Abstract
Outcomes for adults with relapsed/refractory acute lymphoblastic leukemia (ALL) are poor with chemotherapy, particularly in later salvage. The TOWER study examined survival, remission, bridge to allogeneic hematopoietic stem cell transplantation (HSCT), and safety with blinatumomab versus chemotherapy. This report examined outcomes separately for study treatment as first or later salvage. Adults with Philadelphia chromosome-negative B-cell precursor ALL relapsed/refractory to chemotherapy were randomly assigned 2:1 to receive blinatumomab by continuous infusion for 4 weeks in 6-week cycles, or standard salvage chemotherapy. Overall survival for blinatumomab versus chemotherapy was higher both in first salvage and in later salvage. Safety was similar between patients in first salvage and those in later salvage. Blinatumomab as later salvage was associated with higher complete remission rates and served as a bridge to allogeneic HSCT, supporting the use of blinatumomab in both settings. This study is registered at www.clinicaltrials.gov as #NCT02013167.
Introduction
Patients with relapsed/refractory acute lymphoblastic leukemia (ALL) who are treated with standard of care chemotherapy have poor prognosis. After relapse, 31–42% of adults with B-cell precursor (BCP) ALL achieve complete hematologic remission with chemotherapy in first salvage [Citation1–7], 18–25% in second salvage [Citation1,Citation2,Citation8], and 11% in third or greater salvage [Citation1]. One-year survival rates for BCP-ALL after first, second, and third or greater salvage with chemotherapy are 26%, 18%, and 15%, respectively [Citation1].
Blinatumomab is a bispecific T-cell engager immunotherapy that binds simultaneously to CD3-positive cytotoxic T cells and to CD19-positive B cells [Citation9–11]. In single-arm phase 2 studies, blinatumomab induced hematologic remission in the treatment of heavily pretreated, relapsed or refractory, Philadelphia chromosome-negative (Ph–) precursor BCP-ALL [Citation12,Citation13]. The randomized, international phase 3 TOWER study compared blinatumomab and standard of care chemotherapy in salvage treatment of relapsed/refractory Ph– BCP-ALL. Blinatumomab treatment resulted in significantly longer survival and significantly higher rates of hematologic remission compared with chemotherapy [Citation14]. Patients in the blinatumomab group with hematologic remission were also more likely than those in the chemotherapy group to be minimal residual disease (MRD)-negative (76% and 48%, respectively). Twenty-four percent of patients in each treatment group received allogeneic hematopoietic stem cell transplantation (HSCT) after study treatment, including 14% and 9% of patients, respectively, in the blinatumomab and chemotherapy groups who achieved hematologic remission and did not receive another anti-leukemic treatment before allogeneic HSCT.
Approximately half of the patients in the TOWER study received blinatumomab or chemotherapy as first salvage (i.e. no prior salvage at study entry), and the other half received blinatumomab or chemotherapy as second or later salvage (i.e. any prior salvage at study entry). This analysis compared study outcomes for blinatumomab versus chemotherapy, delivered either as first or later salvage.
Materials and methods
Study design and patients
The TOWER study design and primary study results were published previously [Citation14]. In this prospective, randomized, phase 3, international study, investigators enrolled adults (18 years of age or older) with Ph– BCP-ALL that was refractory to chemotherapy, in first relapse after chemotherapy with a first remission <12 months, in second or greater relapse after chemotherapy, or in relapse at any time after allogeneic HSCT. Patients had >5% blasts in bone marrow and Eastern Cooperative Oncology Group (ECOG) performance status of ≤2. Key exclusion criteria included other active cancers, a clinically relevant pathologic condition of the central nervous system, isolated extramedullary disease, autoimmune disease, acute graft-versus-host disease (GVHD) of grade ≥2 or active chronic GVHD, allogeneic HSCT within 12 weeks before randomization, autologous transplantation within 6 weeks before randomization, chemotherapy or radiotherapy within 2 weeks before randomization, use of immunotherapy within 4 weeks before randomization, or ongoing use of investigational treatment. Each patient provided informed consent. An investigational review board or independent ethics committee approved the study design for each study center.
Treatments
Patients were randomly assigned in a 2:1 ratio to receive open-label treatment with either blinatumomab or chemotherapy, stratified by age (<35 versus ≥35 years), previous salvage (yes versus no), and previous allogeneic HSCT (yes versus no). The study sponsor prepared the randomization schedule, which was implemented by the study sites using an interactive voice response system. After two cycles of induction therapy, patients in remission (≤5% bone marrow blasts) could receive up to three cycles of consolidation therapy and up to four cycles of maintenance therapy.
