Abstract
Patients with mantle cell lymphoma (MCL) usually respond to initial combination chemotherapy, but the disease inevitably relapses and often follows an aggressive course. Here, clinical study results published since 2008 for patients with relapsed/refractory MCL were reviewed to compare available evidence for treatment guidance. Most trials identified were non-randomized, phase II studies performed at a limited number of sites, and many evaluated MCL as one of multiple non-Hodgkin lymphoma subtypes. Additional randomized, comparative trials are needed. Treatment selection generally depends on patient need, age and fitness, time of relapse, and line of therapy. Combination regimens typically produce higher response rates than single agents, and adding rituximab generally improves outcomes. The inclusion of ibrutinib, lenalidomide, temsirolimus, and bortezomib, represents an important advance for patients ineligible for, unable to tolerate, or failing high-intensity combination chemotherapy. A high need for effective treatments in relapsed/refractory MCL remains, particularly for elderly and frail patients.
Introduction
Mantle cell lymphoma (MCL) is a distinct histologic type of non-Hodgkin lymphoma (NHL), with a median age of 65 years at diagnosis and predominantly more aggressive course of disease [Citation1,Citation2]. Diagnosis is based on morphology and immunophenotype (CD20+, CD5+, CD23–, and FMC7+), but detection of chromosomal translocation t(11;14)(q13;q32) or the resulting cyclin D1 overexpression is mandatory [Citation3,Citation4]. Although technically categorized as an indolent form of NHL, MCL typically follows an aggressive clinical course and is considered incurable. The majority of patients receive treatment upon diagnosis, except for a small fraction of patients with very indolent disease identifiable by gene expression profiling [Citation5,Citation6] or low-risk characteristics and/or evolution of disease [Citation7,Citation8].
Median overall survival (OS) following initial induction therapy is 3–5 years with the use of dose-intense chemotherapy or combination therapy, incorporation of antilymphoma antibodies, and autologous stem cell transplantation [Citation1,Citation2,Citation9–11]. US guidelines issued by the National Comprehensive Cancer Network® (NCCN®) categorize induction based on aggressive versus less aggressive treatment [Citation12], whereas the European (EU) guidelines categorize induction based on the patient’s age (<65 versus ≥65 years) and status (fit versus frail) [Citation1,Citation2,Citation13]. Aggressive treatment in the US guidelines and treatment of fit patients ≤65 years of age in EU guidelines consist of high-dose chemoimmunotherapy followed by consolidation with high-dose therapy and autologous stem cell transplantation. R-hyperCVAD (rituximab combined with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine) is also recommended as an aggressive regimen in US but not EU guidelines. Less aggressive treatment in the US and treatment of fit, elderly patients in the EU consist of a conventional regimen such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or BR (bendamustine and rituximab).
Although MCL generally responds to initial treatment, the disease inevitably relapses, even after an intensive intervention [Citation14]. Treatment of relapsed or refractory disease, characterized by increasingly shorter periods of remission with successive lines of treatment and progression to more clinically aggressive phenotype, is challenging [Citation1,Citation2,Citation14,Citation15]. This review covers current treatment options in the relapsed/refractory setting, examining selection based on prior therapy, patient characteristics, performance status, and line of therapy. Conventional chemotherapy approaches as well as newer molecular-based therapies are included.
Methods
A review of the literature was carried out using PubMed to identify studies reporting clinical trial results in patients with relapsed/refractory MCL published between January 2008 and July 2017. Search terms included: title mantle cell lymphoma, publication type clinical trial, and language English. Abstracts from major conferences (American Society of Clinical Oncology [ASCO], American Society of Hematology [ASH], European Hematology Association [EHA], and International Conference on Malignant Lymphoma [ICML]) were also evaluated. Trials in previously untreated MCL patients, those with <10 MCL patients, pharmacokinetic studies, and publications such as letters to the editor were excluded, and the resulting list was focused on studies of key marketed and investigational agents. Studies were categorized as monotherapy, chemotherapy/chemoimmunotherapy, chemotherapy/immunotherapy combinations with molecular-based agents, and combination biologic therapies.
Study data
Chemoimmunotherapy
Chemoimmunotherapy trials in relapsed/refractory MCL are shown in , with key results discussed below [Citation16–26]. Many recommendations regarding use of chemoimmunotherapy to treat relapsed/refractory MCL are based on limited studies and few randomized comparative trials. The most commonly used combinations in this setting are CHOP and regimens containing bendamustine, cytarabine, or fludarabine in combination with rituximab. The addition of rituximab to chemotherapy regimens has shown improved OS [Citation27]. These combination regimens tend to be more appropriate in first and second relapse in younger patients and elderly fit patients. While they appear to produce higher response rates than monotherapy, few comparisons have been made in randomized trials.
Table 1. Chemotherapy and chemoimmunotherapy options: clinical trials with ≥10 patients.
