Phosphoros–the Morningstar, what a wonderful world came down to us through the millennia–let us keep and protect it! And Harry Hudson was able to wander between the worlds of arts and science as well. His advice in English history and literature was well accepted, like his profound knowledge in chemistry. We teamed up as partners in a group of chemists named EUROPHOS and combined our labs in London and in Düsseldorf with syntheses, analytics, structures, NMR spectroscopy and biological aspects. We enjoyed mutual visits, supported by binational funds, and looked into α-aminophosphonic acids, α-aminophosphinic acids and corresponding esters, generally abbreviated as NHR1-CHR2-P(O)(R3)ORCitation4. Selected results were published in a review, edited by V. Khukar and H. R. HudsonCitation1 and in several publications.Citation2a–j Molecular modelling, ring current calculations, and NMR were combined to understand complex 1H NMR spectra of NHPh-CHPh-P(O(OEt)2 and related structures.Citation3 Compounds with sterically demanding substituents NH(CHPh2)-CHR2-P(O(OEt)2 (R2 = Ph, Naphthyl, Anthranyl, Pyrenyl) were synthesized.Citation4a–b
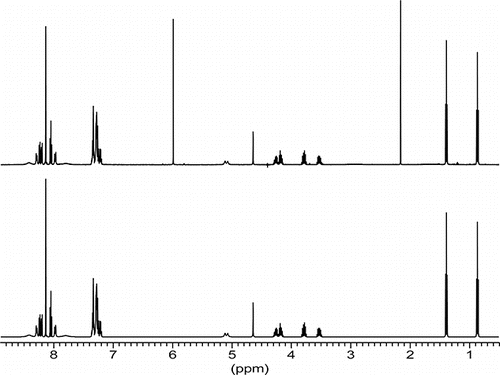
Our particular interests were drawn toward the sterically overcrowded α-N-(diphenylmethyl)amino-α-(1-pyren-yl)methanephosphonic acid diethylester NH(CHPh2)-CH(Pyr)-P(O(OEt)24a which gave rise to a 1H NMR spectrumCitation5 () involving a total of 29 spins ():
Figure 1. 500 MHz-1H-NMR-spectrum of α-N-(diphenylmethyl)amino-α-(1-pyrenyl)-methanephosphonic acid diethyl ester. Upper: experimental (3% in C2D2Cl4, 300 K). Lower: simulated with WIN-DAISY. Two singlet lines: (a) C2HDCl4 (from C2D2Cl4) at 6 ppm; (b) traces from acetone at 2.1 ppm.
Scheme 1. 29 spins of α-N-(diphenylmethyl)amino-α-(1-pyrenyl)methanepho-sphonic acid diethylester NH(CHPh2)-CH(Pyr)-P(O(OEt)2.
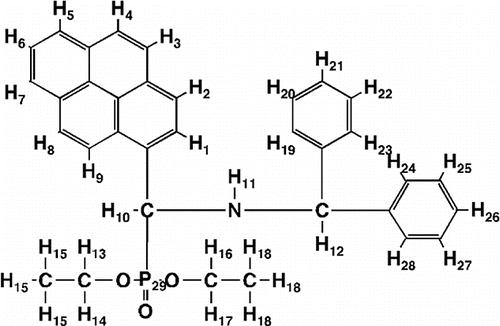
Only the pyrenyl fragment H1 to H9 () and neighboring protons H10 and H11 will be mentioned in more details within this foreword; more details are available in the literatureCitation1,5:
Figure 2. 500 MHz-1H-NMR-spectrum of α-N-(diphenylmethyl)amino-α-(1-pyrenyl)methanephosphonic acid diethyl ester at 300 K. Pyrene range (3% in CDCl3, 300 K).
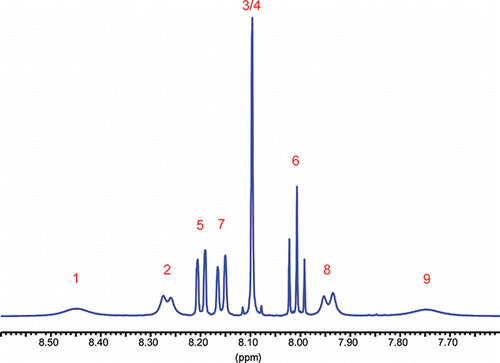
The pyrenyl part of this 1H NMR spectrum may be broken down in a simplified first order approach (neglecting all the small interproton-couplings nJHH with n > 3 and all nJPH) into the superposition of one ABC and three AB type spectra. Those subsystems belong to: (a) H1, H2: AB; (b) H5, H7, H6: ABC; (c) H3, H4: AB very close to A2; (d) H8, H9: AB. Protons H1 and H9 and to a lesser degree H2 and H8 are affiliated with broadened lines possibly pointing toward dynamic exchange. Neighboring protons H10 (CH) and H11 (NH) show broadened lines as well. This might be due to 14N quadrupolar effects, long rang coupling to pyrenyl protons, or indicating again dynamics involving rotation around the pyrenyl-phosphorus bond. And indeed upon warming up to 403°K those broad lines sharpened up. Neglecting dynamics, a full line shape analysis and iteration using DAISY under WINNMR was performed, which yielded NMR parameters of 28 protons H1 to H28. Results for H1 to H11 are listed below in (resonance frequencies, chemical shifts, spectral half widths) and in (coupling constants). Data for the remaining protons H12 to H28 are given in Refs.Citation1,Citation5
Table 1. Resonance frequencies νi, chemical shifts δi, and spectral half widths HWi together with individual errors ±ϵi were obtained from iterating the 500 MHz-1H-NMR-spectrum of α-N-(diphenylmethyl)amino-α-(1-pyrenyl)methanephosphonic acid diethylester at 300 K. Pyrene range. (3% in CDCl3, 300 K, SW = 5170 Hz, NS = 64K, digital resolution: 0.079 Hz/pt corresponding 12.7 pts/Hz).
