Abstract
Healthcare costs are rising due to an increase in chronic diseases, including chronic myeloid leukemia (CML) due to improved survival. In CML care, patient adherence and physician adherence are key elements. We assessed the potential health gain and cost savings when both are improved, using a decision analytic model that integrated various sources of evidence. The current situation was compared to a theoretical situation in which either patient or physician adherence is improved, in terms of costs and quality-adjusted life years (QALYs). Current patient adherence rate is 74%, improvement to 100% resulted in 0.1031 QALYs gained and a saving of €17,509 per patient over a 25-year period. Improving physician adherence from 72% to 100%, resulted in 0.0380 QALYs and €7606. Enhancement of either adherence results in substantial health gain and cost savings. Regarding the rising healthcare costs, new strategies should focus on improving adherence to keep healthcare affordable in the future.
Introduction
The advent of tyrosine kinase inhibitors (TKIs) has drastically improved the treatment of patients with chronic myeloid leukemia (CML). The 10-year overall survival in newly diagnosed CML patients in chronic phase (CML-CP) has improved from historically less than 20% to a current 83.3% when treated with the first approved TKI imatinib [Citation1,Citation2]. As a consequence, the prevalence of CML is steadily rising worldwide and is estimated to reach a plateau at approximately 35 times its incidence by 2050, which would lead to a total of more than 180,000 patients in the United States [Citation3]. The increasing prevalence will result in an increase in costs and health care utilization.
In the current CML care, two elements are of major importance, with the first being physicians’ adherence to the international guidelines of the National Comprehensive Cancer Network (NCCN) and European Leukemia Network (ELN) [Citation4,Citation5]. Multiple studies, however, have shown that guideline adherence in CML care is often suboptimal [Citation6–8].
Preliminary results from a national population-based study showed inadequate monitoring in the first year of TKI treatment in 28% of the newly diagnosed CML-CP patients in the Netherlands, whereas patients who received optimal molecular monitoring had a significant higher overall survival [Citation9]. This is consistent with other studies, showing that 54% of the patients were monitored less than 3 times a year and had a significantly higher risk of progression to advanced phase disease and death [Citation10].
Second, adherence by patients is crucial and a daily challenge in CML management. In the literature, mean adherence rates ranged from 79% to 98% and only small percentages of patients were perfectly adherent whilst many were suboptimal adherent [Citation11–13]. Suboptimal adherence, defined as an intake of less than 90% of the TKIs, is strongly correlated with lower rates of achieving therapeutic goals such as complete cytogenetic response and major and complete molecular responses (MMR and CMR) in patients treated with imatinib [Citation13–15]. This is associated with a higher rate of progression [Citation13,Citation16]. Main reasons described for low adherence are side effects and forgetfulness, resulting in intentional and nonintentional nonadherence, respectively [Citation17–21]. It is clear that better adherence by both the physician and the patient is associated with better outcomes. However, the actual quantitative impact of improving both physician and patient adherence on CML patients as well as on health care costs has not been established yet. Hence, the aim of this study was to assess the potential health benefit and costs of (1) improving patient adherence to TKIs and (2) improving guideline adherence by physicians.
Methods
Population
The current assessment included patients in the Netherlands diagnosed in CML-CP. This comprises approximately 95% of the newly diagnosed patients with CML, resulting in around 160 new patients per year in the Netherlands [Citation22].
Model structure
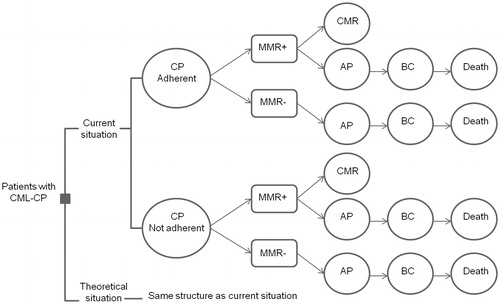
We developed a state transition model to assess the potential value of improving both patient adherence to TKIs and physician adherence to guidelines. A representation of the model structure with different steps is presented in . In the model, costs and effects are estimated for two strategies: the current situation and a situation in which either patient adherence or physician adherence is improved. The potential of improvement of the specific adherence is represented by the difference between the improved and the current situation. In both strategies, patients start in CP. Since adherence rate influences the treatment response, in the model adherence is related to the probability of reaching MMR. Subsequently, whether a patient achieves MMR determines the probability of disease progression to accelerated phase or blast crisis (BC), which results in a higher mortality rate. However, the achievement of MMR can also be followed by a CMR. When CMR is maintained for at least 1 year, in some of the patients TKI treatment could be discontinued; called treatment-free remission. For both patient adherence and physician adherence, the same model structure was used, though with different probabilities. To every state, a certain quality of life (QOL) (utility) value and costs were assigned.
