Abstract
This retrospective study compared effectiveness of (brentuximab vedotin) BV to other chemotherapies in patients with rrHL following an autologous stem cell transplant (ASCT). Data originated from a medical chart review of patients treated in real-world clinical settings at 50 sites in the United Kingdom and Germany. Inverse probability of treatment weights based on propensity scores were used to adjust for differences in baseline characteristics between treatment groups. Among 312 rrHL patients included, 196 received BV and 116 received physicians’ choice chemotherapy. Median PFS was significantly longer (27.0 months vs. 13.4 months; p = .0144) and 12-month OS survival greater (78.1% vs. 65.9%; p = .0129) with BV compared to chemotherapy. Documented adverse events included leukopenia (12.8%) and peripheral neuropathy (8.7%) for BV and leukopenia (12.1%), anemia (5.2%) and diarrhea (5.2%) for chemotherapy. In this real-world study, rrHL patients treated for relapse after ASCT with BV had longer median PFS and 12-month OS than patients receiving chemotherapy.
Introduction
Hodgkin lymphoma (HL) is a rare hematologic malignancy that is diagnosed at a rate of 2.3 cases per 100,000 patients per year in Europe and is most common in young adults and patients >60 years of age [Citation1]. Patients with HL are typically diagnosed at early disease stages and can achieve long-term remission after chemotherapy and radiotherapy; however, up to 20% of patients progress or relapse after initial treatment [Citation2–5]. The standard of care for patients with relapsed or refractory HL (rrHL) is high-dose chemotherapy followed by autologous stem cell transplant (ASCT), which is successful in inducing long-term remission in approximately 50% of patients [Citation6–8]. Prognosis for patients who fail to respond to high-dose chemotherapy or progress quickly after ASCT is particularly poor, with 71% of patients dying in year 1 and 90% by year 2, and a median post-progression survival of 1.3 years [Citation9,Citation10].
Brentuximab vedotin (BV) is an antibody-drug conjugate targeted to CD30, a cell surface marker that is highly expressed on malignant HL cells [Citation11,Citation12], with limited expression in healthy tissue and on resting leukocytes [Citation13,Citation14], making it a rational target for antibody-based therapies. BV is indicated for the treatment of CD30 + rrHL following ASCT (post-ASCT patients) or following at least two prior therapies in patients who are ineligible to receive an ASCT. In a pivotal phase-II trial, patients with rrHL receiving BV had a complete response (CR) rate of 34% and an overall response rate (ORR) of 75% [Citation15]. Median progression-free survival (PFS) was 5.6 months, and 89% and 41% of patients were alive at 12 months and 5 years, respectively [Citation15,Citation16]. Observational data from the BV Named Patient Program (NPP) found a CR rate of 19% and ORR of 74% in 43 post-ASCT patients with rrHL [Citation17]. While no randomized, head-to-head trials have compared BV to other therapeutic options for rrHL, a retrospective study found prolonged overall survival with BV (91.49 months) compared to a historical control group treated prior to the availability of BV (27.99 months) [Citation18]. Moreover, a recent systematic review of studies evaluating treatment of post-ASCT relapse found that five-year survival was highest in patients receiving BV in comparison to alternative interventions including chemotherapy, allogeneic stem cell transplant and repeat ASCT [Citation19,Citation20].
In the absence of randomized trials, retrospective, real-word medical chart data can be helpful in evaluating the comparative effectiveness of rrHL treatment options to inform treatment choices. The objective of this study was thus to describe outcomes and compare effectiveness in patients with rrHL post-ASCT receiving BV to those receiving other physicians’ choice chemotherapy in a real-world clinical setting in the United Kingdom and Germany. Further, to our knowledge, this is the first multisite real-world efficacy study of BV.
