ABSTRACT
Mastitis is the main cause of antibiotic use in dairy sheep. Post-milking teat disinfection is adopted to prevent intra-mammary infections. Use of chemical disinfectants is associated with biocide and antibiotic resistance. Terpinen-4-ol (T-4-ol), component of the Melaleuca alternifolia essential oil, shows antibacterial properties without inducing resistance. Aim of the trial was to evaluate the efficacy of a T-4-ol post-dipping compound in preventing bacterial mastitis in dairy sheep. In two different farms, two groups of 35 ewes were recruited as experimental and control arm (exposed to chlorhexidine-based disinfectant). No differences were observed during the follow-up. In one livestock, during the first year, 30-day incidence of mastitis was significantly lower in the T-4-ol group. Post-milking teat disinfection with T-4-ol showed equivalent efficacy in terms of mastitis incidence. Disinfectants based on natural compounds for mastitis prevention could potentially reduce environmental pollution, avoid drug residues in milk and decrease the antimicrobial resistance development.
Introduction
Mastitis, which is the most important disease of dairy sheep, can cause animal suffering and economic and financial losses owing to reduced milk yield, poor milk quality, treatment-related costs, milk disposal, animals’ death and premature culling (Citation1–Citation3).
Following its suspect or clinical/laboratory confirmation, antibiotics are frequently prescribed, contributing to the risk of the emergence and spread of antimicrobial resistant bacteria, as well as the risk of antimicrobial residues in dairy food (Citation4–Citation7). In the majority of the cases, the disease occurs following an inflammatory response in the mammary gland against an intra-mammary infection (IMI) caused by contagious (e.g. Staphylococcus aureus), opportunistic (e.g. coagulase-negative staphylococci (CNS), coryneform bacteria), and environmental bacteria (e.g. Streptococcus uberis, Pseudomonas spp, Enterobacteriaceae, Enterococcus spp, Streptococcus spp). Infections can be favored by host – (i.e. udder shape, age, local immunity, feeding) and/or environment-related risk factors (i.e. weather, stress, bedding) (Citation8).
For this, the implementation of measures aimed to mastitis prevention and control plays a crucial role.
Post-milking teat disinfection (post-dipping), which is an effective and cheap intervention usually performed in dairy cows, is increasingly recommended in the small ruminant dairy sector to decrease skin bacterial contamination and to prevent IMI. It is routinely performed with conventional chemical disinfectants (e.g. chlorhexidine, iodine compounds, quaternary ammonium salts) associated with lenitive and/or moisturizer products (Citation9).
However, several studies showed the risk of cross-resistances between disinfectants and antibiotics caused by similar resistance pathways (Citation10–Citation12).
In addition, the intra or inter species spread of resistant genes can further increase when resistance genes are included in mobile genetic elements (e.g. plasmids) (Citation13–Citation16).
On this basis, it could be worthwhile to exploit, in both veterinary and human field, alternative treatments based on unconventional antibacterials, proved ineffective to induce resistances to the usually prescribed antibiotics.
Essential oils are derived from plants. Their antimicrobial and anti-inflammatory properties have been extensively studied in the years proving, some of them, extremely effective on pathogens (Citation17).
In particular, based on studies published to date, M. alternifolia essential oil (Tea tree oil-TTO), approved in Australia since 1920s, shows bactericidal activity against both drug-susceptible and -resistant bacteria (Citation18–Citation21), fungi (Citation22,Citation23), viruses (Citation24). Its biological activity has been thoroughly studied on S. aureus and Escherichia coli (Citation25,Citation26). Furthermore, some anti-tumoral (Citation27,Citation28) and anti-inflammatory properties (Citation29–Citation31) were evidenced.
Finally, the potential insecticidal, acaricidal, and repellent efficacy (Citation32–Citation34) so as anti-parasites (Citation35) were also demonstrated.
The wide range of activities of TTO has mainly been associated with the component Terpinen-4-ol (T-4-ol). As a consequence, due to the high variability of composition of the natural oils, to harmonize TTO quality assessment, the international standard ISO 4730:2004 (Citation36) was issued establishing the acceptable ranges of its main components.