Figure 1. Structure of the model and its steps. CP: chronic phase; AP: accelerated phase; BC: blast crisis; CMR: complete molecular response; MMR+: major molecular response (on 18 months in model for patient adherence, and 12 months in model for guideline adherence); MMR−: no major molecular response (on 18 months in model for patient adherence, and 12 months in model for guideline adherence).
Utilities
Health-related QoL was the outcome measure in this model and is expressed as a utility value on a scale from 0 (representing death) to 1 (representing perfect health). The use of utility scores allows the calculation of quality-adjusted life years (QALYs) [Citation23]. Utility values for patients in CP and in disease progression (AP and BC) are described: 0.854 and 0.595, respectively [Citation24].
Costs
Two types of costs were included in the model: costs of treatment and costs of hospital visits. The volumes of use differ in the several phases of CML. Cost prices for polymerase chain reaction (PCR), TKIs, stem cell transplantation (SCT), hematologist visits and hospital days are shown in Appendix Table 4. Since the price of generic imatinib is negotiable, sensitivity analyses were performed in which the price of imatinib varied. The assumptions made are described in Appendix A.
Probabilities: patient adherence
Adherence and outcome. Patient adherence to TKIs was defined as an intake of more than 90% of the prescribed medication. The probability that a patient is adherent to TKIs is 73.6% [Citation16]. Adherence influences the chance of reaching MMR at 18 months: the probability is 57.8% in adherent patients versus 8.7% in nonadherent patients. This association is only reported for imatinib and no data are available for adherence to TKIs used in the second-line, such as dasatinib and nilotinib, in relation to treatment outcomes. Therefore, we assumed the same difference as for imatinib in the probability of reaching MMR between adherent and nonadherent patients.
Progression into accelerated phase. The probability of progression from CP to the next phase in the disease, i.e. accelerated phase (AP), is reported to be 0.9% in patients who have reached MMR at 18 months. When MMR at 18 months is not achieved, this probability increases to 9.9% [Citation25]. In case of progression, it is likely to happen within the first 3 years after the 18-month response period. Therefore, we assumed a linear progression rate in these first 3 years.
Progression into BC. In case of progression, we assumed that patients went in AP before going into BC. This probability is 75% [Citation26] and we assumed an average of 12 months for progression to take place. BC has a high mortality: at 3 years after diagnosis of BC, 76% of the patients has died [Citation27]. The median survival after BC diagnosis is 9 months. For the model, we assumed an average survival of 1 year (Appendix Table 1). The other part of BC patients comprises long-time survivors which were assumed to have a survival equal to that of the general population (Appendix Table 2) [Citation28].
Achieving CMR. The probability that patients will reach CMR is also influenced by patients’ adherence. Adherent patients have a 6-year probability of 43.8% to achieve CMR, whereas no nonadherent patients will reach CMR [Citation16]. We conservatively assumed that after 6 years the chance to achieve CMR is zero. The probabilities to achieve CMR per year are shown in Appendix Table 1. Patients that stay in CP, AP or reach CMR were assumed to have a survival which is equal to that of the general population (Appendix Table 2) [Citation28].
Probabilities: physician adherence
Physician adherence was defined as adherence to the ELN guidelines [Citation4]. In these guidelines, a series of specific time-dependent molecular treatment response criteria are established; for the first 3 years of treatment they are defined as a BCR-ABL1 of ≤10%, ≤1%, and ≤0.1% (MMR) at 3, 6, and 12 months, respectively. When these response criteria are not met and patient adherence is optimal, the TKI received as current treatment should be substituted with an alternative TKI. In the Netherlands, a rate of patients receiving adequate molecular monitoring of 72% was observed [Citation9].
