks, CA: Sage.
- Sutfin, E. L., McCoy, T. P., Reboussin, B. A., Wagoner, K. G., Spangler, J., & Wolfson, M. (2011). Prevalence and correlates of waterpipe tobacco smoking by college students in North Carolina. Drug and Alcohol Dependence, 115, 131–136. doi:10.1016/j.drugalcdep.2011.01.018
- U.S. Department of Health and Human Services (2016). E-cigarette use among youth and young adults: A report of the Surgeon General. Atlanta, GA: USDHHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
- Vogeltanz-Holm, N., Holm, J. E., White Plume, J., & Poltavski, D. (2009). Confirmed recall and perceived effectiveness of tobacco countermarketing media in rural youth. Prevention Science, 10, 325–334. doi:10.1007/s11121-009-0134-0
- Wackowski, O. A., & Delnevo, C. D. (2015). Young adults’ risk perceptions of various tobacco products relative to cigarettes: Results from the National Young Adult Health Survey. Health Education & Behavior, 43, 328–336. doi:10.1177/1090198115599988
- Wagoner, K. G., Cornacchione, J., Wiseman, K. D., Teal, R., Moracco, K. E., & Sutfin, E. L. (2016). E-cigarettes, hookah pens and vapes: Adolescent and young adult perceptions of electronic nicotine delivery systems. Nicotine & Tobacco Research, 18, 2006–2012. doi:10.1093/ntr/ntw095
- Walsh, M. M., Hilton, J. F., Ellison, J. A., Gee, L., Chesney, M. A., Tomar, S. L., & Ernster, V. L. (2003). Spit (smokeless) tobacco intervention for high school athletes: Results after 1 year. Addictive Behaviors, 28, 1095–1113. doi:10.1016/S0306-4603(02)00228-9
- Waziry, R., Jawad, M., Ballout, R. A., Al Akel, M., & Akl, E. A. (2016). The health effects of waterpipe tobacco smoking on health outcomes: An updated systematic review and meta-analysis. International Journal of Epidemiology, 46, 32–43. doi:10.1093/ije/dyw021
References in the systematic review
- Adkison, S. E., Bansal-Travers, M., Smith, D. M., O’Connor, R. J., & Hyland, A. J. (2014). Impact of smokeless tobacco packaging on perceptions and beliefs among youth, young adults, and adults in the U.S: findings from an internet-based cross-sectional survey. Harm Reduction Journal, 11, 2. doi:10.1186/1477-7517-11-2
- Agaku, I. T., Singh, T., Rolle, I. V., & Ayo-Yusuf, O. A. (2016). Exposure and response to current text-only smokeless tobacco health warnings among smokeless tobacco users aged ≥18years, United States, 2012-2013. Preventive Medicine, 87, 200–206. doi:10.1016/j.ypmed.2016.02.014
- Anjum, Q., Ahmed, F., & Ashfaq, T. (2008). Knowledge, attitude and perception of water pipe smoking (Shisha) among adolescents aged 14-19 years. The Journal of the Pakistan Medical Association, 58, 312–317. Retrieved from http://www.jpma.org.pk/PdfDownload/1419.pdf
- Biener, L., Bogen, K., & Connolly, G. (2007). Impact of corrective health information on consumers’ perceptions of “reduced exposure” tobacco products. Tobacco Control, 16, 306–311. doi:10.1136/tc.2006.019240
- Biener, L., Nyman, A. L., Stepanov, I., & Hatsukami, D. (2014). Public education about the relative harm of tobacco products: an intervention for tobacco control professionals. Tobacco Control, 23, 385–388. doi:10.1136/tobaccocontrol-2012-050814
- Borland, R., Li, L., Cummings, K. M., O’Connor, R., Mortimer, K., Wikmans, T., … McNeill, A. (2012). Effects of a fact sheet on beliefs about the harmfulness of alternative nicotine delivery systems compared with cigarettes. Harm Reduction Journal, 9, 19. doi:10.1186/1477-7517-9-19
- Boyle, R. G., Stilwell, J., Vidlak, L. M., & Huneke, J. T. (1999). “Ready to quit chew?” Smokeless tobacco cessation in rural Nebraska. Addictive Behaviors, 24, 293–297. doi:10.1016/S0306-4603(98)00043-4
- Brubaker, R. G., & Mitby, S. K. (1990). Health-risk warning labels on smokeless tobacco products: Are they effective? Addictive Behaviors, 15, 115–118. doi:10.1016/0306-4603(09)90014-O
- Callery, W. E., Hammond, D., O’Connor, R. J., & Fong, G. T. (2011). The appeal of smokeless tobacco products among young Canadian smokers: The impact of pictorial health warnings and relative risk messages. Nicotine & Tobacco Research, 13, 373–383. doi:10.1093/ntr/ntr013