Hammer et a. found that the occurrence of antibiotic resistant strains did not increase following the exposure to all oil components, including T-4-ol (Citation37).
The anti-bacterial activity of T-4-ol is based on the disruption of the cell membrane permeability causing the loss of the bacterial chemiosmotic control. In particular, its efficacy was demonstrated on mastitogenic, drug-susceptible and -resistant, bacteria (Citation38).
Several studies were performed to evaluate the effectiveness of essential oils in dairy animals, particularly in cows (Citation39–Citation41); however, no studies have been carried out on the preventative efficacy of the T-4-ol against bacterial mastitis in dairy ewes. T-4-ol was the active principle of a post-dipping formula developed by Istituto Superiore di Sanità (Patent application n° 08425431.7) for the prevention of mastitis in dairy animals. The present work describes the studies performed to evaluate the efficacy of the formula in the prevention of mastitis in dairy sheep.
Materials and methods
Experimental design
A randomized, parallel-group, 2-year field study was performed to compare the efficacy of the T-4-ol post-dipping respect to a conventional one (i.e. chlorhexidine 0.5%) in preventing mastitis in dairy sheep half-udders.
Animal selection criteria
A 2-year trial was carried out in two well-managed extensive dairy sheep herds located in North Sardinia, Italy, from February to June (concomitantly with middle and late lactation season). The livestocks consisted of 500 (Farm A) and 750 (Farm B) Sarda breed sheep, respectively. In each farm 70 ewes, balanced for age, production and health status were recruited for the experimental (i.e. treated with the T-4-ol compound) and the control arm (i.e. treated with a 0.5% chlorexidine-based disinfectant). During the 60-day follow-up, animals were clinically visited every month and bacteriological and somatic cell content (SCC) determinations of the half-udder milk were carried out.
Post-dipping formula
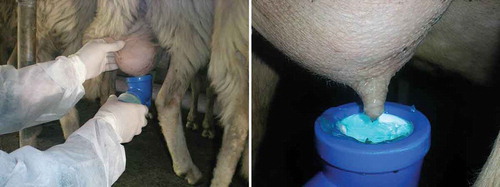
The original formula of the T-4-ol post-dipping was proved to be a ready-to-use product effective for the sanitation of the teat-end and showing a wide spectrum of antimicrobial activity. High coating power and persistence were achieved through the addition of a gelling agent acting also as a skin protective barrier. A food grade blue color was also included in the formula to ease the traceability of treated teats during the experiments (). The post-dipping was dispensed in the amount of approximately 1 kg in non-transparent plastic containers labeled according to the public veterinary authority requirements.
Teat dipping
After each milking by milking machines, the experimental group was treated dipping the teat-ends into the T-4-ol product (), while the control group was exposed to the commercial chlorhexidine-based disinfectant.
Milk sampling procedure
Immediately before the regular milking, a half-udder milk sample (40 mL) was aseptically collected after a through teat disinfection and the discard of the first foremilk streams. Samples were transported in refrigerated condition to the ISO/IEC 17025 accredited laboratory of the Istituto Zooprofilattico Sperimentale della Sardegna (Sassari, Italy) and analyzed within 12 h from milking.
SCC determination in milk
SCC was assessed in a flow cytometry somatic cell counter Fossomatic FC instrument (Foss Electric, Hillerød, Denmark) according to ISO 13366–2:2007 (IDF 148–2: 2006) (Citation42).
Bacteriological analysis
Samples were tempered to room temperature and 10 μL of milk were spread onto a blood agar plate (5% sheep blood). All plates were aerobically incubated at 37 °C for 24 h and examined for bacterial growth. In absence of visible growth, the incubation was prolonged to 48 h. Samples were considered positive for mastitis when the inoculated plates showed at least one colony of suspected primary pathogens (i.e. S. aureus), also concomitantly with other colonies, or more than five colonies of CNS (corresponding to > 500 CFU/mL). In case of plates showing at least two different species of non-primary pathogens, and in absence of mastitogenic species, a sampling contamination was supposed and samples considered negative.