Blinatumomab induction and consolidation were administered in 6-week cycles, consisting of continuous infusion for 4 weeks (9 μg/day during week 1 of induction cycle 1, and 28 μg/day thereafter) and no treatment for 2 weeks. Blinatumomab maintenance was administered in 12-week cycles, with continuous infusion for 4 weeks (28 µg/day) and no treatment for 8 weeks. Patients in the blinatumomab group with high tumor load during screening (>50% bone marrow blasts or peripheral blood blast count ≥15,000/μL) received dexamethasone for up to 21 days before the first infusion to prevent cytokine release syndrome. All patients in the blinatumomab group received dexamethasone within one hour before each infusion to prevent infusion reactions. Intrathecal prophylaxis for central nervous system disease was given according to institutional or national guidelines. Interruption or discontinuation of blinatumomab infusion was required if neurologic events or other selected adverse events occurred.
Patients in the chemotherapy group received the investigator’s choice of one of the following regimens: fludarabine, high-dose cytosine arabinoside, and granulocyte colony-stimulating factor with or without anthracycline; a high-dose cytosine arabinoside-based regimen; a high-dose methotrexate-based regimen; or a clofarabine-based regimen. Chemotherapy could be discontinued at any time after the first treatment cycle. The patient could subsequently undergo allogeneic HSCT if determined to be in the patient’s best interest per investigator discretion. Chemotherapy dose adjustment was permitted but was not required for specific events.
Assessments
Complete hematologic remission was defined as ≤5% bone marrow blasts and no evidence of disease, as follows: CR, full hematologic recovery (platelet count >100,000/µL and absolute neutrophil count >1000/µL); CRh, partial hematologic recovery (platelet count >50,000/µL and absolute neutrophil count >500/µL); or CRi, incomplete hematologic recovery (either platelet count >100,000/µL or absolute neutrophil count >1000/µL). MRD was measured by a central laboratory at the end of each treatment cycle using flow cytometry for centers in the United States and Canada (64 patients), and polymerase chain reaction for other centers (341 patients), with a minimum sensitivity of 0.01%. MRD response was defined as <0.01% leukemic cells and complete MRD response was defined as the absence of detectable leukemia. Adverse events were graded with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Adverse events of interest, which were identified by a steering committee, included neutropenia, infection, elevated liver enzyme, neurologic event, cytokine release syndrome, infusion reaction, and lymphopenia.
Statistical analysis
Endpoints included overall survival (time from randomization to death from any cause), complete hematologic remission within 12 weeks after initiation of treatment, complete MRD response, event-free survival (EFS; time from randomization until relapse after CR/CRh/CRi or death; patients who did not achieve remission were assigned an EFS of 0 months), duration of remission (CR/CRh/CRi), rate of subsequent allogeneic HSCT, and adverse events. Efficacy analyses included randomized patients (intention-to-treat population). Safety analyses included patients who received at least one dose of trial treatment (as-treated population). Remission rates were compared by stratified two-sided Cochran–Mantel–Haenszel’s tests. Survival estimates were calculated by the Kaplan–Meier method and treatment groups were compared by two-sided stratified log-rank tests. Treatment effect was expressed as a hazard ratio with a 95% confidence interval, estimated by a stratified Cox regression model.
Each analysis was conducted separately in patients who received study treatment either in first salvage or in second or later salvage. Due to discrepancies observed between the randomization stratification variable for salvage in the primary analysis [Citation14] and investigator-reported data for salvage therapy, a panel of three medical experts with in-depth knowledge of the trial adjudicated the number of prior salvage regimens. Two experts reviewed the treatment and disease histories and the third expert reconciled any discrepancy between the other two. Overall concordance between the investigator-reported strata for randomization and the adjudicator-reported results was 89% in the blinatumomab group (6% were stratified as first salvage and adjudicated as second or later salvage; 5% were stratified as second or later salvage and adjudicated as first salvage) and 90% in the chemotherapy group (2% were stratified as first salvage and adjudicated as second or later salvage; 8% were stratified as second or later salvage and adjudicated as first salvage). Results are presented according to the expert adjudication for number of prior salvage regimens.