The alkylating agent bendamustine in combination with rituximab (BR regimen) has high activity in relapsed/refractory MCL [Citation28]. Phase II findings with BR within this time period showed 92% overall response rate (ORR), 55% complete response (CR)/CR unconfirmed (CRu), and median duration of response (DOR) of 19 months in relapsed/refractory MCL [Citation17]. Subsequent studies of BR showed significantly prolonged progression-free survival (PFS), producing a significantly higher ORR compared with fludarabine plus rituximab (FR) in a phase III study conducted in patients with relapsed follicular, indolent, and mantle cell lymphomas [Citation18]. Among MCL patients who were randomized to BR or FR, ORR was 71% (38% CR) and 26% (13% CR), respectively, and median PFS was 17.6 and 4.7 months, respectively (p = .01). BR was also associated with improved OS (median 35.3 versus 20.9 months). Among all patients treated with BR, the most common grade 3/4 adverse events (AEs) were leukopenia (13%), neutropenia (9%), and nausea and emesis (4%). In a phase II study with relapsed or refractory MCL (median 2 prior therapies), BR treatment resulted in an 82% ORR (40% CR), median PFS of 17.2 months, and a 3-year OS rate of 55% [Citation19]. Among patients evaluated by positron emission tomography (PET) scan, complete metabolic response was observed in 75%. Grade 3/4 neutropenia and lymphopenia occurred in 44 and 89% of patients, respectively. Serious AEs occurred in 40% of patients, but only three patients withdrew from the study due to AEs. The use of BR in the treatment of relapsed/refractory MCL has increased steadily based on recent results from the multicenter, randomized, phase III non-inferiority StiL and BRIGHT studies, which reported significantly prolonged PFS and CR rates, respectively, in comparison to R-CHOP or R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone), in the small subsets of previously untreated MCL [Citation29–32].
Novel monotherapy treatment options for relapsed/refractory MCL
Pivotal trials of monotherapy options in relapsed/refractory MCL are shown in and results from key studies are discussed below [Citation33–54]. Multiple treatment options are available in this setting, but no clear standard of care is recognized in EU or US treatment guidelines [Citation1,Citation2,Citation12], ibrutinib, lenalidomide, and temsirolimus are approved in the EU [Citation55–57], whereas three molecular-based agents are approved by the US Food and Drug Administration: ibrutinib [Citation58], lenalidomide [Citation59], and bortezomib [Citation60].
Table 2. Targeted monotherapy options: clinical trials with ≥10 patients.
Ibrutinib, an inhibitor of Bruton’s tyrosine kinase (BTK), has been approved by the European Medicines Agency (EMA) for the treatment of adult patients with relapsed or refractory MCL and in the US for patients with MCL who have received at least one prior therapy [Citation56,Citation58]. Ibrutinib was evaluated in a phase II trial involving MCL patients with a median of three prior regimens [Citation33,Citation34]. ORR and CR rates improved over time on therapy, reaching 68 and 21%, respectively. For the entire cohort, median PFS was 13.9 months and median OS was not reached. Response rates did not differ between bortezomib-naïve versus pre-treated patients, although trends toward longer DOR and PFS were observed in patients who had received prior bortezomib. The most common grade 3/4 toxicities were neutropenia (16%) and thrombocytopenia (11%). The activity of ibrutinib in patients with MCL progressing after bortezomib therapy was confirmed in another phase II trial [Citation61].
Both primary and secondary resistance to ibrutinib in relapsed/refractory MCL has been observed [Citation33]. Two separate retrospective reviews reported poor outcomes for patients with ibrutinib-resistant MCL after subsequent salvage therapy, with a median OS of 5.8 [Citation62] and 8.4 months [Citation63] after ibrutinib cessation. Effective therapy for patients with relapsed MCL with ibrutinib resistance therefore represents an important unmet medical need.
Multiple phase II studies have been conducted with lenalidomide alone and in combination with rituximab (R2 regimen) [Citation35–42,Citation64]. The pivotal phase II MCL-001 (EMERGE) study in the US established the activity of lenalidomide in relapsed/refractory MCL, including patients who received prior bortezomib (median 4 prior regimens) [Citation35]. Lenalidomide provided an ORR of 28% (8% CR/CRu) by independent central review, with a median DOR of 16.6 months. Median PFS was 4.0 months, and median OS (longer-term follow-up) was 20.9 months [Citation36]. The most common grade 3/4 toxicities were neutropenia (43%) and thrombocytopenia (27%).
In the phase II, multicenter, open-label MCL-002 (SPRINT) pivotal trial in the EU, patients with relapsed or refractory MCL were randomized 2:1 to lenalidomide monotherapy or investigator’s choice (IC) monotherapy (rituximab, gemcitabine, fludarabine, chlorambucil, or cytarabine) [Citation37]. At a median follow-up of 15.9 months, lenalidomide significantly improved PFS compared with IC (median 8.7 versus 5.2 months, p = .004). ORR was 40% (5% CR/CRu) for lenalidomide and 11% (0% CR/CRu) for IC. Median OS (27.9 versus 21.2 months) also favored lenalidomide. The most common grade 3 or 4 AEs were neutropenia (44% versus 34%) without increased risk of infection, thrombocytopenia (18% versus 28%), leukopenia (8% versus 11%), and anemia (8% versus 7%) in the lenalidomide and IC groups, respectively. Analysis of subgroups and regression analyzes associated superior PFS with lenalidomide over IC therapy irrespective of prior treatment history [Citation65].
