Abstract
Consolidation chemotherapy in acute myeloid leukemia (AML) aims at eradicating residual leukemic cells and mostly comprises high-dose cytarabine with or without the addition of anthracyclines, including daunorubicin. Immunogenic cell death (ICD) may contribute to the efficacy of anthracyclines in solid cancer, but the impact of ICD in AML is only partly explored. We assessed aspects of ICD, as reflected by calreticulin expression, in primary human AML blasts and observed induction of surface calreticulin upon exposure to daunorubicin but not to cytarabine. We next assessed immune phenotypes in AML patients in complete remission (CR), following consolidation chemotherapy with or without anthracyclines. These patients subsequently received immunotherapy with histamine dihydrochloride (HDC) and IL-2. Patients who had received anthracyclines for consolidation showed enhanced frequencies of CD8+ TEM cells in blood along with improved survival. We propose that the choice of consolidation therapy prior to AML immunotherapy may determine clinical outcome.
Introduction
Acute myeloid leukemia (AML) is characterized by rapid accumulation of immature myeloid cells in the bone marrow and other organs. At diagnosis, patients typically receive induction chemotherapy aiming at attaining the microscopic disappearance of leukemic cells and the re-appearance of normal hematopoiesis (complete remission (CR)). The ensuing phase of consolidation chemotherapy aims at eradicating undetectable leukemic cells to maintain CR [Citation1–3]. Leukemic relapses, which are assumed to result from the expansion of leukemic cells that have escaped chemotherapy, are common in the post-consolidation phase and herald poor long-term survival [Citation4,Citation5].
The sensitivity of AML cells to the anti-leukemic activity of cytotoxic lymphocytes such as T cells and NK cells [Citation6–10] along with the purported role of these immune effector cells in the surveillance of leukemic cells [Citation11,Citation12] have inspired the development of strategies to boost cellular immunity in the post-consolidation phase for relapse prevention [Citation13–16]. The NOX2 inhibitor histamine dihydrochloride (HDC) in conjunction with the T and NK cell activating cytokine interleukin-2 (HDC/IL-2) is approved in AML for relapse prevention in Europe, and recent studies imply that aspects of T and NK cell immunity are relevant to the clinical efficiency of this combinatorial immunotherapy [Citation16–19].
The standard treatment for newly diagnosed AML patients comprises cytarabine combined with an anthracycline during induction therapy and high-dose cytarabine, alone or combined with other cytostatic drugs, including anthracyclines, in the ensuing consolidation phase [Citation3,Citation20,Citation21]. In recent years it has become recognized that chemotherapies are important not only to directly eliminate tumor cells but also to induce or reinforce immune responses that may be crucial for the eradication of malignant cells. This phenomenon is referred to as immunogenic cell death (ICD) [Citation22,Citation23] and comprises cell surface expression or release of damage-associated molecular pattern molecules (DAMPs), including calreticulin, ATP, and HMGB1 [Citation24]. Surface-exposed calreticulin functions as an ‘eat me’ signal via binding to LDL-receptor-related protein (LRP) on the engulfing cells, while ATP and HMGB1 recruit and activate antigen-presenting cells (APCs). This stimulates antigen uptake and presentation for priming of antigen-specific T cells [Citation24,Citation25]. Several chemotherapeutics have been reported to induce ICD including anthracyclines, oxaliplatin, and bortezomib [Citation26].
Here, we report that the anthracycline daunorubicin, but not the non-anthracycline cytarabine, triggers a robust upregulation of calreticulin on the surface of primary human CD34+ AML blasts. Furthermore, our results suggest that AML patients receiving consolidation chemotherapy that includes an anthracycline show increased levels of cytotoxic T cells with a TEM phenotype in peripheral blood. A high frequency of cytotoxic TEM cells after consolidation was associated with a favorable clinical outcome in a clinical phase IV trial where AML patients in CR were treated by immunotherapy with HDC/IL-2.