Results
Study population and treatment
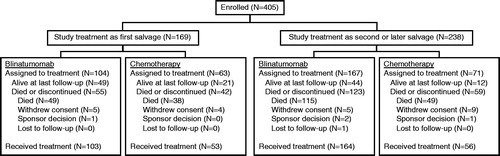
Intention-to-treat analyses for efficacy included 167 patients (104 blinatumomab, 63 chemotherapy) in first salvage and 238 patients (167 blinatumomab, 71 chemotherapy) in second or later salvage (). As-treated analyses for safety included 156 patients (103 blinatumomab, 53 chemotherapy) in first salvage and 220 patients (164 blinatumomab, 56 chemotherapy) in second or later salvage (). Patients were less likely to withdraw consent after randomization to open-label blinatumomab treatment than to open-label chemotherapy, either as first salvage (4.8% [five of 104] versus 6.3% [four of 63], respectively) or as second or later salvage (3.0% [five of 167] versus 12.7% [nine of 71], respectively).
Demographic and baseline characteristics were balanced between the blinatumomab and chemotherapy groups, both in first salvage and in second or later salvage (). In both treatment groups, patients in second or later salvage were younger, and they were more likely to be male, have ECOG performance status scores greater than 0, have refractory disease, and to have received previous allogeneic HSCT compared with patients in first salvage.
Table 1. Demographic and baseline characteristics.
After first salvage, allogeneic HSCT was administered to a similar proportion of patients in the blinatumomab and chemotherapy groups (), both overall (28.8% versus 30.2%; p = .89), and in continuous hematologic remission (CR/CRh/CRi) without subsequent anti-leukemic therapy (15.4% versus 17.5%; p = .71). In first salvage, allogeneic HSCT was administered both pre-study and on-study to 3.8% of patients in the blinatumomab group and 4.8% of patients in the chemotherapy group.
Table 2. Patient incidence of postbaseline allo-HSCT.
After second or later salvage, allogeneic HSCT was administered to a similar proportion of patients in the blinatumomab and chemotherapy groups overall (21.0% versus 18.3%; p = .64) (), and to more patients in continuous hematologic remission from blinatumomab versus chemotherapy without subsequent anti-leukemic therapy (13.2% versus 1.4%; p = .004). In second or later salvage, allogeneic HSCT was administered both pre-study and on-study to 6.3% of patients in the blinatumomab group and 8.5% of patients in the chemotherapy group.
Overall survival
Median overall survival for first salvage was 11.1 months for blinatumomab (95% CI, 8.2 months to not estimable) and 5.5 months for chemotherapy (95% CI, 3.7–9.0 months), for a hazard ratio of 0.59 (95% CI, 0.38–0.91; p = .016; ). Median overall survival for second or later salvage was 5.1 months for blinatumomab (95% CI, 3.2–7.1 months) and 3.0 months for chemotherapy (95% CI, 2.1–4.0 months), for a hazard ratio of 0.72 (95% CI, 0.52–1.01; p = .055; ).
Figure 2. Outcomes by line of salvage. (A) Overall survival. (B) Event-free survival (patients without remission were assigned an event-free survival of one day). (C) Duration of remission (includes only patients who achieved remission). (D) Overall survival by MRD response and by line of salvage in the blinatumomab group, in a landmark analysis starting at day 82. CI: confidence interval; HR: hazard ratio; K–M: Kaplan–Meier; MRD–: MRD response; MRD+: MRD non-response; NE: not estimable; NR: not reached; S1: first salvage; S2+: second or later salvage; +, censored patients.
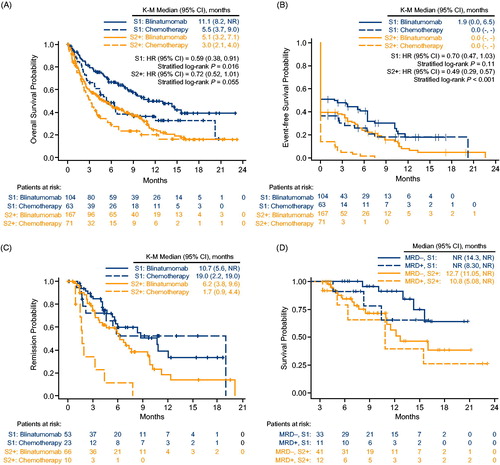
Similar results were obtained for median overall survival after censoring for post-baseline allogeneic HSCT. Median overall survival for first salvage, censored for allogeneic HSCT, was 9.6 months for blinatumomab (95% CI, 7.0–15.6 months) and 4.5 months for chemotherapy (95% CI, 3.7–6.4 months), for a hazard ratio of 0.49 (95% CI, 0.31–0.78; p = .002). Median overall survival for second or later salvage, censored for allogeneic HSCT, was 4.7 months for blinatumomab (95% CI, 3.2–7.1 months) and 2.9 months for chemotherapy (95% CI, 2.1–4.0 months), for a hazard ratio of 0.71 (95% CI, 0.49–1.02; p = .065).