Temsirolimus, an mTOR inhibitor, was the first agent registered by the EMA for relapsed/refractory MCL [Citation55]. In a pivotal phase III study, temsirolimus was compared with investigator’s choice of therapy in 162 patients with relapsed/refractory MCL who had received a median of three prior regimens [Citation43]. Temsirolimus was administered at a dose of 175 mg weekly for 3 weeks followed by either 75 or 25 mg weekly. In the control arm, the investigators selected from prospectively approved options, most commonly gemcitabine and fludarabine. Patients receiving the higher dose of temsirolimus had significantly longer PFS than those in the investigator’s choice arm (4.8 versus 1.9 months; p = .0009); median OS did not differ significantly (12.8 versus 9.7 months; p = .35). The most frequent grade 3/4 toxicities with the higher dose of temsirolimus were thrombocytopenia (59%), anemia (20%), neutropenia (15%), and asthenia (13%). When patients were retrospectively classified according to the MCL International Prognostic Index, a validated predictor of survival [Citation66], the higher temsirolimus dose increased PFS by 7.9, 2.8, and 1.1 months compared with investigator’s choice therapy in low-, intermediate-, and high-risk groups, respectively.
An international, randomized, open-label, phase III study compared ibrutinib with temsirolimus in patients with relapsed or refractory MCL [Citation46,Citation47]. Temsirolimus was administered at a dose of 175 mg weekly for 3 weeks followed by 75 mg weekly. At a median follow-up of 39 months, ORR was significantly improved in the ibrutinib group (77% versus 47%, p < .0001), as was PFS (15.6 versus 6.2 months, p < .0001) [Citation47]. Ibrutinib was better tolerated, with lower rates of grade 3 or 4 thrombocytopenia (9% versus 42%), anemia (8% versus 20%), neutropenia (13% versus 17%), and fatigue (4% versus 7%) than temsirolimus, with fewer AEs leading to discontinuation (6% versus 26%) [Citation46]. Major bleeding was more frequent in the ibrutinib group (10% versus 6%), but after adjusting for longer ibrutinib exposure, event rates were similar.
In the pivotal phase II PINNACLE study, the proteasome inhibitor bortezomib was evaluated in patients with relapsed MCL who had received a median of one prior regimen [Citation48]. ORR was 32% and included 8% with CR/CRu [Citation49]. Median PFS was 6.5 months for all patients and median OS was 23.5 months. Toxicity was generally manageable; grade 3/4 lymphopenia occurred in 34% of patients. The most common grade 3/4 non-hematologic toxicity was peripheral neuropathy (13%). Four deaths occurred within 28 days of the last dose of bortezomib that were probably attributable to study drug (3 due to non-neutropenic sepsis, 1 due to respiratory failure).
Several newer investigational agents are noteworthy, and although clinical data are limited, they show promise for use in combination with other agents based on their mechanism(s) of action. Obinutuzumab (GA101) is a glycoengineered humanized anti-CD20 monoclonal antibody that exhibited superior activity compared with rituximab in MCL xenograft models [Citation67]. In the phase II GAUGUIN study, obinutuzumab was administered in a subset of MCL patients [Citation53]. Two different dosing regimens were evaluated: 1600 mg on days 1 and 8 of cycle 1 and 800 mg on day 1 of cycles 2–8, or 400 mg at all infusions. Best ORR with obinutuzumab for MCL patients was 4/15 (27%), with 2/15 (13%) reaching a CR/CRu and including response durations ranging from 5.5 to 30.5 + months. Obinutuzumab exhibited an acceptable safety profile for all patients; grade 3/4 infusion-related reactions occurred in 3 (8%). Obinutuzumab may represent an alternative to rituximab as a future option in combination therapies.
Idelalisib acts via the B-cell receptor (BCR) pathway and is a selective inhibitor of phosphatidylinositol 3-kinase delta isoform (PI3Kδ). In a phase I study, patients with relapsed or refractory MCL (median 4 prior treatments) were treated with 50–350 mg oral idelalisib once or twice daily [Citation54]. ORR was 40% (5% CR), and median PFS was 3.7 months (1-year PFS 22%). The most frequent grade 3/4 AEs were increased alanine aminotransferase (ALT; 20%), diarrhea (18%), increased aspartate aminotransferase (AST; 15%), and anorexia (15%), with 18% of patients discontinuing due to AEs. Due to limited efficacy, potential toxicity, and the availability of other targeted agents, the future of idelalisib in MCL is doubtful.
Venetoclax (ABT-199), a selective inhibitor of the anti-apoptotic protein BCL-2, received accelerated approval in the US as monotherapy for patients with chronic lymphocytic leukemia with 17p deletion and at least one prior therapy [Citation68], based on results of an uncontrolled phase II study [Citation69]. Although based on a small number of patients, this agent also showed a very high rate of response (75%) in relapsed/refractory MCL, comparable to the efficacy of BTK inhibitors, and would be an attractive option for this group of patients [Citation70].
Novel agents combined with chemoimmunotherapy
The addition of molecular-based agents such as ibrutinib, lenalidomide, temsirolimus, and bortezomib to chemoimmunotherapy may be appropriate for some patients, though careful attention for potential unexpected toxicity is important during initial assessments. These combinations are being examined in multiple phase II studies () [Citation71–80] some of which are described in detail below.
Table 3. Combined targeted and chemoimmunotherapy regimens: clinical trials with ≥10 patients.