- Capella, M. L., Taylor, C. R., & Kees, J. (2012). Tobacco harm reduction advertising in the presence of a government-mandated warning. Journal of Consumer Affairs, 46, 235–259. doi:10.1111/j.1745-6606.2012.01229.x
- Gupta, P. C., Mehta, F. S., Pindborg, J. J., Aghi, M. B., Bhonsle, R. B., Daftary, D. K., … Sinor, P. N. (1986). Intervention study for primary prevention of oral cancer among 36,000 Indian tobacco users. Lancet, 1(8492), 1235–1239. https://doi.org/10.1016/S0140-6736(86)91386-3
- Islam, F., Salloum, R. G., Nakkash, R., Maziak, W., & Thrasher, J. F. (2016). Effectiveness of health warnings for waterpipe tobacco smoking among college students. International Journal of Public Health. doi:10.1007/s00038-016-0805-0
- Jawad, M., Abass, J., Hariri, A., & Akl, E. A. (2015). Social media use for public health campaigning in a low resource setting: The case of waterpipe tobacco smoking. BioMed Research International, 2015. doi:10.1155/2015/562586
- Jawad, M., Bakir, A., Ali, M., & Grant, A. (2015). Impact of waterpipe tobacco pack health warnings on waterpipe smoking attitudes: A qualitative analysis among regular users in London. BioMed Research International, 2015, 745865. doi:10.1155/2015/745865
- Johnson, S. E., Wu, C. C., Coleman, B. N., & Choiniere, C. J. (2014). Self-reported exposure to tobacco warning labels among U.S. middle and high school students. American Journal of Preventive Medicine, 47, S69–75. doi:10.1016/j.amepre.2014.05.005
- Lipkus, I. M., Eissenberg, T., Schwartz-Bloom, R. D., Prokhorov, A.V., & Levy, J. (2011). Affecting perceptions of harm and addiction among college waterpipe tobacco smokers. Nicotine & Tobacco Research, 13, 599–610. doi:10.1093/ntr/ntr049
- MacKinnon, D. P., & Fenaughty, A. M. (1993). Substance use and memory for health warning labels. Health Psychology, 12, 147–150. doi:10.1037/0278-6133.12.2.147
- Mays, D., Moran, M. B., Levy, D. T., & Niaura, R. S. (2016). The impact of health warning labels for Swedish snus advertisements on young adults’ snus perceptions and behavioral intentions. Nicotine & Tobacco Research, 18, 1371–1375. doi:10.1093/ntr/ntv140
- Mays, D., Tercyak, K. P., & Lipkus, I. M. (2016). The effects of brief waterpipe tobacco use harm and addiction education messages among young adult waterpipe tobacco users. Nicotine & Tobacco Research, 18, 777–784. doi:10.1093/ntr/ntv223
- Mohammed, H. T. (2013). The efficacy of viewing health warnings on shisha smoking among shisha smokers. Retrieved from https://uwspace.uwaterloo.ca/handle/10012/7419
- Murukutla, N., Turk, T., Prasad, C. V. S., Saradhi, R., Kaur, J., Gupta, S., … Wakefield, M. (2012). Results of a national mass media campaign in India to warn against the dangers of smokeless tobacco consumption. Tobacco Control: An International Journal, 21, 12–17. doi:10.1136/tc.2010.039438
- Mutti, S., Reid, J. L., Gupta, P. C., Pednekar, M. S., Dhumal, G., Nargis, N., … Hammond, D. (2015). Perceived effectiveness of text and pictorial health warnings for smokeless tobacco packages in Navi Mumbai, India, and Dhaka, Bangladesh: findings from an experimental study. Tobacco Control, 25, 437–443. doi:10.1136/tobaccocontrol-2015-052315
- Oswal, K. C., Raute, L. J., Pednekar, M. S., & Gupta, P. C. (2011). Are current tobacco pictorial warnings in India effective? Asian Pacific Journal of Cancer Prevention, 12, 121–124. Retrieved from http://journal.waocp.org/article_25485_80da31ac5474deda485e2f02daf63cea.pdf
- Parr, V. & Ell, P. (2011). Market testing of new health warnings and information messages for tobacco product packaging: Premium cigars, cigarillos/little cigars and roll your own. Sydney, Australia. Retrieved from http://www.tobaccolabels.ca/wp/wp-content/uploads/2013/12/Australia-2011-Market-testing-Graphic-health-warnings-on-products-other-than-cigarettes.pdf
- Popova, L. (2014). Scaring the snus out of smokers: Testing effects of fear, threat, and efficacy on smokers’ acceptance of novel smokeless tobacco products. Health Communication, 29, 924–936. doi:10.1080/10410236.2013.824063
- Popova, L., Kostygina, G., Sheon, N. M., & Ling, P. M. (2014). A qualitative study of smokers’ responses to messages discouraging dual tobacco product use. Health Education Research, 29, 206–221. doi:10.1093/her/cyt150