Bacterial identification was run according to conventional standard procedures: Gram stain, microscopy, coagulase test (Oxoid and SCLAVO Diagno-stics International), biochemical confirmations kits (BioMérieux’s API®), oxidase test (Bactident oxidase test strip, Merck) and catalase tests (hydrogen peroxide solution 3%) (Citation43,Citation44).
Criteria for mastitis diagnosis
Clinical mastitis was diagnosed when udder clinical symptoms occurred (i.e. swelling, heat, hardness, redness, or pain) and/or when milk alteration (i.e. watery appearance, flakes, clots, blood or pus) was observed. Subclinical mastitis was diagnosed when bacterial pathogens and/or SCC > 500,000 cell/mL (Citation45) were detected in milk samples.
Statistical analysis
Categorical variables were described with absolute and relative frequencies and differences were computed with the Chi square or the Fisher exact test, when appropriate. Incidence and relative risk were calculated. A p-value < 0.05 (two-tailed) was considered statistically significant. Statistical analyses were performed with Stata® 13.0 (StataCorp, College Station, TX, USA).
Sample-size
The sample size was computed considering every single half udder as a statistical unit. The total number was estimated taking into account a parallel group study aimed to evaluate the efficacy of the T-4-ol post-dipping disinfection in preventing mastitis incidence in comparison with the standard treatment. Moreover, a power of the study of 80%, an α-error < 5%, and a drop-out of 10% were assumed.
Results
Episodes of clinical mastitis did not occur in the experimental and control arms in both farms during the 2-year trial.
No statistically significant differences in bacterial isolation were found between post-dipping treatments in both milking seasons (): CNS were the most frequently bacterial isolate, followed by S. uberis, Enterococcus faecalis, Pseudomonas aeruginosa, and Corynebacterium spp. ().
Table 1. Half-udder milk samples positive to bacteriological analysis.
Table 2. Bacterial isolates from positive half-udder milk samples.
Incidences and relative risks of subclinical mastitis were similar in both arms (); however, in Farm B, a statistically significant lower incidence of mastitis was found in the T-4-ol group 30 days after treatment start, showing a relative risk of 0.06 (CI 95%: 0.01–0.45; p-value = 0.006) () during the first year of the trial.
Table 3. Incidence [n(%)] and relative risk [RR (CI 95%)] of subclinical mastitis in half-udders.
During the first year, owing to a blue-tongue virus outbreak, the follow-up in Farm A was interrupted 30 days after the treatment initiation.
Discussion
Bacterial mastitis represents the main disease affecting dairy animals and is frequently treated with antimicrobial drugs, whose inappropriate prescription and misuse can contribute to the development of antibiotic resistance (Citation46). Prevention and control of mastitis are essentially based on the implementation of good milking practices and hygiene standards (Citation2).
Post-milking teat disinfection, routinely performed with disinfectants, is an effective tool in the prevention of new IMI episodes (Citation47).
However, the systematic exposure to chemical biocides has been reported to favor the development of bacterial resistance to antibiotics (Citation48).
The antimicrobial efficacy of some essential oils has been proved, whereas no clear evidence has been reported on the induction of bacterial resistance. T-4-ol is the main antimicrobial component of M. alternifolia whose antimicrobial and anti-inflammatory properties have been showed both in human and veterinary fields (Citation49).
The efficacy of a T-4-ol post-dipping formulation in the prevention of bacterial mastitis in dairy sheep was compared with that of a commercial chlorhexidine-based disinfectant. A 2-year trial was performed in two dairy sheep farms located in Sardinia, a region characterized by the highest number (> 3,000,000) of ewes in Italy, corresponding to 44% of the total.
The efficacy of the T-4-ol post-dipping compound in preventing both clinical and subclinical mastitis in dairy sheep was equivalent to the commercial chlorhexidine-based product.