Patients, materials, and methods
Primary AML blasts
Peripheral blood was collected from newly diagnosed AML patients at the Department of Hematology at Sahlgrenska University Hospital, after informed consent was obtained. The peripheral blood was diluted 1:1 in sterile PBS, carefully layered on top of Ficoll-Paque and thereafter centrifuged (820 g, 20 min in room temperature). Peripheral blood mononuclear cells (PBMCs) were collected from the interface and subsequently frozen in liquid nitrogen.
The cryopreserved AML cells from newly diagnosed patients were thawed quickly and resuspended in IMDM (Iscove’s modified Dulbeccos Medium) supplemented with 10% human AB serum. Four hundred thousand PBMCs from the newly diagnosed AML patients were seeded in each well and exposed to either cytarabine or daunorubicin. After incubation overnight, the cells were stained for calreticulin, CD34, CD14 and CD3, a live/dead marker and were then analyzed on a 4-laser BD LSRFortessa SORP flow cytometer (405, 488, 532, and 640 nm; BD Biosciences, Stockholm, Sweden).
Clinical trial patients, study design, and objectives
Eighty-four adult patients (age 18–79) with confirmed AML in first CR who were not eligible for allogeneic stem cell transplantation were enrolled in the Re:Mission trial (NCT01347996, registered at www.clinicaltrials.gov). provides information about the prior induction and consolidation chemotherapy. Further patient characteristics are accounted for in previous publications [Citation17,Citation18,Citation27]. In this single-armed multicenter phase IV study, patients received 10 consecutive 21-d cycles of HDC and low-dose IL-2 for 18 months or until relapse or death using a regime identical to that employed in a previous phase III trial [Citation28]. Patients were enrolled at a median of 46 d after the completion of consolidation (19–172 d after the last day of consolidation) and were followed-up for at least until 24 months after the onset of immunotherapy. Patients who discontinued prematurely from the study (n = 14) were censored at the last captured follow-up date. The primary trial endpoints included the quantitative and qualitative effects of HDC/IL-2 on T and NK cell populations in peripheral blood during treatment cycles. The present analyses of effects of induction and consolidation regimes and T cell subpopulations on clinical outcome (leukemia free survival (LFS) and overall survival (OS)) were performed post-hoc. Individual induction and consolidation regimens were based on local guidelines at each recruiting center. Relapse was defined as at least 5% blast cells in the bone marrow or presence of extramedullary leukemia.
Table 1. Induction and consolidation chemotherapy.
Isolation of PBMCs, staining, and flow cytometry
Peripheral blood was collected immediately before the first HDC/IL-2 treatment cycle from patients in the Re:Mission clinical trial. PBMCs were isolated and cryopreserved at local sites and shipped on dry ice to the central laboratory at the TIMM Laboratory, University of Gothenburg for analysis. Cryopreserved samples were quickly thawed, washed, and stained with LIVE/DEAD fixable yellow stain (Life Technologies, Grand Island, NY). Thereafter, cells were washed and incubated with an antibody cocktail for surface markers in PBS containing 0.5% BSA and 0.1% EDTA or in Brilliant stain buffer (BD Biosciences, Stockholm, Sweden). The following anti-human monoclonal antibodies were used for phenotyping of PBMCs: CD4-APC-H7 (RPA-T4), CD8-PerCP-Cy5.5 (RPA-T8/SK1) CD45RA-APC (HI100), CD45RO-PE (UCHL1) (all from BD Biosciences, Stockholm, Sweden). CCR7-PE-Cy7 (G043H7) from Biolegend, San Diego, CA and CD3-Pacific Blue (S4.1) from Life Technologies, Carlsbad, CA. A 4-laser BD LSRFortessa SORP flow cytometer (405, 488, 532, and 640 nm; BD Biosciences, Stockholm, Sweden) was employed to analyze samples. Data analysis was performed by using FlowJo Software version 7.6.5 or later (TreeStar, Ashland, OR). Blood samples were available from 81 out of 84 patients. Phenotype analysis of T cell subsets was performed on PBMC from 44 patients who were selected based on the availability of frozen PBMCs with sufficient viability.
