In this issue of Leukemia and Lymphoma, Wimazal et al. Citation[1] describe the intramedullary distribution of vascular endothelial growth factor (VEGF) expression in patients with myelodysplastic syndrome (MDS). VEGF is of great interest for neoplasia in general and myeloid disorders in particular, because it plays a key role as mediator of the neoangiogenesis necessary to sustain cell proliferation and survival Citation[2],Citation[3]. Furthermore, increased intramedullary microvessel density is associated with unfavorable prognosis in MDS Citation[4],Citation[5], myeloproliferative disorders Citation[6],Citation[7] acute myeloid leukemia (AML) Citation[8] and several other hematologic malignancies Citation[9]. Ironically, the clonal cell-derived angiogenic cytokines reinforce neoplastic proliferation as well as promoting an intense stromal reaction, suppressing production of mature blood cells to fill the new blood vessels.
Several groups previously demonstrated expression of VEGF and its receptors () in MDS marrow Citation[10], but the precise pathobiologic significance of this finding is unknown, and uncertainty remains about the microarchitectural distribution of this expression Citation[11]. Wimazal et al. Citation[1] extend prior observations by confirming VEGF expression in the marrow of 46 patients with various types of MDS. The investigators show that, at least by immunohistochemistry, VEGF is predominantly expressed in myeloid progenitor cells, immature monocytic cells and megakaryocytes, but is absent in the erythroid precursors and maturing granulocytes that comprise the predominant cells in lower-risk MDS marrow.
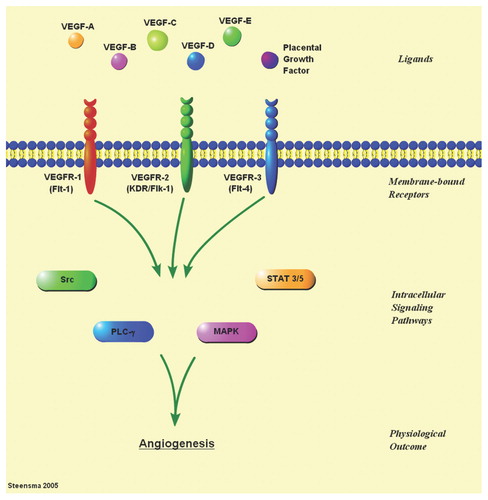
Figure 1. There are three well-described membrane-bound VEGF receptors (VEGFR-1 to -3), and at least six growth factor ligands (VEGF-A to -E, and placental growth factor); some of the ligands have multiple isoforms. Ligand-receptor binding induces signaling through multiple interacting intracellular pathways that include Src, MAP kinase (MAPK), signal transduction and activator of transcription (STAT), and phospholipase C (PLC), ultimately resulting in angiogenesis.
Multiple inhibitors of VEGF signaling are currently in preclinical or clinical development for patients with myeloid neoplasia, including MDS Citation[12]. As a group, these agents target the autophosphorylation and downstream signaling induced by VEGF binding to its receptors. Most of the agents in development are small-molecule receptor tyrosine kinase inhibitors, which have varying degrees of specificity for VEGF receptors, often cross-inhibiting c-kit and the platelet derived growth factor receptor. This broader inhibitory specificity makes it challenging to isolate true anti-VEGF treatment activity. Regardless, this class of drugs has yet to live up to its preclinical therapeutic promise in myeloid diseases, at least when used as single agents.
SU-5416 (semaxanib; small molecule inhibitor of VEGFR-2) is the only VEGF inhibitor to have completed Phase II trials in MDS and refractory AML, with occasional transient hematologic responses Citation[13] that were not enough to stave off discontinuation of its development; the more potent inhibitor SU-11248 (sunitinib; multi-targeted tyrosine kinase inhibitor) completed Phase I trials, induced a few brief remissions in AML patients Citation[14], and is now being focused at solid tumors. The Cancer and Leukemia Group B is currently studying vatalnib (PTK787/ZK222584; another broad-spectrum VEGF receptor antagonist) in MDS, despite the absence of objective responses in a Phase I trial enrolling high-risk MDS and relapsed/refractory/elderly AML Citation[15]. This study is still accruing patients (http://www.clinicaltrials.gov/ct/show/NCT00072475?order=15">). A few other small molecules are in earlier stages of development. Finally, the monoclonal antibody bevacizumab, which blocks VEGF binding to its receptors, has been studied in at least one small trial in MDS, with a few major erythroid responses Citation[16]. Given the extensive cross-talk and redundancy between VEGF signaling and other proliferative pathways, it appears likely that these agents will be most valuable when used in combination with chemotherapy or other inhibitors. The new study by Wimazal et al. Citation[1] suggests that patients with low-risk MDS might be the least likely to respond to VEGF inhibition, which has important implications for future trial design.