Adherence and outcome. To determine the value of improving physician adherence, we compared a nonadherent situation versus an adherent situation. For the first and nonadherent situation, we used a data from the IRIS trial [Citation25], in which patients used only imatinib and could not switch to second-line TKI if they failed the targets. In this study, 40% of the patients reached MMR at 12 months [Citation29], which we assumed to be an indicator for the probability of reaching MMR in a nonadherent situation.
For the second and adherent situation, we used data from the TIDEL-II study [Citation30]. When treatment outcomes were not reached, patients switched to a second-line TKI. At 12 months, 64% reached MMR. The difference in MMR could be partially due to the higher dose of imatinib (600mg vs. 400mg in IRIS study) and not only to the possibility of switching TKIs. This could result in an overestimation of the difference in MMR between the adherent and nonadherent situation. Therefore, we performed a sensitivity analysis in which we used data from the TIDEL-I study for the nonadherent situation. In this study patients received 600 mg of imatinib and could not switch to a second line TKI in case of low response. MMR at 12 months was reached by 47%. However, in this study patients did get a dose escalation (to 800 mg) in case of low response and this could, therefore, result in an underestimation of the effect of adherence.
Progression. The effect of achieving MMR on the probability of disease progression at 12 months is similar to that at 18 months [Citation25]. Therefore, we used the same probabilities as we used for patient adherence, based on achievement of MMR at 18 months instead of 12 months.
Achieving CMR. When only imatinib was used, i.e. the nonadherent situation (IRIS study), 30% of the patients reached CMR after 5 years [Citation16]. In the TIDEL-II study, i.e. the adherent situation, the 5-year probability of CMR was 50%. Hence, we assumed a difference in probability of achieving CMR of 20% in patients who underwent adequate versus inadequate monitoring. For the sensitivity analysis, where 47% MMR of the TIDEL-I study as non-adherent situation was used instead of the 40% from the IRIS study, we also included a lower difference in the probability of achieving CMR (14% instead of 20%). Since the CMR rate was not shown in the TIDEL-I study, this reduction in CMR was in proportion to the difference in MMR between the IRIS and TIDEL-I study.
An overview of the probabilities is shown in Appendix Table 3.
Analysis
Using the decision analytic model, we compared the current situation with a theoretical situation in which either patient adherence or physician adherence was perfect (100%). Additionally, we calculated the value of each percentage point increase in adherence. The outcomes costs and effects were defined in euros and QALYs, respectively. They were determined both per patient and over a total of 160 new patients per year. Since no costs were assigned for a new strategy in the theoretical situation, the differences in costs represent the room for investment in a new strategy. That is, the costs that could be made in order to improve adherence without making CML care more expensive. Future costs and effects were discounted at 4%, and 1.5% annually, following the Dutch guideline for economic evaluation [Citation31]. Sensitivity analyses with different prices of imatinib were performed for both patient adherence and physician adherence. A sensitivity analysis with a different percentage of patients reaching MMR (and a difference in probability of reaching CMR) in the non-adherent situation was performed for physician adherence.
Results
Patient adherence
In current clinical practice, 73.6% of the patients are adherent [Citation16]. Over a period of 25 years, in the current situation patients yielded on average 13.83 QALYs and the mean costs per patient were €422,066. Compared to this, perfect adherence resulted in a gain of 0.1031 QALYs and a saving of €17,509 per patient (). Over 25 years, for each yearly cohort of patients, improving adherence could result in a total cost saving of approximately €2.8 million and a gain of 16.5 QALYs maximum.
Table 1. Room for improvement in patient adherence over 25 years.
Each percentage point increase in patient adherence resulted in 0.0039 QALYs gained and a saving of €666 per patient. Over 25 years, for each yearly cohort, this resulted in a total cost reduction of €106,560 and gain of 0.624 QALY per percentage point improvement in adherence (). When the price of imatinib is reduced by 90%, in a situation with 100% patient adherence, sensitivity analyses showed that potential cost saving was approximately halved: €9037 ().
Table 2. Gain for each percentage point increase in patient adherence over 25 years.