This is the first study aimed to evaluate the efficacy of the T-4-ol in the prevention of mastitis in dairy ewes. However, the reliability of the findings can be hindered by some study limitations, particularly the logistical and organizational conditions which could have influenced the animal management. Another limitation could be represented by the low frequency of sampling during the follow-up due to the concomitant farming practices. However, the large sample size, the randomization at the study baseline, and the two-year duration in two different farms could contribute to reduce the above-mentioned bias.
An important goal of the dairy business is to produce the high quantities of high-quality and safe milk, characterized by a low burden of somatic cells, low bacterial counts, and by the absence of pathogens and antibiotic residues.
Antibiotics are prescribed to treat mastitis episodes and several molecules are available. They are administered locally (i.e. intra-mammary infusion) and/or systemically, frequently at high dosages to achieve the effective concentration in the udder. The massive use in the animals can cause a selective pressure favoring the emergence of antibiotic resistant strains and reflecting the ‘tuning of the microorganisms to antibiotic polluted eco-systems’ (Citation50).
Antibiotic resistance is posing challenges worldwide for both human and animal health and WHO recognises it as a major threat to public health (Citation51).
An efficient mastitis preventive approach would reduce the need of therapeutic antibiotic treatments, thus limiting their use and, then, the selection of resistant strains. The use of preventive molecules unable to increase the probability of emergence and spread of bacterial drug-resistant strains is expected to play a strategic role.
No evidence of induced resistance is available at moment for T-4-ol and a spontaneous development of resistance is very unlikely to occur, considering that it acts as a membrane permeabilizer leading to loss of chemiosmotic control in both gram-positive and gram-negative bacteria (Citation19,Citation20,Citation52).
Conclusion
Our results confirmed that T-4-ol could be usefully used as active principle of post-dipping formula also in dairy sheep as a valid alternative to chemical products routinely applied for mastitis prevention, contributing to both milk quality and to animal welfare.
Finally, the positive results of the trial fully fulfill the One-Health approach, considering the strong interconnections among the health of humans, animals and ecosystem.
Acknowledgments
This research was approved and funded by the Italian Ministry of Health (Project n. IZS SA 08/10RC).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- D. Bergonier, R. De Crémoux, R. Rupp, G. Lagriffoul and X. Berthelot, Mastitis of dairy small ruminants. Veterinary Research, Sep-Oct 34(5), 689–716 (2003).
- A. Contreras, D. Sierra, A. Sanchez, J.C. Corrales, J.C. Marco, M.J. Paape and C. Gonzalo, Mastitis in small ruminants. Small Ruminant Research : the Journal of the International Goat Association, 68(Issues 1–2), Pages 145–153 (2007).
- H. Hogeveen, K. Huijps and T.J. Lam, Economic aspects of mastitis: new developments. New Zealand Veterinary Journal, Jan 59(1), 16–23 (2011).
- D. Chandrasekaran, P. Venkatesan, K.G. Tirumurugaan, A.P. Nambi, P.S. Thirunavukkarasu, K. Kumanan, S. Vairamuthu and S. Ramesh, Pattern of antibiotic resistantmastitis in dairy cows. VeterinaryWorld, 7(6), 389–394 (2014).
- T.F. Landers, B. Cohen, T.E. Wittum and E.L. Larson, A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Reports, 127(1), 4–22 (2012).
- K.B. Martins, P.Y. Faccioli, M.F. Bonesso, 3. Fernandes, A.A. Oliveira, A. Dantas, L.F. Zafalon and M.L. Cunha, Characteristics of resistance and virulence factors in different species of coagulase-negative staphylococci isolated from milk of healthy sheep and animals with subclinical mastitis. Journal Dairy Sciences Mar, 100(3), 2184–2195 (2017).
- S.A. Lollai, M. Ziccheddu, I. Duprè and D. Piras, Characterization of resistance to tetracyclines and aminoglycosides of sheep mastitis pathogens: study of the effect of gene content on resistance. Journal of Applied Microbiology, Oct 121(4), 941–951 (2016).
- A.I. Gelasakis, V.S. Mavrogianni, I.G. Petridis, N.G. Vasileiou and G.C. Fthenakis, Mastitis in sheep – the last 10 years and the future of research. Veterinary Microbiology, Dec 14 181(1–2), 136–146 (2015).