Statistical analyses
The impact of the choice of previous chemotherapy on the distribution of CD8+ T cell phenotypes at the onset of immunotherapy was performed using Student’s t-test. The logrank test was utilized to determine the impact of the number of induction and consolidation courses, the type of consolidation therapy or the frequency of CD8+ T cell subsets on LFS and OS at the trial closing date (13 October 2014). The impact of the inclusion of anthracyclines in the consolidation phase and the distribution of cytotoxic T cell phenotypes in blood on LFS and OS were further assessed using Cox univariable and multivariable regression analyses. To select factors to include in the multivariable Cox regression model, univariable analysis was utilized to determine the impact of age, risk group classification according to recommendations by the European LeukemiaNet [Citation29] along with the number of induction courses required to achieve CR, the number of consolidation courses given and dose of cytarabine used in consolidation on LFS and OS. Prognostic factors achieving a p value below .1 in univariable analysis for LFS, i.e. age and number of induction courses, were included as potential confounders in the multivariable analysis.
All indicated p values are 2-sided. This study was conducted according to principles outlined in the Declaration of Helsinki and was approved by the Ethics Committees of each participating institution. All patients gave written informed consent before enrollment.
Results
Daunorubicin triggers robust calreticulin expression on primary CD34+ AML blasts
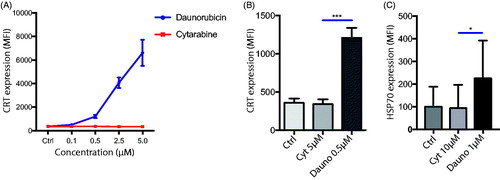
Cell surface expression of CRT is regarded as a hallmark of ICD [Citation24]. To elucidate if cell surface levels of CRT differed on primary AML cells after exposure to anthracyclines as compared to cytarabine, primary AML cells were treated with varying concentrations of either the anthracycline daunorubicin or the non-anthracycline cytarabine. Over a wide concentration range, daunorubicin, but not cytarabine, triggered an upregulated expression of surface calreticulin on the primary AML blasts (). We also assessed the expression of the associated DAMP HSP70 (Heat Shock Protein-70.) In line with the CRT data, we observed a significant upregulation of HSP70 upon exposure to daunorubicin but not to cytarabine ().
Figure 1. Daunorubicine upregulate calreticulin in vitro on primary AML blasts. (A) Primary AML PBMCs were cultured overnight with increasing concentrations of either daunorubicin or cytarabine (n = 5). Daunorubicin induced a robust upregulation of CRT on live CD34+ AML blasts, defined as Live/dead-CD34+CD3−CD14− cells. (B) Comparison of CRT levels on live cells AML blasts after exposure to 0.5 µM daunorubicin or 5 µM cytarabine which yielded similar levels of cell death (29.8 ± 11.5% vs. 26.9 ± 7.5% dead cells, respectively (mean ± SEM)). (C) HSP70 up-regulation on live CD34+ AML blasts after exposure to 1.0 µM daunorubicin or 10 µM cytarabine. Paired t-test. *p < .05, ***p < .001.
Impact of induction chemotherapy on clinical outcome in HDC/IL-2 AML trial
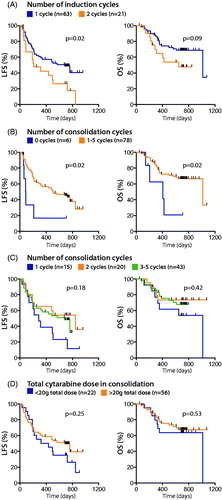
Information about the induction and consolidation strategies were available for 84 AML patients participating in a clinical phase IV trial using post-consolidation immunotherapy with HDC/IL-2. The induction strategies were similar for the vast majority of patients and included cytarabine in all cases. For all but two patients, cytarabine was combined with anthracyclines (daunorubicin, n = 67; idarubicin, n = 11; or both, n = 4). Fourteen patients received cytarabine and anthracyclines with additional chemotherapy (). The two patients who did not receive anthracyclines for induction both experienced relapse. Sixty-three patients attained CR after the first induction cycle and two induction cycles were required in 21 patients. In agreement with previous studies [Citation30], patients who had attained CR after one course of induction showed significantly superior outcome ().