Two studies have evaluated the combination of rituximab, lenalidomide, and bendamustine (R2-B) in MCL [Citation71,Citation72]. A phase I/II study in patients age >65 years (n = 51) with untreated stage II–IV MCL assessed induction with six cycles of R2-B followed by maintenance with ≤7 cycles of single-agent lenalidomide. Inclusion criteria allowed treatment with one cycle of chemotherapy or radiation therapy. Because of a high number of AEs (especially allergic and cutaneous reactions) in the phase I dose escalation, the protocol was amended to omit lenalidomide during the first cycle and to include corticosteroids and Pneumocystis carinii pneumonia prophylaxis in subsequent cycles. In the combined phase I and II parts of the study, 74% completed the induction phase and 24% of patients completed the maintenance phase. Best responses were 80% ORR and 64% CR/CRu. At a median follow-up of 31 months, the PFS was 42 months and the 3-year OS was 73%. Of concern, many patients had a grade 3–5 infection, including three patients with opportunistic infections, and 16% had a second primary malignancy. In a phase II study of second-line therapy, patients responding to R2-B induction received R2 consolidation, and those with CR or partial response (PR) were then treated with lenalidomide maintenance [Citation72]. In elderly patients (median age 70 years), ORR was 79% (55% CR) after consolidation, and 24-month PFS and OS rates were 43 and 67%, respectively. Toxicities were predominantly hematologic: grade 3/4 neutropenia (71% in induction/consolidation; 72% during maintenance) and grade 3/4 thrombocytopenia (14% in induction/consolidation; 7% during maintenance). Thirty-six percentage of responding patients achieved minimal residual disease (MRD) negativity in bone marrow following induction and consolidation.
A multicenter phase Ib study of temsirolimus in combination with rituximab-chemotherapy in patients with relapsed/refractory MCL reported ORRs of 56, 42, and 80% for the combination of temsirolimus with R-CHOP, RFC (rituximab, fludarabine, cyclophosphamide), and R-DHA (rituximab, dexamethasone, and high-dose cytarabine), respectively [Citation73]. The combination of temsirolimus with BR produced a 94% ORR (39% CR) and a median PFS of 22 months in a recent phase I/II study [Citation74]. In the full patient population, which included nine patients with follicular lymphoma, the most common grade 3/4 AEs were leukopenia (32%), neutropenia (24%), and thrombocytopenia (21%).
Most evidence exists for chemotherapy regimens combined with bortezomib. Based on encouraging first line data [Citation81], a multicenter, phase II study randomized patients with relapsed MCL who had received one treatment prior to CHOP or bortezomib (days 1 and 8) plus CHOP [Citation75]. The addition of bortezomib improved ORR (83% versus 48%), CR rates (35% versus 22%), median PFS (16.5 versus 8.1 months), and median OS (35.6 versus 11.8 months). The difference in OS was statistically significant (p = .01), although higher rates of grade 3/4 neutropenia (30% versus. 20%) in the bortezomib-CHOP arm translated into increased febrile neutropenia (39% versus 17%).
In patients unwilling or unable to receive first-line dose-intensive therapy, the combination RiBVD (rituximab, bendamustine, bortezomib, and dexamethasone; no maintenance) in a phase II study of newly diagnosed MCL patients >65 years of age has also shown notable efficacy (84% ORR with a 24-month PFS of 70% at a median follow-up of 52 months), but was hampered by prominent infectious complications [Citation80].
Molecular-based combinations
With the availability of novel agents and biologic therapy, treatment of relapsed/refractory MCL has moved toward a molecular-based approach rather than a high-dose, intense chemotherapy strategy. In addition to monotherapy as discussed above, several agents have been evaluated in combination mostly with rituximab () [Citation64,Citation82–92].
Table 4. Combined targeted regimens: clinical trials with ≥10 patients.
Ibrutinib was combined with rituximab in a single-center, phase II trial enrolling patients with relapsed/refractory MCL [Citation82]. An 88% ORR (44% CR) was reported, with atrial fibrillation (12%) being the only grade 3/4 AE occurring in more than one patient. One on-study death (septic shock) may have been treatment related. Recent report of the MCL6 PHILEMON phase II study from the Nordic Lymphoma Group showed that ibrutinib combined with R2 was active (83% ORR, 41% CR) and well tolerated in relapsed/refractory MCL patients, and was associated with molecular remission in approximately half of MRD-evaluable patients [Citation83]. The phase II AIM trial of ibrutinib and venetoclax in patients with relapsed or refractory MCL showed a 71% ORR (63% CR) at week 16, along with 80% of complete responders with negative bone marrow by flow cytometry [Citation84]. The 8-month PFS and OS were 74% and 81%, respectively. Although most AEs were grade 1/2, 25% of patients had grade 3/4 neutropenia and tumor lysis syndrome occurred in two patients requiring dose reduction of venetoclax from a 50–20 mg/day starting dose.