Physician adherence
The current physician adherence to guidelines regarding adequate molecular monitoring in the Netherlands is 72% [Citation9]. The current situation yields 13.95 QALY and mean costs of €452,578 per patient over a period of 25 years. When comparing the situation where adherence is 100%, 0.0380 QALYs were gained and €7606 was saved per patient. Over the time period of 25 years and with a total of 160 patients per year this would result in a potential cost saving of €1.2 million and a gain of 6.08 QALYs ().
Table 3. Room for improvement in physician adherence over 25 years.
Each percentage point increase in adherence resulted in 0.0014 QALYs gained and a saving of €272 per patient. The total potential cost reduction, for each yearly cohort, was €43,520 (). In the case of a 90% price reduction of imatinib, in a situation with 100% adherence, sensitivity analyses showed a potential cost saving of €607 per patient (). These results were based on the difference in MMR rate at 12 months between the IRIS study (as nonadherent situation, 40% MMR) and the TIDEL-II study (as adherent situation, 64% MMR). Sensitivity analyses were performed with the results of the TIDEL-I study representing the nonadherent situation (47% MMR), resulting a gain in QALY of 0.0223 and saving €5323 per patient with 100% adherence.
Table 4. Gain for each percentage point increase in physician adherence over 25 years.
Table 5. Sensitivity analysis.
Discussion
For patient adherence, the total room for improvement in costs is approximately €2.8 million for each yearly cohort of 160 patients over a time period of 25 years with a potential gain of 16.496 QALYs. For guideline adherence, the potential improvement in costs was €1.2 million with a potential gain of 6.08 QALYs. Given the reported adherence rates in international studies of patients to TKIs, with only 20–53% of the patients fully (i.e. 100%) adherent and most patients being suboptimal adherent [Citation11], current therapy may not be used to its full potential. With the estimated gain in costs and QALYs per percentage point increase in adherence, one could argue that exploring strategies to improve adherence to current therapies and thereby enhancing their potentials should be of main focus.
In the model, several assumptions had to be made. First, the difference between adherent and nonadherent patients in treatment response has only been studied in patients using imatinib [Citation16]. This difference in response between adherent and nonadherent patients could be different for second-generation TKIs. It is difficult to predict whether this could have resulted in an overestimation or underestimation of the potential value of increasing adherence. Secondly, since physician adherence has never been directly compared to physician nonadherence there are no other data available than of indirect comparisons. Therefore, we compared data of the IRIS trial [Citation32] with data of TIDEL-II trial [Citation30]. In this indirect comparison, other factors such as variety in population could play a role and may have resulted in an under- or overestimation of the potential value of improving physician adherence. The higher dose of imatinib used in the TIDEL-II trial may have resulted in an overestimation of the potential value. Hence, we performed sensitivity analyses with results of the TIDEL-I study instead of the IRIS trial, resulting in a lower potential value of improving physician adherence and a potential underestimation. Additionally, at the time of developing the model preliminary results showed a rate of suboptimal monitoring 2% higher than the final published study [Citation22], which could lead to an overestimation of the potential. Third, we assumed the possibility of discontinuing TKIs in patients in CMR. Yet, currently, a more often used indicator is the deep molecular response (DMR). However, there are no data available on the influence of physician and patient adherence on the probability of achieving DMR. DMR is a less strict definition compared to CMR; therefore, patients are more likely to achieve DMR than CMR resulting in a possible underestimation of the potential. Last, although side effects are often reported by patients as a reason for nonadherence in general, there are no data available on the direct influence of side effects of TKIs on the utility (i.e. QALY) of patients. Therefore, the model does not cover the negative influence of possible side effects on QALYs. By increasing TKI adherence patients could experience more side effects and have a decrease in QALY gain. Nevertheless, the decrease in side effects when TKI therapy can be discontinued has not been included in the model either. Since TKI adherence is crucial in achieving treatment goals and subsequently eligibility for treatment discontinuation, the actual health benefits of improving adherence could be higher.
A possible solution may come from e-Health technology. With forgetfulness being the main reason of unintentional nonadherence, investments in e-Health interventions that, for example, remind patients to take their medication, could provide a straightforward solution.