Figure 2. Impact of induction and consolidation therapy on clinical outcome. Patients were separated into groups depending on (A) the number of induction courses, (B) the presence or absence of consolidation chemotherapy, (C) the number of consolidation courses or (D) a total dose of cytarabine in consolidation of > or <20 g. LFS and OS were analyzed by the logrank test (A, B and D) or logrank test for trend (C).
Use of anthracyclines in consolidation therapy predicts favorable outcome in the HDC/IL-2 AML trial
Seventy-eight patients received consolidation chemotherapy comprising 1 (n = 15), 2 (n = 20), 3 (n = 37), 4 (n = 5), or 5 (n = 1) courses. Five out of the six patients who did not receive post-remission chemotherapy relapsed within 7 months (); for five of these patients two rounds of induction therapy had been required to attain CR. Among the 78 patients who had received consolidation chemotherapy, there was a non-significant trend toward improved LFS for patients given 2 vs. 1 round of consolidation, but no further improvement of LFS was apparent for those receiving 3–5 rounds of consolidation (). In patients receiving cytarabine for consolidation, 56 out of 78 patients received higher doses (1.7–6.6 g/d for 3–7 d), with a total dose (all cycles counted) of >20 g. Low to intermediate doses were given to 22 patients (0.18–2 g/d for 3–7 d, with a total dose of <20 g). There was a non-significant trend toward improved outcome for patients receiving a total dose of >20 g (), but no further improvement was noted for patients receiving >40 g (Supplementary Figure 1(A)). Furthermore, patients receiving gram doses of cytarabine (at least 1 g/m2 for at least one dose; n = 62 vs. patients receiving <1 g/m2 in all doses; n = 16) did not show superior outcome (Supplementary Figure 1(B)).
The majority of patients received anthracyclines i.e. daunorubicin (n = 48), idarubicin (n = 10), or mitoxantrone (n = 2), together with cytarabine also during consolidation (n = 60). In addition to receiving cytarabine and anthracyclines in consolidation, one patient was also given gemtuzumab ozogamicin and two patients received fludarabine (). The clinical outcome was similar for the patients receiving daunorubicin or idarubicin, while both patients treated with mitoxantrone relapsed.
Eighteen patients received cytarabine in consolidation in the absence of anthracyclines. Among these, 13 patients received cytarabine as the single drug, while five patients received cytarabine combined with etoposide + amsacrine (n = 1), methotrexate (n = 1), or fludarabine (n = 3) (). The addition of these non-anthracycline chemotherapies to cytarabine did not result in improved LFS or OS (data not shown). However, when comparing clinical outcomes in patients receiving consolidation with cytarabine, used alone or together with non-anthracyclines, with those receiving cytarabine together with an anthracycline, the inclusion of anthracyclines was associated with significantly improved OS (). Potential confounders such as age, risk group classification, FAB classification, number of induction courses, and number of consolidation courses did not differ significantly between patients receiving cytarabine in combination with anthracycline and those receiving only cytarabine in consolidation (). The use of anthracyclines during consolidation independently predicted OS in multivariable analysis ().
Figure 3. Patients receiving anthracyclines for consolidation show altered distribution of CD8+ T cell subsets and improved clinical outcome. (A) The outcome for patients treated by cytarabine in combination with anthracyclines in consolidation was compared with the outcome for patients receiving cytarabine, but without anthracyclines, using the logrank test. (B–D) The percentage of naïve (TN; CD45RA+CCR7+), central memory (TCM; CD45RO+CCR7+), effector memory (TEM; CD45RO+CCR7−), and effector (Teff; CD45RA+CCR7−) CD8+ T cells in blood before the first cycle of HDC/IL-2 treatment was determined by flow cytometry. Patients were dichotomized by the median based on the frequency of CD8+ TEM (B) or Teff (C) cells at the onset of HDC/IL-2 treatment, followed by analyses of LFS and OS by the logrank test. (D) The frequency of the T cell subsets for patients receiving no consolidation, consolidation chemotherapy not containing anthracyclines, or consolidation chemotherapy including anthracyclines, was compared using Student´s t-test. *p < .05, **p < .01.