Table 2. Coupling constants nJik (nJHH and nJPH) and individual errors ±ϵik of α-N-(diphenylmethyl)amino-α-(1-pyrenyl)methanephosphonic acid diethylester.
Data listed in and are formal results obtained by line shape iteration. A few coupling constants are small, close to digital resolution, might be near to or equal to zero. 5JHH of 5 Hz between protons H1 and H9 appears too large and not realistic, but it is the formal result and consequence from spectral half widths HW1 = ca. 40 Hz and HW9 = ca. 59 Hz. It is worth mentioning that 1H NMR spectra of less crowded compounds α-N-(diphenylmethyl)amino-α-(1-Ar)methanephosphonic acid diethylester with Ar = Ph, Napthyl, and Anthranyl do not show those dyanamic phenomena. Hence spectral analysis of such systems is more simple.Citation1,Citation5
Harry Hudson´s pyrenyl problem ended up in a thorough test of WIN-DAISY coming to the limits of iterability for complex multi spin systems, where fragment techniques, chemical, and magnetical equivalence in connection with fast line shape iterators were requested.Citation5,Citation6
Toward the end of this foreword one more of Harry´s favourite structures should be mentioned: PNL62, which is the betainic form CH3CH2CH(NH3+)PO3H− of the biorelevant α-aminopropanephosphonic acid CH3CH2CH(NH2)PO3H2 and received considerable attention.Citation7a–f Dissociation and stability constants were determined followed by NMR controlled titrations leading to ion specific chemicals shifts δP. The HR 1H NMR spectrum of CH3CH2CH(NH3+)PO3H− was analyzed and iterated by automated lines shape procedures. And of course one side-question concerning the sign of coupling constant 2JPH was settled by double resonance: the sign of 2JPH is negative, while the sign of 3JPH is positive. And this is true for related structures, a fact worth remembering when publishing on α-aminoalkylphosphonic acids and similar compounds.
Harry Hudson´s work was a catalyst for adjunct research, and we will not forget this fine, productive and pleasant cooperation.
References
- Hägele G. Physical properties and NMR-spectroscopic characterization of aminophosphonates and aminophosphinates. In: V. P. Kukhar and H. R. Hudson Eds., Aminophosphonic and Aminophosphinic Acids; Wiley & Sons ltd.: Chichester, 2000. Chapter 8, pp. 217–284.
- ( a) Hägele, G.; Gruß, U.; Dronia, H. J. Fluorine Chem. 1991, 54, 285. ( b) Caccamese, S.; Failla, S.; Finocchiaro, P.; Hägele, G.; Principato, G. J. Chem. Res. (S) 1992, 242–243. ( c) Gruß, U.; Dronia, H.; Hägele, G. Phosphorus Sulfur Silicon Relat. Elem. 1993, 77, 318. ( d) Gruß, U.; Hägele, G. Phosphorus Sulfur Silicon Relat. Elem. 1994, 97, 209–221. e) ibid. 1996, 111, 159. f) Dronia, H.; Gruß, U.; Hägele, G.; Friedrich, T.; Weiss, H. J. Comput.-Aided Mol. Des. 1996, 10, 100–106. ( g) Green, D. St. C.; Gruß, U.; Hägele, G.; Hudson, H. R.; Lindblom, L.; Pianka, P. Phosphorus Sulfur Silicon Relat. Elem. 1996, 113, 179–207. ( h) Vaubaillon, V.; Giraud, G.; Rabiller, C.; Gruß, U.; Haas, A.; Hägele, G. Phosphorus Sulfur Silicon Relat. Elem. 1997, 126, 177–183. ( i) Gruß, U. Dissertation 1997, Heinrich-Heine-University Düsseldorf. ( j) Osthaus, N. P. Dissertation 1998, Heinrich-Heine-University Düsseldorf.
- Dronia, H.; Failla, S.; Finocchiaro, P.; Gruß, U.; Hägele, G. Phosphorus Sulfur Silicon Relat. Elem. 1995, 101, 149–160.
- ( a) Hudson, H. R.; Lee, R. J.; Matthews, R. W. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 1691–1709. ( b) Hudson, H. R.; Lee, R. J. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 1149–1155.
- Hottgenroth, A. Dissertation 1998, Heinrich-Heine-University Düsseldorf.
- Spiske, R. Dissertation 1996, Heinrich-Heine-University Düsseldorf.
- ( a) Dronia H.; Hägele G.; Hudson H. R.; Matthews R. W. Proc. XIII Internat. Conf. Phosphorus Chem. Jerusalem, 16–21 July, 1995, ed. E. Breuer, Phosphorus Sulfur and Silicon Relat. Elem. 1996, 111, 23. ( b) Hudson H. R. Aminophosphonic and Aminophosphinic acids and their derivatives as Agrochemicals. In: V. P. Kukhar and H. R. Hudson Eds., Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; Wiley: Chichester, 2000, Chapter 13 pp. 443–482. ( c) Hudson, H. R.; Ismail, F.; Pianka, M. Phosphorus Sulfur Silicon Relat. Elem. 2001, 173, 143–162. ( d) Hudson, H. R.; Ismail, F. J. Labelled Compd. Radiopharm. 2001, 44, 549–552. ( e) Hudson, H. R.; Volckman, J. F. Periodica Polytechnica Chem. Eng. 2010, 54, 9–14. ( f) Bashall, A. P.; Crowder, J.; Dronia, H.; Hägele, G.; Hudson, H. R.; Lee, R. J.; McPartlin, M.; Matthews, R. W.; Ollig, J. Heteroatom Chem. 2010, 21, 314–325.