References
- Wimazal F, Krauth M T, Vales A, Böhm A, Agis H, Sonneck K, et al. Immunohistochemical detection of VEGF in the bone marrow in patients with myelodysplastic syndromes: correlation between VEGF expression and the FAB category. Leuk Lymphoma 2006; 47: 451–460
- Estey E H. Modulation of angiogenesis in patients with myelodysplastic syndrome. Best Pract Res Clin Haematol 2004; 17: 623–639
- Aguayo A, Giles F, Albitar M. Vascularity, angiogenesis and angiogenic factors in leukemias and myelodysplastic syndromes. Leuk Lymphoma 2003; 44: 213–222
- Pruneri G, Bertolini F, Soligo D, Carboni N, Cortelezzi A, Ferrucci P F, et al. Angiogenesis in myelodysplastic syndromes. Br J Cancer 1999; 81: 1398–1401
- Korkolopoulou P, Apostolidou E, Pavlopoulos P M, Kavantzas N, Vyniou N, Thymara I, et al. Prognostic evaluation of the microvascular network in myelodysplastic syndromes. Leukemia 2001; 15: 1369–1376
- Mesa R A, Hanson C A, Rajkumar S V, Schroeder G, Tefferi A. Evaluation and clinical correlations of bone marrow angiogenesis in myelofibrosis with myeloid metaplasia. Blood 2000; 96: 3374–3380
- Mesa R A, Hanson C A, Li C Y, Yoon S Y, Rajkumar S V, Schroeder G, Tefferi A. Diagnostic and prognostic value of bone marrow angiogenesis and megakaryocyte c-Mpl expression in essential thrombocythemia. Blood 2002; 99: 4131–4137
- Loges S, Heil G, Bruweleit M, Schoder V, Butzal M, Fischer U, et al. Analysis of concerted expression of angiogenic growth factors in acute myeloid leukemia: expression of angiopoietin-2 represents an independent prognostic factor for overall survival. J Clin Oncol 2005; 23: 1109–1117
- Moehler T M, Ho A D, Goldschmidt H, Barlogie B. Angiogenesis in hematologic malignancies. Crit Rev Oncol Hematol 2003; 45: 227–244
- Bellamy W T, Richter L, Sirjani D, Roxas C, Glinsmann-Gibson B, Frutiger Y, et al. Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood 2001; 97: 1427–1434
- Mangi M H. Misleading information about ALIP and VEGF in myelodysplasia. Blood 2001; 98: 1272–1273
- Gore S D. Inhibitors of signaling in myelodysplastic syndrome. Best Pract Res Clin Haematol 2004; 17: 613–622
- Giles F J, Stopeck A T, Silverman L R, Lancet J E, Copper M A, Hannah A L, et al. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood 2003; 102: 795–801
- Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann O G, O'Farrell A M, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood 2005; 105: 986–993
- Roboz G J, List A F, Giles F, Rae P E, Dugan M H, Greenberg J, et al. Phase I trial of PTK787/ZK 222584, an inhibitor of vascular endothelial growth factor receptor tyrosine kinases in acute myeloid leukemia and myelodysplastic syndrome. Blood 2002; 100: 337
- Gotlib J, Jamieson C HM, List A, Cortes J, Albitar M, Sridhar K, et al. Phase II study of bevacizumab (anti-VEGF humanized monoclonal antibody) in patients with myelodysplastic syndrome (MDS): preliminary results. Blood 2003; 102: 425