Materials and methods
We conducted a retrospective medical chart review study of patients with rrHL at 50 clinical sites in the United Kingdom and Germany. The study enrolled patients who were at least 18 years old at the time of HL diagnosis and received an ASCT for rrHL between 1 January 2008 and 30 June 2014. Surviving patients were required to have at least 12 months of follow-up from the initiation of post-ASCT treatment in order to be included in the study; patients who died within 12 months of ASCT were included. In addition, study investigators were required to certify that complete HL-related medical record history and treatment details were available for the patient from the time of HL diagnosis to most recent follow-up. Patients enrolled in an HL-related clinical trial during the study period were excluded from the study. Ethics approval for the study was granted by the Freiburg Ethics Commission International (FECI) in Germany and the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
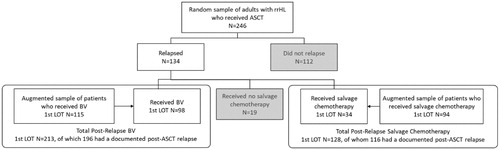
The study population consisted of patients who had relapsed after ASCT, who were selected using two methods. Patients who relapsed after ASCT were first selected from a random sample of all rrHL patients undergoing ASCT. Due to the orphan nature of rrHL, resulting in a small number of rrHL patients within the first sample, this cohort of patients was then augmented with a second random sample from the population of patients who relapsed after ASCT. These patient groups were combined to form the analytic sample for this study (). Patients without documentation of clinical characteristics at the time of post-ASCT relapse and those who did not receive any treatment following the relapse were excluded from the analysis.
Study investigators within the United Kingdom and Germany were hematologists and oncologists who were systematically screened and recruited. Specifically, every 46th and 42nd record in a database compiled from oncology organizations in the United Kingdom and GE, respectively, was contacted to determine eligibility to participate in the study. If the selected physician did not qualify and/or refused to participate in the study, the next record was contacted and screened for eligibility until the quota of eligible physicians was achieved. Physicians were required to contemporaneously manage or treat at least 10 patients with HL, at least five who have received an ASCT, and at least three who have been treated with BV.
Patients were selected at random by the study investigator who selected study subjects from the entire pool of eligible patients within their center. Each study investigator was assigned a random birth month and selected patients born in that month. A standardized case report form was developed for use by investigators to collect medical chart data, and was pilot tested prior to implementation. Data collected included demographic and baseline clinical characteristics, all prior regimens administered for the treatment of HL, best response to therapy as assessed by the study investigator and date of relapse for each treatment received, adverse events (AEs) occurring during each treatment regimen, dates of progression and death and healthcare resource utilization.
Patients in the United Kingdom and Germany were pooled for all analyses. Patients were grouped according to the first line of therapy received after post-ASCT relapse (BV, chemotherapy or no treatment). In the absence of randomization, inverse probability of treatment weights (IPTW) based on propensity scores were used to adjust for differences in demographics, clinical characteristics and other risk factors for relapse, [Citation8,Citation21–24] between patients receiving BV and those receiving chemotherapy [Citation25,Citation26]. IPTW was selected due to an insufficient sample size for direct matching based on propensity scores.
Standardized mean differences (SMD) between covariates were assessed before and after propensity score weighting using SAS. SMD ≥0.20, indicative of nonsignificant difference in patient characteristics between the BV and chemotherapy groups, was applied to ensure that characteristics were well balanced before and after weighting.
Clinical outcomes compared included best response to therapy (CR, partial response [PR], stable disease [SD] or progressive disease [PD]), progression-free survival (PFS) and overall survival (OS) as reported by the treating physician. Weighted Cox proportional hazards models were used to model survival outcomes, after validation of the proportionality assumption. AEs occurring during treatment were described and, due to the retrospective nature of the study, no assessment of causality was made.