- P. Menzies, J. Jansen, C. Fitzpatrick, M. Foran, P. Wilman and V.A. Taylor Guide to Udder Health for Dairy Sheep.Web Version. (2014) http://www.uoguelph.ca/~pmenzies/Dairy_Sheep/DairySheep.html (2017)
- S.C.E.N.I.H.R. Scientific, Committee on Emerging and Newly Identified Health Risks. Assessment of the Antibiotic Resistance Effects of Biocides. European Commission, Brussels, Belgium 1–87. (2009) http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_021.pdf (2017)
- A.D. Russell, Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. Journal of Applied Microbiology, 92, 121S-35S (2002).
- A.P. Fraise, Biocide abuse and antimicrobial resistance: a cause for concern? Journal Antimicrobial Chemotherc, 49(1), 11–12 (2002).
- M. Braoudaki and A.C. Hilton, Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. Journal of Clinical Microbiology, Jan 42(1), 73–78 (2004a).
- M. Braoudaki and A.C. Hilton, Low level of cross-resistance between triclosan and antibiotics in Escherichia coli K-12 and E. coli O5 compared to E. coli O157. FEMS Microbiology Letters, Jun 15 235(2), 305–309 (2004b).
- R. Chuanchuen, K. Beinlich, T.T. Hoang, A. Becher,R.R. Karkhoff-Schweizer and H.P. Schweizer, Cross-resistance between Triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to Triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrobial Agents and Chemo-therapy, 45(2), 428–432 (2001).
- O. Tkachenko, J. Shepard, V.M. Aris, A. Joy, A. Bello, I. Londono, J. Marku, P. Soteropoulos and M.A. Peteroy-Kelly, A triclosan-ciprofloxacin cross-resistant mutant strain of Staphylococcus aureus displays an alteration in the expression of several cell membrane structural and functional genes. Research in Microbiology, Oct-Nov 158, 651–658 (2007).
- J. Sharifi-Rad, A. Sureda, G.C. Tenore, M. Daglia, M. Sharifi-Rad, M. Valussi, R. Tundis, M. Sharifi-Rad, M.R. Loizzo, A.O. Ademiluyi, R. Sharifi-Rad, S.A. Ayatollahi and M. Iriti, Biological Activities of Essential Oils: from Plant Chemoecology to Traditional Healing Systems. Molecules (Basel, Switzerland), 22(1), 70 (2017).
- S.A. Burt and R.D. Reinders, Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Letters in Applied Microbiology, 36(3), 162–167 (2003).
- S.D. Cox, C.M. Mann, J.L. Markham, H.C. Bell, J.E. Gustafson, J.R. Warmington and S.G. Wyllie, The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). Journal of Applied Microbiology, Jan 88(1), 170–175 (2000).
- C.F. Carson, B.J. Mee and T.V. Riley, Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother, Jun 46(6), 1914–1920 (2002).
- A.M. Ferrini, V. Mannoni, P. Aureli, G. Salvatore, E. Piccirilli, T. Ceddia, E. Pontieri, R. Sessa and B. Oliva, Melaleuca alternifolia essential oil possesses potent anti-staphylococcal activity extended to strains resistant to antibiotics. International Journal of Immunopathology and Pharmacology, Jul-Sep 19(3), 539–544 (2006).
- F. Pisseri, A. Bertoli, S. Nardoni, L. Pinto, L. Pistelli, G. Guidi and F. Mancianti, Antifungal activity of tea tree oil from Melaleuca alternifolia against Trichophyton equinum: an in vivo assay. Phytomedicine : International Journal of Phytotherapy and Phytopharmacology, Nov 16(11), 1056–1058 (2009).
- K. Ninomiya, N. Maruyama, S. Inoue, H. Ishibashi, T. Takizawa, H. Oshima and S. Abe, The essential oil of Melaleuca alternifolia (tea tree oil) and its main component, terpinen-4-ol protect mice from experimental oral candidiasis. Biological & Pharmaceutical Bulletin, 35(6), 861–865 (2012).