The feasibility of administering lenalidomide in combination with rituximab (R2 regimen) was demonstrated in a phase I/II study [Citation64]. In the phase II portion, R2 was given to MCL patients, producing an ORR of 57% (36% CR), with a median PFS and OS of 11.1 and 24.3 months, respectively. Patients who subsequently underwent stem cell transplantation, achieved a 100% CR. R2 was well tolerated, with the most common grade 3/4 toxicities being neutropenia (66%), lymphopenia (36%), leukopenia (30%), and thrombocytopenia (23%). Of note, a single-center phase II study of R2 in rituximab-resistant MCL, with oral lenalidomide daily for 8 weeks followed by four weekly treatments with rituximab, with lenalidomide treatment continued during and after rituximab, reported a 55% ORR, all CR/CRu, and a median PFS of 24.4 months. The regimen was well tolerated, with the most common grade 3/4 AE being neutropenia [Citation85]. Promising early data have been reported with temsirolimus/rituximab combinations [Citation88].
The combination of rituximab with bortezomib was evaluated in the BRIL06 study [Citation89]. Patients with relapsed/refractory MCL or marginal zone lymphoma not eligible for high-dose chemotherapy with stem cell transplant were treated with weekly bortezomib and standard rituximab for 4 weeks followed by two courses of four-weekly bortezomib alone. Among the MCL patients, ORR was 64% and 5-year PFS was 17%.
Treatment options
Treatments for younger patients without comorbidities
Nearly one-half of all MCL patients are younger (<65 years) with no comorbidities (i.e. fit) [Citation93]. In first relapse, the treatment goal in patients not receiving upfront transplant (and who are primary refractory or early relapsed patients) is to achieve the best possible remission as a bridge to either autologous or allogeneic transplant (). For transplant-ineligible patients, the objective is to obtain the best possible response to treatment to induce long-lasting remission. Patients with an early first relapse (i.e. within 1–2 years after initial therapy) are considered high risk. In this setting, the treatment of choice is chemoimmunotherapy, such as BR, FCR, R-BAC (bendamustine, rituximab, cytarabine), or targeted therapy, such as ibrutinib. Patients with longer remission (i.e. relapse >2 years after initial therapy) may be treated with a high-dose cytarabine-containing regimen in combination with rituximab. Ongoing clinical trials are exploring whether adding a molecular-based and immunomodulator agents, such as ibrutinib, lenalidomide, temsirolimus, and bortezomib to these chemoimmunotherapy regimens will improve outcome in younger patients.
Figure 1. Overview of treatment options in relapsed/refractory MCL based on patient age and fitness. allo: allogeneic; Ara-C: cytarabine; BR: bendamustine + rituximab; CIT: chemoimmunotherapy; clb: chlorambucil; MCL: mantle cell lymphoma; R-BAC: rituximab, bendamustine, cytarabine (Ara-C); SCT: stem cell transplantation.
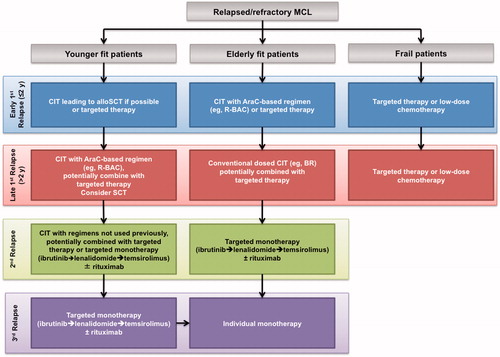
In second relapse (if allogeneic transplantation is not an option), the goal is to achieve the best possible remission with chemotherapy or molecular-based regimens not used in first- or second-line therapy, as tolerated by the patient. Treatment options in this setting include rituximab combined with ibrutinib, lenalidomide, temsirolimus, or bortezomib.
Disease control and palliation becomes the goal of treatment for subsequent relapses. In this setting, the selection of treatment depends on the reported efficacy but with a greater emphasis on minimizing toxicity and ease of administration. Accordingly, the highest unmet need among younger patients is better treatments for third and later relapses. If no cross-resistance has been demonstrated in earlier lines of therapy, monotherapy agents (± rituximab) may be considered for these patients.
Treatments for elderly fit patients
Approximately 40–50% of MCL patients are aged >65 years without significant comorbidities and are no longer candidates for transplantation. In first relapse, the treatment goal is to achieve and maintain the best response for the longest possible duration. The toxicity of the treatment needs to be taken into consideration, as these patients are generally not considered suitable for transplantation. Treatment regimens for these patients are generally similar to those for fit younger patients, including cytarabine-containing chemoimmunotherapy (e.g. R-BAC) in early first relapse, with possible addition of a targeted agent for elderly patients rather than allogeneic stem cell transplantation (SCT). Although no maintenance therapies are currently approved, clinical studies have explored the use of rituximab and lenalidomide with favorable results [Citation41,Citation94]. For late first relapse, a conventional dosing with chemoimmunotherapy with/without a targeted agent is employed.
In contrast to younger patients, elderly patients in second relapse are given targeted monotherapy (ibrutinib, lenalidomide, or temsirolimus) with/without rituximab. Alternatively, experimental therapies in a clinical study setting may be investigated. The highest unmet need in elderly, fit patients is for better, more active and tolerable treatment for second and third relapse.