Results
A total of 360 patients were included in the study (199 in the United Kingdom, 161 in Germany). Of these, 213 received BV (108 United Kingdom, 105 Germany), 128 received other treatments, (76 United Kingdom, 52 Germany), and 19 received no treatment for post-ASCT rrHL (15 United Kingdom, 4 Germany) (). Twenty-nine patients (17 in the BV group and 12 in the chemotherapy group) were excluded from the analysis due to insufficient documentation of the post-ASCT relapse, leaving a total of 312 patients (196 receiving BV, 116 receiving chemotherapy). Study investigators represented primarily hospital-based practice (100% in the United Kingdom, 90% in Germany) and were commonly affiliated with academic/teaching hospitals (60% in the United Kingdom, 70% in Germany).
Patients in the study were predominantly male, received ABVD-based front-line therapy and had bulky disease at the time of pre-ASCT relapse (). Prior to propensity score weighting, patients receiving BV as the first line of therapy following ASCT relapse were significantly more likely to receive radiotherapy in combination with first- or second-line chemotherapy and receive more than two lines of chemotherapy prior to ASCT. After propensity score weighting, there were no statistically significant differences in these or any other characteristics between groups.
Table 1. Patient characteristics before and after propensity score weighting.
Patients in the BV group received a median of 7.5 infusions (range, 5-11 infusions), while patients in the chemotherapy group received a median of four cycles (range, 3–6 cycles). In the physicians choice chemotherapy group, the most common regimens received in the first-line post-ASCT relapse were gemcitabine based (41.4%), CHOP (9.5%) and ICE (9.5%) (). Thirty-three patients in the BV group and 13 patients in the chemotherapy group later received an allogeneic stem cell transplant (allo-SCT). Patients receiving BV were more likely to be alive at 12 months compared than those on chemotherapy both among patients who did receive allo-SCT (97% in the BV group vs 83% in the chemotherapy group; p = .135) and those who did not (73% vs. 62%; p = .003).
Table 2. Physicians’ choice chemotherapy regimens used in study patients.
Best response to therapy
The overall response rate was 80.6% in patients receiving BV and 68.2% in patients receiving physician’s choice chemotherapy (p < .05; ). Complete response was similar in the BV (45.4%) and chemotherapy (44.2%) groups. Patients receiving BV were more likely to achieve partial response, and less likely to experience progressive disease, compared to patients receiving chemotherapy.
Table 3. Best response to treatment with BV or chemotherapy, propensity djusted.
Survival outcomes
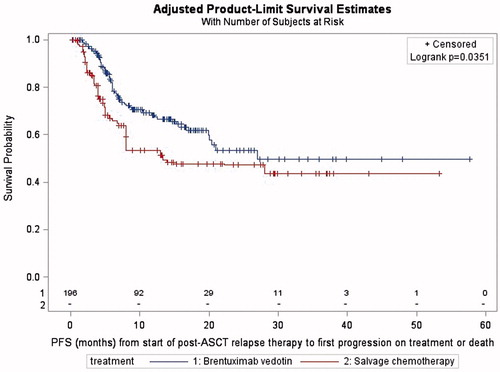
Patients were followed for a median of 10.7 months from the time of postrelapse therapy initiation to death or loss to follow-up (interquartile range [IQR], 6.2–17.7 months) in the BV group and 8.0 months (IQR, 4.0–22.1 months) in the chemotherapy group. The proportional hazards assumption was met for PFS. Adjusted median PFS was significantly longer in patients receiving BV (27.0 months) compared to those receiving chemotherapy (13.4 months; p = .0441; ).
The proportional hazards assumption was not met for OS, and thus, a hazard ratio was not calculated. Unadjusted median OS could not be estimated in the BV group due to more than 50% of patients being alive at the last follow-up. In the chemotherapy group, the median unadjusted OS was 29.9 months. The adjusted proportion of patients alive at 12 months after initiating BV or chemotherapy was significantly higher in patients receiving BV (78.1% vs. 65.9%; p = .0129).