Among patients who achieved CR/CRh/CRi, median overall survival censored at HSCT was not reached by the end of the study for either blinatumomab first salvage (95% CI, 10.0 months to not estimable) or chemotherapy first salvage (95% CI, 5.3 months to not estimable) (Figure S1). In second or later salvage, median overall survival was 12.3 months for blinatumomab (95% CI, 7.6–15.0 months) and 5.0 months for chemotherapy (95% CI, 0.9 months to not estimable; Figure S1).
Minimal residual disease
In first salvage, complete hematologic remission (CR/CRh/CRi) rates during the first 12 weeks were 51.0% for blinatumomab and 36.5% for chemotherapy (p = .069; ). Among patients who achieved CR/CRh/CRi with first salvage, complete MRD response was achieved by 26 of 53 (49.1%) patients in the blinatumomab group and nine of 23 (39.1%) patients in the chemotherapy group (p = .53; ). In second or later salvage, complete hematologic remission (CR/CRh/CRi) rates during the first 12 weeks were 39.5% for blinatumomab and 14.1% for chemotherapy (p < .001; ). Among patients who achieved CR/CRh/CRi with second or later salvage, complete MRD response was achieved by 32 of 66 (48.5%) patients in the blinatumomab group and one of 10 (10.0%) patients in the chemotherapy group (p = .008; ).
Table 3. Best hematologic response and minimal residual disease response within 12 weeks of treatment initiation.
In the blinatumomab group, overall survival was longer for MRD responders than for MRD non-responders, with a greater difference in first salvage than in second or later salvage (). In first salvage, median overall survival was not reached in the blinatumomab group, either for MRD responders (95% CI, 14.3 months to not estimable) or for MRD nonresponders (95% CI, 8.3 months to not estimable). In second or later salvage, median overall survival in the blinatumomab group was 12.7 months for MRD responders (95% CI, 11.1 months to not estimable) and 10.8 months for MRD nonresponders (95% CI, 5.1 months to not estimable). This landmark analysis started at day 82 (i.e. after scheduled MRD assessment); no patient in the blinatumomab group died before day 82 and was excluded from analysis. In the chemotherapy group, the number of MRD responders (13 in first salvage and 3 in second or later salvage) was too small for a meaningful analysis of overall survival by MRD response.
Event-free survival and duration of remission
In first salvage, median EFS was 1.9 months for blinatumomab (95% CI, 0.0–6.5 months) and 0.0 months for chemotherapy (a majority of chemotherapy patients did not achieve remission and were assigned an EFS of 0 months) (). Estimated EFS at 6 months was 40.8% for blinatumomab (95% CI, 31.0–50.3%) and 25.9% for chemotherapy (95% CI, 15.3–37.8%), and the hazard ratio for EFS was 0.70 (95% CI, 0.47–1.03; p = .11). Within the subset of patients with CR/CRh/CRi (n = 53 for blinatumomab and n = 23 for chemotherapy), median duration of remission was 10.7 months for blinatumomab (95% CI, 5.6 months to not estimable) and 19.0 months for chemotherapy (95% CI, 2.2–19.0 months) (). Median duration of remission, censored at HSCT, was 10.8 months for blinatumomab (95% CI, 5.6 months to not estimable) and not reached for chemotherapy (95% CI, 1.8 months to not estimable; Figure S2).
In second or later salvage, median EFS was 0.0 months in each treatment group. Estimated EFS at 6 months was 24.0% for blinatumomab (95% CI, 17.4–31.3%) and 1.6% for chemotherapy (95% CI, 0.1–7.5%), and the hazard ratio for EFS was 0.49 (95% CI, 0.29–0.57; p < .001; ). Within the subset of patients with CR/CRh/CRi (n = 66 for blinatumomab and n = 10 for chemotherapy), median duration of remission was 6.2 months for blinatumomab (95% CI, 3.8–9.6 months) and 1.7 months for chemotherapy (95% CI, 0.9–4.4 months) (). Median duration of remission, censored at HSCT, was 6.2 months for blinatumomab (95% CI, 3.8–7.6 months) and 1.7 months for chemotherapy (95% CI, 0.9–4.4 months; Figure S2).