Treatments for frail patients with comorbidities
Approximately 10% of MCL patients, irrespective of age, have significant comorbidities. In first relapse, the treatment goal is disease control and palliation to provide the best quality of life. Treatment options are limited, and combination chemotherapy is typically inappropriate. For patients in early relapse, low-dose chemotherapy (e.g. dose-reduced BR of chlorambucil/rituximab) or targeted monotherapy (ibrutinib, lenalidomide, or temsirolimus) with/without rituximab are considered. For patients with later relapse, the latter molecular-based monotherapy with/without rituximab may be more appropriate.
Conclusions
Although clinical guidelines for treatment of relapsed/refractory MCL are available in the EU and US, evidence supporting specific treatment options requires continued investigation. Most clinical trials in relapsed/refractory MCL are small, non-randomized, phase II studies conducted at a single center, or limited numbers of sites. In many cases, MCL was included as one of multiple NHL subtypes evaluated. Few phase III studies were completed in relapsed/refractory MCL. Clearly, more clinical studies of this type are needed to investigate promising phase II findings more rigorously and to determine whether rationally designed combination regimens offer improved efficacy and safety compared with monotherapy.
Combination regimens have generally provided higher response rates than single agents but often cause increased toxicity, making them difficult to use in elderly, frail, or heavily pre-treated patients. The addition of rituximab to other treatments has generally proven beneficial to patient outcome. However, many patients are not eligible for combination chemotherapy regimens, typically those with significant comorbidities or a life expectancy less than 6 months; younger, fit patients in third relapse; elderly, fit patients in second relapse; and patients with chemotherapy-refractory disease. For such patients, monotherapy with newer molecular-based agents, such as ibrutinib, lenalidomide, temsirolimus, and bortezomib represent an appropriate treatment choice. These agents can also be combined with rituximab or steroids based on promising responses in multiple clinical trials. Oral agents may be preferred in this setting as patients can be treated at home. Thus, despite considerable advances, a high unmet need for effective treatments remains, particularly in the elderly and frail patient populations.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1403602.
ICMJE Forms for Disclosure of Potential Conflicts of Interest
Download Zip (14.4 MB)Acknowledgements
Editorial support for this manuscript was provided by Bio Connections LLC, which was funded by Celgene Corporation.
References
- Dreyling M, Geisler C, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii83–iii92.
- Dreyling M, Jerkeman M, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2017;28(Suppl 4):iv62–iv71.
- Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390.
- Avivi I, Goy A. Refining the mantle cell lymphoma paradigm: impact of novel therapies on current practice. Clin Cancer Res. 2015;21:3853–3861.
- Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–197.
- Fernandez V, Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res. 2010;70:1408–1418.
- Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209–1213.
- Abrahamsson A, Albertsson-Lindblad A, Brown PN, et al. Real world data on primary treatment for mantle cell lymphoma: a Nordic Lymphoma Group observational study. Blood. 2014;124:1288–1295.
- Chandran R, Gardiner SK, Simon M, et al. Survival trends in mantle cell lymphoma in the United States over 16 years 1992-2007. Leuk Lymphoma. 2012;53:1488–1493.
- Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–518.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN® Guidelines) for Non-Hodgkin's Lymphoma V.3.2017. © National Comprehensive Cancer Network, Inc. 2017. All rights reserved. Accessed September 5, 2017. To view the most recent and complete version of the guideline, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
- McKay P, Leach M, Jackson R, British Committee for Standards in H, et al. Guidelines for the investigation and management of mantle cell lymphoma. Br J Haematol. 2012;159:405–426.
- Goy A, Kahl B. Mantle cell lymphoma: the promise of new treatment options. Crit Rev Oncol Hematol. 2011;80:69–86.
- Trneny M, Klener P, Belada D, et al. The outcome of mantle cell lymphoma patients after treatment failure and prognostic value of secondary mantle cell international prognostic index (sec MIPI). Blood. 2014;124:Abstract 4425.
- Ohmachi K, Ando K, Ogura M, et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101:2059–2064.
- Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4473–4479.
- Rummel M, Kaiser U, Balser C, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol. 2016;17:57–66.
- Czuczman MS, Goy A, Lamonica D, et al. Phase II study of bendamustine combined with rituximab in relapsed/refractory mantle cell lymphoma: efficacy, tolerability, and safety findings. Ann Hematol. 2015;94:2025–2032.
- Visco C, Finotto S, Zambello R, et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol. 2013;31:1442–1449.
- Garbo LE, Flynn PJ, MacRae MA, et al. Results of a Phase II trial of gemcitabine, mitoxantrone, and rituximab in relapsed or refractory mantle cell lymphoma. Invest New Drugs. 2009;27:476–481.
- Gironella M, Lopez A, Merchan B, et al. Rituximab plus gemcitabine and oxaliplatin as salvage therapy in patients with relapsed/refractory mantle cell lymphoma. Blood. 2012;120:Abstract 1627.
- Obrador-Hevia A, Serra-Sitjar M, Rodriguez J, et al. Efficacy of the GemOx-R regimen leads to the identification of Oxaliplatin as a highly effective drug against Mantle Cell Lymphoma. Br J Haematol. 2016;174:899–910.
- Moita F, Santos J, da Silva MG. Fludarabine-based combination chemotherapy in relapsed mantle cell lymphoma – the experience of a single center. Haematologica. 2012;97:Abstract 0256.
- Coleman M, Martin P, Ruan J, et al. Low-dose metronomic, multidrug therapy with the PEP-C oral combination chemotherapy regimen for mantle cell lymphoma. Leuk Lymphoma. 2008;49:447–450.