Since side effects are the main cause of intentional nonadherence, adequate management on reported adverse events is a necessity. Strategies to educate patients on their treatment and possible adverse events and help them manage these using, for instance, e-Health technologies would be worth exploring. By using the assessments as performed in this study one can determine the room for investment by computing the costs savings that come with improved adherence. The number of QALYs gained per patient may not seem that high, however, this was calculated for our whole annual cohort of CML patients, whereas only the nonadherent patients will benefit in QALYs.
Improving physician adherence can save costs and gain QALYs as well. Reported reasons of nonadherence include lack of familiarity, medical resource barriers in implementation as well as high costs of TKI medication and other patient resources [Citation33]. Here too technology can help to improve physician adherence by providing educational programs to improve familiarity and facilitate implementation of guidelines in daily practice [Citation33]. As for patient resources as barrier, the high costs of TKIs will not decrease by developing new ones but by using the ones with generic variants available. Nevertheless, in the same study that explored the reasons of nonadherence to CML guidelines by surveying hematologist-oncologists, additional trainings or efforts to facilitate guideline adherence were not considered important by physicians. This calls for a culture change in CML management by healthcare professionals.
At the time of writing, generic imatinib just became available at a lower price than Gleevec® [Novartis, Basel, Switzerland]. Sensitivity analyses were performed with price reductions up to 90% for generic imatinib and still costs were saved by improving either patient or physician adherence. Except in case of a 99% reduction, where full physician adherence costs money (€93 per patient) because of an increase in TKI switches and hence use of next-line TKIs. Developments in therapies will produce new and patented medication. Which, despite a possible earlier achievement of treatment goals, should demonstrate an advantage in survival rate in order to be prescribed instead of the cheaper generic alternative. This might be quite the challenge since the most recent update of the CML-IV study reported a 10-year relative survival probability of 92% and a 10-year CML-mortality of 6% in patients receiving first-line imatinib [Citation34]. With the survival rates of CML-CP patients approaching the survival rates of the general population the question is raised how many patients one needs to treat in order to attain an advantage in survival over the current TKIs. Besides, even when current TKI therapy is not molecularly monitored at all and subsequently no adjustments are made in case of failure, 3-year progression-free survival was 83% [Citation10]. The current study did not make a direct comparison between the costs savings and QALY gain between improved adherence, either patients’ or physicians’, and new generation TKIs though. In future research, newer generation TKIs should be evaluated through this model as soon as data on probabilities are known. It should include the sensitivity analyses for price reductions through generic alternatives as performed in the current study, combined with the probabilities of sustained molecular remission and discontinuation of TKI treatment. Furthermore, the scope of the model could be broadened, including the probabilities of adverse events of newer generation TKIs, which may be more serious than with current therapies.
With the increasing prevalence of CML and other chronic diseases, healthcare costs and utilization in general are rapidly rising. In this light, the importance of assessments as performed in this study is emphasized in order to determine whether investments in improving adherence seem worthwhile and whether they could be cost-effective. Furthermore, improving adherence, both patients’ and physicians’, show great potential in health-care cost reduction and gained QALYs. Further research on development of interventions improving adherence should be conducted. The results of this study in combination with the current literature demonstrate the gaps in the current care that need to be addressed in order to be able to keep providing the best care possible in the near future.
Potential conflict of interest Disclosure forms provided by the authors are available with the full text of this article online at http://dx.doi.org/10.1080/10428194.2018.1535113.
ICMJE Form for Disclosure of Potential Conflicts of Interest
Download PDF (1.2 MB)Supplementary information
Download MS Word (22.7 KB)References
- Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–927.
- Kantarjian H, O'Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119:1981–1987.
- Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123–3127.
- Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884.
- O'Brien S, Berman E, Borghaei H, et al. NCCN clinical practice guidelines in oncology: chronic myelogenous leukemia. J Natl Compr Canc Netw. 2009;7:984–1023.
- Goldberg SL. Monitoring chronic myeloid leukemia in the real world: gaps and opportunities. Clin Lymphoma Myeloma Leuk. 2015;15:711–714.
- Guerin A, Chen L, Dea K, et al. Association between regular molecular monitoring and tyrosine kinase inhibitor therapy adherence in chronic myelogenous leukemia in the chronic phase. Curr Med Res Opin. 2014;30:1345–1352.