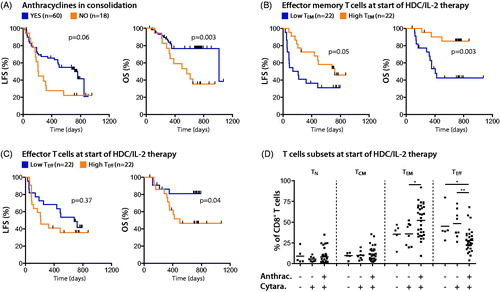
Table 2. Patient characteristics.
Table 3. Univariable and multivariable Cox regression analyses of the impact of type of consolidation and T cell phenotypes on LFS and OS.
Use of anthracyclines in consolidation alters the distribution of cytotoxic T cell phenotypes
A high frequency of effector memory T cells (TEM) after consolidation, but prior to HDC/IL-2 immunotherapy, prognosticated LFS, and OS (), which is in agreement with an earlier report [Citation17]. The survival benefit of a high frequency of TEM cells at onset of immunotherapy remained significant in multivariable analysis (). As shown in low percentage of T effector cells (Teff) in blood at onset of immunotherapy also prognosticated OS.
When analyzing the immune phenotypes of mononuclear cells of patients before the start of immunotherapy, a skewed distribution of CD8+ cytotoxic T cell phenotypes toward a higher ratio of TEM to Teff cells was observed among patients who had received anthracyclines for consolidation (). The administration of cytarabine at high or low doses, or not at all (for the six patients not receiving consolidation therapy), did not impact on the distribution of T cells ( and data not shown).
Discussion
Certain chemotherapeutic agents, in particular anthracyclines, have been shown to trigger cell death by inducing ER stress leading to a distinct phenotype of the apoptotic cells, such as surface expression of calreticulin [Citation31]. These apoptotic cells stimulate APCs and enhance subsequent T cell responses in a process denoted ICD [Citation32]. It was previously shown that high expression of CRT on the cell surface of malignant cells may herald favorable prognosis in AML [Citation31]. It has also been shown that ICD-inducing chemotherapeutic agents generate CRT upregulation on cancer cells in animal models [Citation33]. In this study we show that primary AML cells exposed to the anthracycline daunorubicin, but not to the non-anthracycline cytarabine, expressed high levels of cell surface CRT and HSP70, suggesting that the choice of chemotherapy impacts on the phenotype of dying AML cells.
Induction chemotherapy in AML using a combination of cytarabine and anthracyclines is the standard-of-care worldwide. However, while numerous studies support that consolidation is critical to prevent relapse in CR [Citation34], there is currently no consensus regarding the optimal consolidation strategy. When used as a single agent, high-dose cytarabine for consolidation reduces the relapse risk in the post-remission phase, in particular in core-binding factor leukemias and in patients carrying NPM1 or CERPα mutations in leukemic cells [Citation35,Citation36]. However, it remains uncertain whether or not the addition of anthracyclines during consolidation improves outcome [Citation37–39].
We analyzed a cohort of AML patients treated with HDC/IL-2 immunotherapy for relapse prevention aiming at determining whether or not the inclusion of anthracyclines during consolidation chemotherapy impacted on clinical outcome. In this trial, patients received cycles of induction and consolidation according to local guidelines at the participating centers. All patients were in CR at study entry and all received HDC/IL-2 immunotherapy as remission maintenance therapy.