- A. Garozzo, R. Timpanaro, B. Bisignano, P.M. Furneri, G. Bisignano and A. Castro, In vitro antiviral activity of Melaleuca alternifolia essential oil. Letters in Applied Microbiology, Dec 49(6), 806–808 (2009).
- J.A. Cuaron, S. Dulal, Y. Song, A.K. Singh, C.E. Montelongo, W. Yu, V. Nagarajan, R.K. Jayaswal, B.J. Wilkinson and J.E. Gustafson, Tea tree oil-induced transcriptional alterations in Staphylococcus aureus. Phytotherapy Research: PTR, Mar 27(3), 390–396 (2013).
- S.D. Cox, J.E. Gustafson, C.M. Mann, J.L. Markham, Y.C. Liew, R.P. Hartland, H.C. Bell, J.R. Warmington and S.G. Wyllie, Tea tree oil causes K+ leakage and inhibits respiration in Escherichia coli. Letters in Applied Microbiology, May 26(5), 355–358 (1998).
- C.E. Assmann, F.C. Cadoná, B.D.S.R. Bonadiman, E.B. Dornelles, G. Trevisan and I.B.M.D. Cruz, Tea tree oil presents in vitro antitumor activity on breast cancer cells without cytotoxic effects on fibroblasts and on peripheral blood mononuclear cells. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, Jul 103, 1253–1261 (2018).
- S. Shapira, S. Pleban, D. Kazanov, P. Tirosh and N. Arber, Terpinen-4-ol: A Novel and Promising Therapeutic Agent for Human Gastrointestinal Cancers. PLoS One, Jun 8 11(6), (2016).
- C. Salvatori, L. Barchi, F. Guzzo and M. Gargari, A comparative study of antibacterial and anti-inflammatory effects of mouthrinse containing tea tree oil. Oral & Implantology, Apr 10 10(1), 59–70 (2017).
- J. Wallengren, Tea tree oil attenuates experimental contact dermatitis. Archives of Dermatological Research, Jul 303(5), 333–338 (2011).
- F. Caldefie-Chézet, M. Guerry, J.C. Chalchat, F. C, M.P. Vasson and J. Guillot, Anti-inflammatory effects of Melaleuca alternifolia essential oil on human polymorphonuclear neutrophils and monocytes. Free Radical Research, Aug 38(8), 805–811 (2004).
- V.1. Klauck, R. Pazinato, S. Lm, S. Rc, V. Ra, M.D. Baldissera, R. Raffin, A. Boligon, M. Athayde, D. Baretta, G. Machado and D.A. Silva AS, Insecticidal and repellent effects of tea tree and andiroba oils on flies associated with livestock. Medical and Veterinary Entomology, Aug 28(Suppl 1), 33–39 (2014).
- S.F. Walton, M. McKinnon, S. Pizzutto, A. Dougall, E. Williams and B.J. Currie, Acaricidal activity of Melaleuca alternifolia (tea tree) oil: in vitro sensitivity of sarcoptes scabiei var hominis to terpinen-4-ol. Archives of Dermatology, May 140(5), 563–566 (2004).
- P.J. James and J.T. Callander, Bioactivity of tea tree oil from Melaleuca alternifolia against sheep lice (Bovicola ovis Schrank) in vitro. Veterinary Parasitology, Jul 6 187(3–4), 498–504 (2012).
- T.H. Grando, M.F. de Sá, M.D. Baldissera, C.B. Oliveira, M.E. de Souza, R.P. Raffin, R.C. Santos, R. Domingues, A.P. Minho, M.L. Leal, S.G. Monteiro and J. Helminthol, In vitro activity of essential oils of free and nanostructured Melaleuca alternifolia and of terpinen-4-ol on eggs and larvae of Haemonchus contortus. Journal of Helminthology, May 90(3), 377–382 (2016).
- ISO-4730:2017 - Essential oil of Melaleuca, terpinen-4-ol type (Tea Tree oil). (2017).