Safety
In first salvage, any adverse event was reported in 98.1% of patients in the blinatumomab group and in 100.0% of patients in the chemotherapy group, including any Grade 3 adverse event in 61.2% and 83.0%, respectively, any Grade 4 adverse event in 34.0% and 50.9%, respectively, and any serious adverse event in 57.3% and 41.5%, respectively. In second or later salvage, any adverse event was reported in 98.8% of patients in the blinatumomab group and in 98.2% of patients in the chemotherapy group, including any Grade 3 adverse event in 67.7% and 75.0%, respectively, any Grade 4 adverse event in 36.0% and 53.6%, respectively, and any serious adverse event in 64.6% and 48.2%, respectively.
Within each salvage subgroup, the most commonly reported adverse events of any grade occurred with similar incidences in the blinatumomab and chemotherapy groups (). Adverse events of interest were reported infrequently in first salvage and included Grade 3 neurologic events (7.8% blinatumomab versus 7.5% chemotherapy), Grade 4 neurologic events (1.0% versus 1.9%), or Grade 3 cytokine release syndrome (3.9% versus 0.0%). No patient developed Grade 4 cytokine release syndrome with first salvage. Adverse events of interest were also reported infrequently in second or later salvage and included Grade 3 neurologic events (9.1% blinatumomab versus 7.1% chemotherapy), Grade 4 neurologic events (0.6% versus 1.8%), Grade 3 cytokine release syndrome (4.9% versus 0.0%), and Grade 4 cytokine release syndrome (0.6% versus 0.0%).
Table 4. Treatment emergent adverse events.
Grade 3 or 4 neurologic adverse events that were reported for more than one patient in either treatment group in first salvage were encephalopathy (2.9% blinatumomab versus 0.0% chemotherapy) and syncope (0.0% versus 3.8%). Grade 3 or 4 neurologic adverse events that were reported for more than one patient in either treatment group in second or later salvage were confusional state (1.8% blinatumomab versus 0.0% chemotherapy), somnolence (1.8% versus 0.0%), seizure (0.6% versus 3.6%), and headache (0.0% versus 3.6%).
In the chemotherapy group, a Grade 3 or 4 adverse event of decreased immunoglobulins was not reported for any patient either in first salvage or in second or later salvage. In the blinatumomab group, a Grade 3 or 4 adverse event of decreased immunoglobulins was reported for no patient in first salvage and 4.3% of patients in second or later salvage. Mean (SD) values for immunoglobulin G at baseline and at cycle 1 day 29 were as follows: in first salvage, 6.81 (2.55) g/L before and 5.47 (2.18) g/L after cycle 1 of blinatumomab, and 7.73 (2.34) g/L before and 7.77 (2.68) g/L after cycle 1 of chemotherapy; in second or later salvage, 6.40 (2.31) g/L before and 4.97 (1.80) g/L after cycle 1 of blinatumomab, and 6.89 (2.34) g/L before and 6.94 (2.87) g/L after cycle 1 of chemotherapy.
Discussion
In the international, randomized, phase 3 TOWER study, higher rates of complete hematologic remission and longer overall survival were achieved with blinatumomab treatment compared with chemotherapy in adults with Ph– BCP-ALL that was relapsed or refractory to at least one previous therapy [Citation14]. In this subgroup analysis of the TOWER study by salvage regimen, complete hematologic remission, MRD response, EFS, and overall survival consistently favored blinatumomab over chemotherapy both in first salvage and in second or later salvage.