- Saleh MN, Haislip S, Sharpe J, et al. Assessment of treatment and monitoring patterns and subsequent outcomes among patients with chronic myeloid leukemia treated with imatinib in a community setting. Curr Med Res Opin. 2014;30:529–536.
- Geelen ITN, Janssen J, Roosma T, et al. P. CML treatment in hospitals with low patients volumes is associated with a substandard quality of molecular response monitoring. Dutch Hematology Congress; 2016 January; Arnhem, The Netherlands; 2016.
- Goldberg SL, Chen L, Guerin A, et al. Association between molecular monitoring and long-term outcomes in chronic myelogenous leukemia patients treated with first line imatinib. Curr Med Res Opin. 2013;29:1075–1082.
- Hall AE, Paul C, Bryant J, et al. To adhere or not to adhere: rates and reasons of medication adherence in hematological cancer patients. Crit Rev Oncol Hemat. 2016;97:247–262.
- Kekale M, Talvensaari K, Koskenvesa P, et al. Chronic myeloid leukemia patients’ adherence to peroral tyrosine kinase inhibitors compared with adherence as estimated by their physicians. Patient Prefer Adherence. 2014;8:1619–1627.
- Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401–5411.
- Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2014;123:494–500.
- Latremouille-Viau D, Guerin A, Gagnon-Sanschagrin P, et al. Health care resource utilization and costs in patients with chronic myeloid leukemia with better adherence to tyrosine kinase inhibitors and increased molecular monitoring frequency. J Manag Care Spec Pharm. 2017;23:214–224.
- Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388.
- Breccia M, Efficace F, Sica S, et al. Adherence and future discontinuation of tyrosine kinase inhibitors in chronic phase chronic myeloid leukemia. A patient-based survey on 1133 patients. Leuk Res. 2015;39:1055–1059.
- Breccia M, Efficace F, Alimena G. Adherence to treatment is a complex and multifaceted issue that can substantially alter the outcome of chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Leuk Res. 2012;36:804–805.
- Efficace F, Baccarani M, Rosti G, et al. Investigating factors associated with adherence behaviour in patients with chronic myeloid leukemia: an observational patient-centered outcome study. Br J Cancer. 2012;107:904–909.
- Eliasson L, Clifford S, Barber N, et al. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35:626–630.
- Rychter A, Jerzmanowski P, Hołub A, et al. Treatment adherence in chronic myeloid leukaemia patients receiving tyrosine kinase inhibitors. Med Oncol. 2017;34:104.
- Geelen IGP, Thielen N, Janssen J, et al. Treatment outcome in a population-based, ‘real-world’ cohort of patients with chronic myeloid leukemia. Haematologica. 2017;102:1842–1849.
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programme. 3rd ed. Oxford: Oxford University Press; 2005.
- Reed SD, Anstrom KJ, Ludmer JA, et al. Cost-effectiveness of imatinib versus interferon-alpha plus low-dose cytarabine for patients with newly diagnosed chronic-phase chronic myeloid leukemia. Cancer. 2004;101:2574–2583.
- Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010;116:3758–3765.
- Rochau U, Sroczynski G, Wolf D, et al. Cost-effectiveness of the sequential application of tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. Leuk Lymphoma. 2015;56:2315–2325.
- Hehlmann R. How I treat CML blast crisis. Blood. 2012;120:737–747.
- Central Bureau of Statistics (CBS): StatLine, Sterfte; geslacht, leeftijd [Internet]. 2014. Available from: http://statline.cbs.nl/StatWeb/publication/?DM=SLNL&PA=37530NED&D1=1&D2=101-120&D3=0&D4=l&HDR=T,G1&STB=G2,G3&VW=T.
- Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432.
- Yeung DT, Osborn MP, White DL, et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood. 2015;125:915–923.
- Roijen LH, Tan SS, Bouwmans CAM. Handleiding voor kostenonderzoek: methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. Diemen: College Voor Zorgverzekeringen; 2010.
- Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006; 355:2408–2417.
- Goldberg SL, Akard LP, Dugan MJ, et al. Barriers to physician adherence to evidence-based monitoring guidelines in chronic myelogenous leukemia. J Oncol Pract. 2015;11:e398–e404.
- Hehlmann R, Lauseker M, Saußele S, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–2406.