- Wang M, Fayad L, Cabanillas F, et al. Phase 2 trial of rituximab plus hyper-CVAD alternating with rituximab plus methotrexate-cytarabine for relapsed or refractory aggressive mantle cell lymphoma. Cancer. 2008;113:2734–2741.
- Schulz H, Bohlius JF, Trelle S, et al. Immunochemo-therapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99:706–714.
- Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:3383–3389.
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–2952.
- Flinn IW, van der Jagt R, Chang J, et al. First-line treatment of iNHL of MCL patients with BR or R-CHOP/R-CVP: results of the BRIGHT 5-year follow-up study. Hematol Oncol. 2017;101:140–141. Abstract 131.
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210.
- Rummel MJ, Maschmeyer G, Ganser A, et al. Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent lymphomas: nine-year updated results from the StiL NHL1 study. J Clin Oncol (ASCO Annual Meeting Abstracts) 2017;35:abstract 7501.
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516.
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739–745.
- Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31:3688–3695.
- Goy A, Kalayoglu Besisik S, Drach J, et al. Longer-term follow-up and outcome by tumour cell proliferation rate (Ki-67) in patients with relapsed/refractory mantle cell lymphoma treated with lenalidomide on MCL-001(EMERGE) pivotal trial. Br J Haematol. 2015;170:496–503.
- Trneny M, Lamy T, Walewski J, et al. Lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol. 2016;17:319–331.
- Stefoni V, Pellegrini C, Gandolfi L, et al. Lenalidomide in pretreated mantle cell lymphoma patients: an Italian observational multicenter retrospective study in daily clinical practice, the Lenamant study. Blood. 2015;126: Abstract 3946. (ASH proceedings)
- Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol. 2011;22:1622–1627.
- Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24:2892–2897.
- Eve HE, Carey S, Richardson SJ, et al. Single-agent lenalidomide in relapsed/refractory mantle cell lymphoma: results from a UK phase II study suggest activity and possible gender differences. Br J Haematol. 2012;159:154–163.
- Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349.
- Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829.
- Hoy SM, McKeage K. Temsirolimus: In relapsed and/or refractory mantle cell lymphoma. Drugs. 2010;70:1819–1829.
- Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514.
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387:770–778.
- Rule S, Jurczak W, Jerkeman M, et al. Ibrutinib vs temsirolimus: three-year follow-up of patients with previously treated mantle cell lymphoma from the phase 3, international, randomized, open-label RAY study. Hematol Oncol. 2017;101:143–144. Abstract 134.
- Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874.
- Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525.
- O'Connor OA, Moskowitz C, Portlock C, et al. Patients with chemotherapy-refractory mantle cell lymphoma experience high response rates and identical progression-free survivals compared with patients with relapsed disease following treatment with single agent bortezomib: results of a multicentre Phase 2 clinical trial. Br J Haematol. 2009;145:34–39.
- O'Connor OA, Portlock C, Moskowitz C, et al. Time to treatment response in patients with follicular lymphoma treated with bortezomib is longer compared with other histologic subtypes. Clin Cancer Res. 2010;16:719–726.
- Gerecitano J, Portlock C, Moskowitz C, et al. Phase 2 study of weekly bortezomib in mantle cell and follicular lymphoma. Br J Haematol. 2009;146:652–655.
- Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2912–2919.
- Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3Kδ inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. 2014;123:3398–3405.
- TORISEL (temsirolimus) prescribing information. Kent, United Kingdom: Pfizer Limited; 2017.
- IMBRUVICA (ibrutinib) prescribing information. Belgium: Janssen-Cilag International NV; 2017.
- REVLIMID (lenalidomide) prescribing information. United Kingdom: Celgene Europe Limited; 2017.
- IMBRUVICA (ibrutinib) prescribing information. Sunnyvale (CA): Pharmacyclics, Inc.; 2017.
- REVLIMID (lenalidomide) prescribing information. Summit (NJ): Celgene Corporation; 2017.
- VELCADE (bortezomib) prescribing information. Cambridge (MA): Millennium Pharmaceuticals, Inc.; 2017.
- Wang M, Goy A, Martin P, et al. Efficacy and safety of single-agent ibrutinib in patients with mantle cell lymphoma who progressed after bortezomib therapy. Blood (ASH Proceedings). 2014;124:Abstract 4471.
- Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127:1559–1563.
- Cheah CY, Chihara D, Romaguera JE, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol. 2015;26:1175–1179.
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13:716–723.
- Trneny M, Lamy T, Walewski J, et al. Impact of prior treatment on PFS for relapsed/refractory mantle cell lymphoma patients randomized to lenalidomide vs investigator's choice: a subgroup analysis of the phase II MCL-002 (SPRINT) study. Haematologica (EHA Meeting Proceedings). 2015;100:Abstract 4.
- Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565.
- Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402.
- VENCLEXTA (venetoclax) prescribing information. North Chicago (IL): AbbVie Inc.; 2016.
- Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–778.
- Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826–833.
- Albertsson-Lindblad A, Kolstad A, Laurell A, et al. Lenalidomide-bendamustine-rituximab in untreated mantle cell lymphoma >65 years, the Nordic Lymphoma Group phase I + II trial NLG-MCL4. Blood. 2016;128:1814–1820.