- Popova, L., & Ling, P. M. (2014). Nonsmokers’ responses to new warning labels on smokeless tobacco and electronic cigarettes: an experimental study. BMC Public Health, 14, 997. doi:10.1186/1471-2458-14-997
- Popova, L., Neilands, T. B., & Ling, P. M. (2014). Testing messages to reduce smokers’ openness to using novel smokeless tobacco products. Tobacco Control, 23, 313–321. doi:10.1136/tobaccocontrol-2012-050723
- Popper, E. T., & Murray, K. B. (1989). Communication effectiveness and format effects on in-ad disclosure of health warnings. Journal of Public Policy & Marketing, 8, 109–123. Retrieved from http://www.jstor.org/stable/30000316
- Rodu, B., Plurphanswat, N., Hughes, J. R., & Fagerström, K. (2016). Associations of proposed relative-risk warning labels for snus with perceptions and behavioral intentions among tobacco users and nonusers. Nicotine & Tobacco Research, 18, 809–816. doi:10.1093/ntr/ntv168
- Rousu, M. C., O’Connor, R. J., Thrasher, J. F., June, K. M., Bansal-Travers, M., & Pitcavage, J. (2014). The impact of product information and trials on demand for smokeless tobacco and cigarettes: evidence from experimental auctions. Preventive Medicine, 60, 3–9. doi:10.1016/j.ypmed.2013.11.001
- Salloum, R. G., Maziak, W., Hammond, D., Nakkash, R., Islam, F., Cheng, X., & Thrasher, J. F. (2015). Eliciting preferences for waterpipe tobacco smoking using a discrete choice experiment: implications for product regulation. BMJ Open, 5(9), e009497. doi:10.1136/bmjopen-2015-009497
- Sanders‐Jackson, A., Schleicher, N. C., Fortmann, S. P., & Henriksen, L. (2015). Effect of warning statements in e‐cigarette advertisements: An experiment with young adults in the United States. Addiction, 110, 2015–2024. doi:10.1111/add.12838
- Sarkar, S., Sharma, A., & Basu, D. (2013). Comparison of craving between smoked and smokeless tobacco across a variety of cue exposures. Substance Use & Misuse, 48, 233–238. doi:10.3109/10826084.2012.752851
- Shah, V. R., Dave, V. R., & Sonaliya, K. N. (2013). Impact of anti-tobacco warning labels on behaviour of tobacco users in one of the cities in Gujarat, India. Journal of Preventive Medicine & Hygiene, 54, 109–113. Retrieved from http://pubmedcentralcanada.ca/pmcc/articles/PMC4718388/
- Stark, E., Kim, A., Miller, C., & Borgida, E. (2008). Effects of including a graphic warning label in advertisements for reduced-exposure products: Implications for persuasion and policy. Journal of Applied Social Psychology, 38, 281–293. doi:10.1111/j.1559-1816.2007.00305.x
- Strasser, A. A., Orom, H., Tang, K. Z., Dumont, R. L., Cappella, J. N., & Kozlowski, L. T. (2011). Graphic-enhanced information improves perceived risks of cigar smoking. Addictive Behaviors, 36, 865–869. doi:10.1016/j.addbeh.2011.03.005
- Sussman, S., Dent, C. W., Flay, B. R., Burton, D., Craig, S., Mestel-Rauch, J., & Holden, S. (1989). Media manipulation of adolescents’ personal level judgements regarding consequences of smokeless tobacco use. Journal of Drug Education, 19, 43–57. doi:10.2190/51YX-L5HD-Y78B-5E5D
- Truitt, L., Hamilton, W. L., Johnston, P. R., Bacani, C. P., Crawford, S. O., Hozik, L., & Celebucki, C. (2002). Recall of health warnings in smokeless tobacco ads. Tobacco Control, 11, ii59–ii63. doi:10.1136/tc.11.suppl_2.ii59
- Turk, T., Murukutla, N., Gupta, S., Kaur, J., Mullin, S., Saradhi, R., & Chaturvedi, P. (2012). Using a smokeless tobacco control mass media campaign and other synergistic elements to address social inequalities in India. Cancer Causes & Control, 23 Suppl 1, 81–90. doi:10.1007/s10552-012-9903-3
- Vogeltanz-Holm, N., Holm, J. E., White Plume, J., & Poltavski, D. (2009). Confirmed recall and perceived effectiveness of tobacco countermarketing media in rural youth. Prevention Science, 10, 325–334. doi:10.1007/s11121-009-0134-0
- Walsh, M. M., Hilton, J. F., Ellison, J. A., Gee, L., Chesney, M. A., Tomar, S. L., & Ernster, V. L. (2003). Spit (smokeless) tobacco intervention for high school athletes: Results after 1 year. Addictive Behaviors, 28, 1095–1113. doi:10.1016/S0306-4603(02)00228-9