As expected, patients who did not receive consolidation therapy did poorly (5/6 relapses and 4/6 deaths). Notably, these patients were all >60 years old, and 5/6 had required 2 cycles of induction therapy to attain CR. Although cytarabine formed part of the consolidation regimen for all patients there was considerable variation in dose, number of doses and number of cycles between patients. When patients were dichotomized based on the total cytarabine dose, there was a non-significant trend toward improved outcome for those receiving higher doses (>20 g), but outcome was not further improved for patients receiving a total dose of >40 g cytarabine. This is in accordance with previous results showing that the outcome is similar for patients receiving cytarabine in 1 g/m2/dose and 2–3 g/m2/dose during consolidation [Citation40]. Patients receiving anthracyclines in combination with >20 g cytarabine showed the most favorable survival outcome, followed by those receiving anthracyclines combined with <20 g cytarabine, >20 g cytarabine without anthracyclines and <20 g cytarabine without anthracyclines (Supplementary Figure 1(C)). However, a multivariable analysis did not confirm significantly improved OS for patients receiving high total doses of cytarabine, even when taking the presence of anthracyclines in consolidation into account (HR = 0.83, CI 0.54–1.26, p = .38).
In contrast to the weak association between outcome and the dose of cytarabine used for consolidation in this study, there was a significant relationship between a favorable outcome and inclusion of anthracyclines during consolidation. In addition, patients who had received anthracyclines showed a high percentage of cytotoxic TEM cells and a low percentage of Teff cells in the peripheral blood after the completion of consolidation. When separating patients based on the frequency of TEM and Teff cells into three groups, patients with the highest amount of cytotoxic TEM and lowest amount of Teff cells showed significantly superior outcome in terms of LFS and OS (Supplementary Figure 2 (A,B)). A high frequency of CD8+ TEM cells at onset of immunotherapy heralded significantly improved outcome also in multivariable analysis (). Despite that previous studies have shown that calreticulin levels may be elevated in AML patients compared to healthy donors regardless of chemotherapy exposure [Citation22,Citation25], it may be speculated that the altered distribution of T cell subsets in the post-consolidation phase presented in this study reflects anthracycline-induced ICD and enhanced T cell activation [Citation22,Citation25].
In conclusion, we show that anthracyclines trigger upregulation of CRT on primary AML blasts. In addition, our results imply that AML patients receiving consolidation chemotherapy with anthracyclines show an altered distribution of cytotoxic T cells subset in peripheral blood toward a higher frequency of CD8+ TEM cells. A high frequency of CD8+ TEM impacted favorably on the outcome of AML patients receiving post-consolidation immunotherapy, but our study does not exclude other contributing effects of anthracyclines that may favor survival. Our findings merit further studies to clarify whether the choice of chemotherapy in the consolidation phase of AML may determine the anti-leukemic efficiency of T cell immunity.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2019.1599110.
Supplemental Figures
Download PDF (742.5 KB)Additional information
Funding
References
- Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163.
- Schiller G, Gajewski J, Territo M, et al. Long-term outcome of high-dose cytarabine-based consolidation chemotherapy for adults with acute myelogenous leukemia. Blood. 1992;80:2977–2982.
- Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127:53–61.
- Szer J. The prevalent predicament of relapsed acute myeloid leukemia. Hematol Am Soc Hematol Educ Program. 2012;2012:43–48.
- Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse [multicenter study]. J Clin Oncol. 2005;23:1969–1978.
- Hsu KC, Gooley T, Malkki M, et al. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Marrow Transplant. 2006;12:828–836.
- Lion E, Willemen Y, Berneman ZN, et al. Natural killer cell immune escape in acute myeloid leukemia. Leukemia. 2012;26:2019–2026.
- Brune M, Hellstrand K. Remission maintenance therapy with histamine and interleukin-2 in acute myelogenous leukaemia. Br J Haematol. 1996;92:620–626.
- Montagna D, Maccario R, Locatelli F, et al. Emergence of antitumor cytolytic T cells is associated with maintenance of hematologic remission in children with acute myeloid leukemia. Blood. 2006;108:3843–3850.
- Greiner J, Schmitt M, Li L, et al. Expression of tumor-associated antigens in acute myeloid leukemia: implications for specific immunotherapeutic approaches. Blood. 2006;108:4109–4117.