Adverse events
The most common AEs in the BV group were leukopenia (12.8%), anemia (9.2%) and peripheral neuropathy (8.7%). Three of the 17 cases of peripheral neuropathy were judged by the study investigator to be serious and resulted in discontinuation of BV. In the chemotherapy group, 14 patients (12.1%) developed leukopenia and four required hospitalization for management of this AE ().
Table 4. Adverse events occurring during treatment.
Use of BV in later lines of therapy
Among the 116 patients in the chemotherapy group, eight patients received BV as the second line of therapy and one patient received BV as the third line of therapy following ASCT relapse. Overall response rate was 75% in second line (2 patients with CR, 4 with PR and 2 with SD) and 0% in third line (1 patient with SD). PFS from start of BV was 27.0 months in patients receiving BV as second- or third-line therapy following post-ASCT relapse chemotherapy.
Discussion
In this real-world retrospective observational study using medical chart data, patients with rrHL receiving BV post-ASCT in routine clinical care were less likely to experience progressive disease compared to patients receiving chemotherapy, with an overall acceptable safety profile. PFS was extended by 13.6 months in patients with BV compared to the chemotherapy group. ORR was higher in patients receiving BV as the first post-relapse line of therapy compared to those treated with chemotherapy followed by BV, but PFS from initiation of BV was similar in both lines of therapy.
Although median OS was not reached in the BV group, analyses of OS at 12 months post starting BV or chemotherapy, respectively, indicated that BV patients were more likely to survive to 12 months after initiation of therapy. CR with BV in this chart review study was achieved in 45.4% of patients demonstrating a higher response rate than the pivotal phase-II trial (34%) [Citation15]. With more than 5 years of follow-up from the pivotal phase-II trial, a median OS of 39.4 months in patients with PR and 18.3 months in patients with SD was reported; however, median OS has not been estimated in patients with CR due to 64% being alive at 5 years. In addition, 52% of patients achieving CR on BV were progression free at 5 years in this phase-II trial. However, the duration of follow-up in the present study (median, 10.7 months) was insufficient to identify a similar durability of response after a CR to that previously demonstrated in the clinical trial.
ORR in this study (80.6%) was higher than reported the pivotal phase-II trial (75%), despite patients receiving a median of 7.5 infusions compared to 9 in the prospective study. Moreover, PFS was longer in this study compared to the phase-II trial and reports from the BV NPP (median 27.0 vs. 5.6 months and 5–10.5 months, respectively) [Citation15,Citation17]. In addition to investigator versus centrally reviewed response assessment, these differences could also be explained by differences in the patient populations enrolled in this real-world study and the clinical trial: patients in the present study were more likely to have achieved a CR or PR with their most recent chemotherapy regimen (87.2%) compared to the phase-II trial (46%) and were less heavily pretreated (median of 2 vs. 3.5 prior regimens in the phase-II trial), probably indicative of less aggressive disease in the present cohort.
Among patients receiving BV in this study, peripheral neuropathy was reported in 8.7% of patients, an incidence considerably lower than the phase-II trial (55%) and the NPP (28–31%) [Citation16,Citation17]. However, this difference likely reflects the method of data collection in the present study, with investigators identifying AEs retrospectively from the medical chart rather than through monitored, prospective, direct examination of the patient and recording of the data at the time. Results are hence to be interpreted with caution.
To our knowledge, this is the first study comparing BV to chemotherapy regimens administered in real-world clinical practice. The chart review design allowed for the collection of detailed treatment, response and adverse event data by physician investigators who were experienced in the care of HL patients, limiting the amount of missing data. Finally, the study had a sufficient sample size to demonstrate potentially clinically significant differences in OS and PFS between BV and physicians’ choice chemotherapy for post-ASCT rrHL.