- Zaja F, Ferrero S, Stelitano C, et al. Second-line rituximab, lenalidomide, and bendamustine (R2B) in mantle cell lymphoma: a phase II clinical trial of the Fondazione Italiana Linfomi. Haematology. 2017;102:e203–e206.
- Le Gouill S, Bouabdallah K, Cartron G, et al. A multicenter phase Ib dose escalation study to evaluate the safety, feasibility and efficacy of the temsirolimus (Torisel™)-CHOP-rituximab (T-R-CHOP), temsirolimus (Torisel™)-FC-rituximab (T-R-FC) and temsirolimus (Torisel™)- DHA-rituximab (T-R-DHA) for the treatment of patients with relapsed/refractory mantle cell lymphoma (MCL): a LYSA study. Blood. 2014;124:Abstract 4426.
- Hess G, Keller U, Scholz CW, et al. Safety and efficacy of temsirolimus in combination with bendamustine and rituximab in relapsed mantle cell and follicular lymphoma. Leukemia. 2015;29:1695–1701.
- Furtado M, Johnson R, Kruger A, et al. Addition of bortezomib to standard dose chop chemotherapy improves response and survival in relapsed mantle cell lymphoma. Br J Haematol. 2015;168:55–62.
- Kouroukis CT, Fernandez LA, Crump M, et al. A phase II study of bortezomib and gemcitabine in relapsed mantle cell lymphoma from the National Cancer Institute of Canada Clinical Trials Group (IND 172). Leuk Lymphoma. 2011;52:394–399.
- Gerecitano J, Portlock C, Hamlin P, et al. Phase I trial of weekly and twice-weekly bortezomib with rituximab, cyclophosphamide, and prednisone in relapsed or refractory non-Hodgkin lymphoma. Clin Cancer Res. 2011;17:2493–2501.
- Ruan J, Martin P, Coleman M, et al. Durable responses with the metronomic rituximab and thalidomide plus prednisone, etoposide, procarbazine, and cyclophosphamide regimen in elderly patients with recurrent mantle cell lymphoma. Cancer. 2010;116:2655–2664.
- Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28:418–423.
- Gressin R, Daguindau N, Tempescul A, et al. First line treatment by the RiBVD regimen elicits high clinical and molecular response rates and prolonged survival in elderly MCL patients; final results of the LYSA group trial. Hematol Oncol. 2017;101:141–142. Abstract 132.
- Robak T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372:944–953.
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17:48–56.
- Jerkeman M, Hutchings M, Raty R, et al. Ibrutinib-lenalidomide-rituximab in patients with relapsed/refractory mantle cell lymphoma: first results from the Nordic Lymphoma Group MCL6 (PHILEMON) phase II trial. Blood (ASH Annual Meeting Abstracts). 2016;128:Abstract 148.
- Tam CS, Roberts AW, Anderson MA, et al. Combination ibrutinib and venetoclax for the treatment of mantle cell lymphoma: primary endpoint assessment of the phase 2 AIM study. J Clin Oncol. 2017;35:Abstract 7520.
- Chong EA, Ahmadi T, Aqui NA, et al. Combination of lenalidomide and rituximab overcomes rituximab resistance in patients with indolent B-cell and mantle cell lymphomas. Clin Cancer Res. 2015;21:1835–1842.
- Morrison VA, Jung SH, Johnson J, et al. Therapy with bortezomib plus lenalidomide for relapsed/refractory mantle cell lymphoma: final results of a phase II trial (CALGB 50501). Leuk Lymphoma. 2015;56:958–964.
- Zaja F, De Luca S, Vitolo U, et al. Salvage treatment with lenalidomide and dexamethasone in relapsed/refractory mantle cell lymphoma: clinical results and effects on microenvironment and neo-angiogenic biomarkers. Haematologica. 2012;97:416–422.
- Ansell SM, Tang H, Kurtin PJ, et al. Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: a phase 2 study. Lancet Oncol. 2011;12:361–368.
- Chiappella A, Pregno P, Zinzani PL, et al. The combination of weekly infusion of rituximab and bortezomib is effective in relapsed or refractory indolent and mantle cell lymphoma: long-term results of phase II BRIL06 study of the Fondazione Italiana Linfomi (FIL). Blood. 2015;126:Abstract 2735.
- Baiocchi RA, Alinari L, Lustberg ME, et al. Phase 2 trial of rituximab and bortezomib in patients with relapsed or refractory mantle cell and follicular lymphoma. Cancer. 2011;117:2442–2451.
- Agathocleous A, Rohatiner A, Rule S, et al. Weekly versus twice weekly bortezomib given in conjunction with rituximab, in patients with recurrent follicular lymphoma, mantle cell lymphoma and Waldenström macroglobulinaemia. Br J Haematol. 2010;151:346–353.
- Lamm W, Kaufmann H, Raderer M, et al. Bortezomib combined with rituximab and dexamethasone is an active regimen for patients with relapsed and chemotherapy-refractory mantle cell lymphoma. Haematologica. 2011;96:1008–1014.
- Harel S, Delarue R, Ribrag V, et al. Treatment of younger patients with mantle cell lymphoma. Semin Hematol. 2011;48:194–207.
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520–531.