- Barrett AJ, Savani BN. Does chemotherapy modify the immune surveillance of hematological malignancies? Leukemia. 2009;23:53–58.
- Tajima F, Kawatani T, Endo A, et al. Natural killer cell activity and cytokine production as prognostic factors in adult acute leukemia. Leukemia. 1996;10:478–482.
- Arpinati M, Curti A. Immunotherapy in acute myeloid leukemia. Immunotherapy. 2014;6:95–106.
- Ruggeri L, Parisi S, Urbani E, et al. Alloreactive natural killer cells for the treatment of acute myeloid leukemia: from stem cell transplantation to adoptive immunotherapy. Front Immunol. 2015;6:479.
- Austin R, Smyth MJ, Lane SW. Harnessing the immune system in acute myeloid leukaemia. Crit Rev Oncol Hematol. 2016;103:62–77.
- Martner A, Thoren FB, Aurelius J, et al. Immunotherapeutic strategies for relapse control in acute myeloid leukemia. Blood Rev. 2013;27:209–216.
- Sander FE, Rydstrom A, Bernson E, et al. Dynamics of cytotoxic T cell subsets during immunotherapy predicts outcome in acute myeloid leukemia. Oncotarget. 2016;7:7586–7596.
- Martner A, Rydstrom A, Riise RE, et al. Role of natural killer cell subsets and natural cytotoxicity receptors for the outcome of immunotherapy in acute myeloid leukemia. Oncoimmunology. 2016;5:e1041701.
- Aurelius J, Martner A, Brune M, et al. Remission maintenance in acute myeloid leukemia: impact of functional histamine H2 receptors expressed by leukemic cells. Haematologica. 2012;97:1904–1908.
- Rowe JM. Optimal induction and post-remission therapy for AML in first remission. Hematol Am Soc Hematol Educ Program. 2009;2009:396–405.
- Schlenk RF. Post-remission therapy for acute myeloid leukemia. Haematologica. 2014;99:1663–1670.
- Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701.
- Kepp O, Senovilla L, Vitale I, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3:e955691.
- Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875.
- Galluzzi L, Buque A, Kepp O, et al. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714.
- Garg AD, Nowis D, Golab J, et al. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71.
- Martner A, Rydstrom A, Riise RE, et al. NK cell expression of natural cytotoxicity receptors may determine relapse risk in older AML patients undergoing immunotherapy for remission maintenance. Oncotarget. 2015;6:42569–42574.
- Brune M, Castaigne S, Catalano J, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108:88–96.
- Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474.
- Jourdan E, Reiffers J, Stoppa AM, et al. Outcome of adult patients with acute myeloid leukemia who failed to achieve complete remission after one course of induction chemotherapy: a report from the BGMT Study Group. Leuk Lymphoma. 2001;42:57–65.
- Fucikova J, Truxova I, Hensler M, et al. Calreticulin exposure by malignant blasts correlates with robust anticancer immunity and improved clinical outcome in AML patients. Blood. 2016;128:3113–3124.
- Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61.
- Lu X, Ding ZC, Cao Y, et al. Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells. J Immunol. 2015;194:2011–2021.
- Rowe JM. Consolidation therapy: what should be the standard of care? Best Pract Res Clin Haematol. 2008;21:53–60.
- Lowenberg B, Pabst T, Vellenga E, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364:1027–1036.
- Bloomfield CD, Lawrence D, Byrd JC, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–4179.
- Kim DS, Kang KW, Lee SR, et al. Comparison of consolidation strategies in acute myeloid leukemia: high-dose cytarabine alone versus intermediate-dose cytarabine combined with anthracyclines. Ann Hematol. 2015;94:1485–1492.
- Schaich M, Parmentier S, Kramer M, et al. High-dose cytarabine consolidation with or without additional amsacrine and mitoxantrone in acute myeloid leukemia: results of the prospective randomized AML2003 trial. J Clin Oncol. 2013;31:2094.
- Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31:3360–3368.
- Schiffer CA. Optimal dose and schedule of consolidation in AML: is there a standard? Best Pract Res Clin Haematol. 2014;27:259–264.