However, there are important limitations that must be considered when interpreting its results which firstly include that this was a retrospective study and patients were not randomized to treatment with BV or chemotherapy. The IPTW adjusts for observed differences between groups, but cannot adjust for any differences in characteristics not documented in the medical chart. There are also limitations to the surrounding AE data collection. Patients in the United Kingdom and Germany were pooled for analysis of clinical outcomes, but selection of front-line and postrelapse chemotherapy regimens differed between countries and could have influenced outcomes in the respective countries. Response to therapy was based on the study investigator’s assessment using the data in the medical chart, without specific criteria provided. Finally, the comparator group in this study was heterogeneous with respect to the chemotherapy administered, and thus, a conclusion regarding the relative efficacy of BV with any specific other therapeutic regimen cannot be made.
The results of this study suggest that rrHL patients receiving BV as the first line of therapy after post-ASCT relapse are more likely to achieve overall response and have longer PFS and better 12-month OS than patients receiving physicians’ choice chemotherapy regimens. In conclusion, and in the absence of randomized studies, this observational study provides an assessment of comparative effectiveness in a real-world setting.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1382698.
ICMJE Forms for Disclosure of Potential Conflicts of Interest
Download Zip (5 MB)Acknowledgments
This study was sponsored by Takeda Pharmaceutical Company. The authors would like to acknowledge Melissa Hagan, PhD, MPH, Associate Medical Director, Health Economics and Outcomes Research, Ogilvy CommonHealth Worldwide; Caitlyn Solem, PhD, Senior Director of Health Economics and Outcomes Research, Pharmerit International; and Pete Wolthoff, Director of Research Operations, Medical Data Analytics, for their contributions to this study.
Additional information
Funding
References
- Eichenauer DA, Engert A, Andre M, et al. Hodgkin’s lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii70–iii75.
- Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015;385:1418–1427.
- von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. JCO. 2012;30:907–913.
- Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607.
- Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799.
- Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–1072.
- Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054.
- Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol. 2002;20:221–230.
- Arai S, Fanale M, DeVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533.
- Arai S, Fanale M, deVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531--2533.
- Francisco JA, Cerveny CG, Meyer DL, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–1465.
- Wahl AF, Klussman K, Thompson JD, et al. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin’s disease. Cancer Res. 2002;62:3736–3742.
- Falini B, Pileri S, Zinzani PL, et al. ALK + lymphoma: clinico-pathological findings and outcome. Blood. 1999;93:2697–2706.
- Stein H, Foss HD, Durkop H, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–3695.
- Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–2189.
- Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128:1562–1566.
- Zinzani PL, Sasse S, Radford J, et al. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma: an updated review of published data from the named patient program. Crit Rev Oncol Hematol. 2016;104:65–70.
- Karuturi MS, Arai S, Chen RW, et al. Overall survival benefit for patients with relapsed hodgkin lymphoma treated with brentuximab vedotin after autologous stem cell transplant. Blood. 2012;120:3701.
- Bonthapally V, Wu E, Macalalad A, et al. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma post-autologous transplant: meta-analysis versus historical data. Curr Med Res Opin. 2015;31:993–1001.
- Zagadailov E, Halfpenny NJ, Taylor A, et al. Treatment outcomes of relapsed/refractory hodgkin’s lymphoma post-ASCT: a systematic review. Value Health. 2016;19:A139.
- Bröckelmann PJ, Müller H, Casasnovas O, et al. Risk factors and a prognostic score for progression free survival after treatment with autologous stem cell transplantation (ASCT) in patients with relapsed or refractory hodgkin lymphoma (RRHL). Blood. 2015;126:1978.
- Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623.
- Hahn T, McCarthy PL, Carreras J, et al. Simplified validated prognostic model for progression-free survival after autologous transplantation for hodgkin lymphoma. Biol Blood Marrow Transplant. 2013;19:1740–1744.
- Josting A, Rueffer U, Franklin J, et al. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood. 2000;96:1280–1286.
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424.
- Curtis LH, Hammill BG, Eisenstein EL, et al. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:(10 Supl 2):S103–107.