- K.A. Hammer, C.F. Carson and T.V. Riley, Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single- and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob Agents Chemother, Feb 56(2), 909–915 (2012).
- A.M. Ferrini, V. Mannoni, F. Superti, M.G. Ammendolia, S. Fuselli, R. Morlino, P. Aureli, S. Amatiste, G. Giacinti, A. Tammaro, R. Rosati ., G. Varisco, B. Oliva, E. Pontieri, M. Montel, I. Verdier-Mets and P. Pradel, Terpinen-4-olo come principio attivo di formulazioni veterinarie per la prevenzione delle mastiti. Convegno nazionale Sostanze naturali: Dalla ricerca di base all’applicazione clinica. Istituto Superiore di Sanità. Roma, 23-25 marzo 2009. Istituto Superiore di Sanità;. (ISTISAN Congressi 09/C1), Riassunti. Roma 80–81. (2009).
- S.E. Mason, K.A.E. Mullen, K.L. Anderson, S.P. Washburn, J.L. Yeatts and R.E. Baynes, Pharmacokinetic analysis of thymol, carvacrol and diallyl disulfide after intramammary and topical applications in healthy organic dairy cattle. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, May 34(5), 740–749 (2017).
- F.H. Santos, M.R. De Paula, D. Lezier, J.T. Silva, G. Santos and C.M. Bittar, Essential oils for dairy calves: effects on performance, scours, rumen fermentation and intestinal fauna. Animal : an International Journal of Animal Bioscience, Jun 9(6), 958–965 (2015).
- A.C. Smith, C.L. Wood, K.J. McQuerry and J.M. Bewley, Effect of a tea tree oil and organic acid footbath solution on digital dermatitis in dairy cows. Journal of Dairy Science, 97(4), 2498–2501 (2014).
- ISO 13366-2/IDF 148-2: 2006, Milk - Enumeration of Somatic Cells – Part 2: Guidance on the Operation of Fluoro-Opto-Electronic Counters). (2006).
- P.J. Quinn, M.E. Carter, B. Markey and G.R. Carter, Clinical Veterinary Microbiology. Wolf/Mosby, London (1994).
- J.C. Holt, N.R. Krieg, P.H.A. Sneath, J.T. Staley and S.T. Williams, Bergey’s Manual of Determinative Bacteriology, 9th. William & Wilkins, Baltimore (1994).
- X. Berthelot, G. Lagriffoul, D. Concordet, F. Barillet and D. Bergonier, Physiological and pathological thresholds of somatic cell counts in ewe milk. Small Ruminant Research : the Journal of the International Goat Association, Volume 62(Issues 1–2), Pages 27–31 (2006). March
- C.L. Ventola, The Antibiotic Resistance Crisis: part 1: causes and threats. Pharmacy and Therapeutics, 40(4), 277–283 (2015).
- F.K. Neave, F.H. Dodd, R.G. Kingwell and D.R. Westgarth, Control of mastitis in the dairy herd by hygiene and management. Journal of Dairy Science, May 52(5), 696–706 (1969).
- A. Davin-Regli and J.M. Pagès, Cross-resistance between biocides and antimicrobials: an emerging question. Revue Scientifique Et Technique (International Office of Epizootics), Apr 31(1), 89–104 (2012).
- C.F. Carson, K.A. Hammer and T.V. Riley, Melaleuca alternifolia (Tea Tree) Oil: a Review of Antimicrobial and Other Medicinal Properties. Clinical Microbiology Reviews, Jan 19(1), 50–62 (2006).
- P. Courvalin, The Garrod Lecture. Evasion of antibiotic action by bacteria. Antimicrobial Chemotherapy, May 37(5), 855–869 (1996).
- WHO factsheet on antimicrobial resistance (AMR). Available from:http://www.who.int/mediacentre/factsheets/fs194/en/ (2017)
- J. Reichling, M. Harkenthal, H. Geiss, T. Hoppe-Tichy and R. Saller, Electron microscopic and biochemical investigations on the antibacterial effects of Australian tea tree oil against -Staphylococcus aureus. Current Topics Phytochem, 5, 77–84 (2002).