In first salvage, which comprised 41.2% of the study population overall (38.4% of the blinatumomab group and 47.0% of the chemotherapy group), median overall survival was 11.1 months for blinatumomab and 5.5 months for chemotherapy, suggesting that blinatumomab may be particularly beneficial for overall survival in first salvage. Previous studies reported that median overall survival in adults with ALL in first relapse is 4.5 months overall [Citation6] and 8.4 months among those in relapse who are treated with first salvage chemotherapy [Citation2]. Thus, overall survival for chemotherapy in first salvage in this study was consistent with previously reported results, despite the high prevalence of risk factors for worse outcomes in this study population, such as refractory disease or early relapse. In first salvage, blinatumomab was also associated with a higher rate of complete hematologic remission than chemotherapy (51.0% versus 36.5%) and a higher rate of MRD response (49.1% versus 39.1%). Allogeneic HSCT rates were similar between the treatment groups in first salvage. Duration of remission did not favor blinatumomab over chemotherapy in first salvage, but these analyses were complicated by the small subset of patients that achieved remission with chemotherapy in first salvage. Analyses of MRD response and duration of remission were more descriptive than comparative because the analysis set for this outcome depended on having a post-randomization event (CR/CRh/CRi), which was achieved by a greater proportion of patients in the blinatumomab group than in the chemotherapy group. Thus, a nonrandom subgroup of the full analysis set was analyzed for duration of remission. Analyses of EFS, which included all patients and represented the differences in remission rates, addressed this issue.
In second or later salvage, the overall survival benefit for blinatumomab compared with chemotherapy was less pronounced. Blinatumomab was nevertheless substantially more effective than chemotherapy in second or later salvage for complete hematologic remission (39.5% versus 14.1%) and EFS. While rates of allogeneic HSCT were similar overall in the blinatumomab and chemotherapy groups, patients in second or later salvage were more likely to receive allogeneic HSCT in continuous remission after blinatumomab than after chemotherapy, with a large difference between treatment groups, suggesting that blinatumomab may be a better bridge to transplant in these patients with advanced disease.
In both treatment groups, remission rates and other outcomes appeared to be worse in second or later salvage than in first salvage, which was consistent with previous reports [Citation1,Citation7,Citation8]. These results were also consistent with responses to prior therapy at baseline: patients in second or later salvage were nearly twice as likely as those in first salvage to have ALL that was refractory to prior treatment. Regardless of prior salvage, neurologic adverse events or cytokine release syndrome occurred infrequently with blinatumomab and safety results in each salvage subgroup were consistent with those previously reported for the overall study population [Citation14]. Immunoglobulin G appeared to decrease from baseline to the end of cycle 1 in the blinatumomab group, but not in the chemotherapy group, with Grade 3 or 4 adverse events of decreased immunoglobulins in 4% of patients who received blinatumomab in first salvage. Decreased immunoglobulins have been reported previously with blinatumomab treatment; patients should be monitored and treated appropriately for signs and symptoms of infection.
MRD response as a prognostic factor was stronger in first salvage than in second or later salvage. However, MRD response was a post-randomization variable, complicating interpretation. The study was designed and powered to compare the primary efficacy endpoint, overall survival, between the blinatumomab and chemotherapy groups for all patients who underwent randomization [Citation14]. The randomization was stratified by line of salvage therapy (first versus second or later), as well as age and previous allogeneic HSCT. The study was not powered for statistical comparisons between treatment groups within each salvage subgroup, limiting the interpretation of p values, particularly for secondary endpoints such as hematologic response and MRD response.
In either first salvage or in second or later salvage, blinatumomab was associated with greater remission or survival than chemotherapy and similar or higher transplantation rates. Blinatumomab was associated with similar or better safety than chemotherapy in both populations. Salvage therapy with blinatumomab may be particularly beneficial compared with chemotherapy for overall survival as first salvage in adults with Ph– precursor BCP-ALL, and it may serve as a bridge to transplant in patients in later salvage.
Data deposition
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article online at http:\\doi:10.1080/10428194.2019.1576872
Supplemental Material
Download Zip (258.6 KB)Acknowledgements
Jonathan Latham of PharmaScribe, LLC (on behalf of Amgen Inc.) assisted the authors with the preparation and submission of the manuscript. Robert Dawson of CACTUS (on behalf of Amgen Inc.) assisted the authors with preparation of the images.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Gökbuget N, Dombret H, Ribera JM, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2016;101:1524–1533.
- Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032–2041.
- Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–1230.
- Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21:1907–1914.
- Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950.
- Oriol A, Vives S, Hernández-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95:589–596.
- Kantarjian HM, Thomas D, Ravandi F, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer. 2010;116:5568–5574.
- O'Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113:3186–3191.
- Dreier T, Lorenczewski G, Brandl C, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100:690–697.
- Löffler A, Gruen M, Wuchter C, et al. Efficient elimination of chronic lymphocytic leukaemia B cells by autologous T cells with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Leukemia. 2003;17:900–909.
- Hoffmann P, Hofmeister R, Brischwein K, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98–104.
- Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–4140.
- Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66.
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–847.