Abstract
This trial evaluated quality of life (QoL) using the EORTC QLQ-C30 and the EORTC QLQ-MY20 instruments in 90 patients with relapsed/refractory multiple myeloma during induction and maintenance therapy with eight cycles of ixazomib-thalidomide-dexamethasone, followed by 12 months of ixazomib maintenance therapy. When patient’s baseline QoL was compared with data of the general population, a significant impairment in health-related QoL, physical, role, and social functioning and several other dimensions, as well as more pain and fatigue, was noted. Induction therapy resulted in significant improvement of pain and worsening of neuropathy, with no significant variation of other parameters. During maintenance treatment, scores for most dimensions including health-related QoL, physical functioning and pain, improved, while for neuropathy no improvement was observed. Time to deterioration (≥10 score points) of health-related QoL, physical functioning, pain, and neuropathy was distinctly shorter than time to progression. Health-related QoL and physical functioning at baseline correlated with overall survival.
Introduction
The aim of multiple myeloma (MM) treatment is to control the disease, prolong survival, reduce disease-related symptoms, and improve quality of life (QoL) [Citation1]. Several studies revealed that physicians often underestimate the impact of cancer associated symptoms such as nausea, fatigue, or pain on patient’s well-being [Citation2]. Hence, patient-reported outcomes (PRO) have become the gold standard for the evaluation of the burden of disease and of the benefits and side effects of therapy [Citation3]. Maintaining reasonable QoL is particularly important when treatments improve progression-free survival (PFS), but do not prolong the overall survival (OS), because in these instances, documentation of a substantial benefit in terms of QoL is required [Citation4–6], otherwise the treatment would be of no or only questionable value. The impact of a specific treatment on QoL in patients with MM depends not only on the depth of response, but to a significant part on the tolerance and toxicity of individual drugs used for therapy, and also on the patient’s individual biological robustness and possible morbidities. In this study, we aimed to evaluate changes in several predefined dimensions of QoL in patients with relapsed/refractory multiple myeloma (RRMM) treated with a triple combination employing ixazomib-thalidomide-dexamethasone, followed by ixazomib maintenance therapy. Dexamethasone is part of almost every myeloma treatment regimen, and after introduction of the low-dose regimen with once-weekly dosing, relatively well tolerated. Ixazomib, when combined with lenalidomide-dexamethasone (Rd), was found to prolong PFS in a large phase 3 trial with limited additional toxicity [Citation7], and was well tolerated and did not result in impairment of QoL with the exception of a higher incidence of diarrhea, when compared to Rd alone [Citation8].
Ixazomib maintenance therapy after autologous transplantation improved PFS by more than 5 months, was equally effective in patients with high- and standard risk cytogenetics, and was well tolerated with minimal increase in serious adverse events [Citation9], but information on its impact on QoL is only limited until now. Thalidomide is still the most widely used immunomodulatory drug (IMiD) worldwide because it is simply the most affordable one, but is associated with several side effects, particularly neuropathy, which is the clinically most relevant. Side effects of thalidomide depend on dose, particularly cumulative dose, and on patient’s individual susceptibility to thalidomide-induced toxicities [Citation10]. In the Myeloma IX study, the combination of thalidomide with cyclophosphamide and dexamethasone (CTD) significantly reduced fatigue at 3 months and improved physical functioning at 12 months compared with the non-thalidomide containing combination of cyclophosphamide-vincristine-adriamycin-dexamethasone (CVAD), while in the non-intensive pathway attenuated cyclophosphamide-thalidomide-dexamethasone (CTD) was superior to melphalan-prednisone (MP) in terms of pain at 3 months. Thalidomide maintenance therapy improved pain at 12 months but decreased global health status/quality of life versus observation [Citation11]. Unfortunately, results on polyneuropathy, the clinically most relevant side effect of thalidomide, were not reported. Here, we present the analysis of the clinically most relevant dimensions of QoL in patients with RRMM [Citation12] during planned treatment with eight cycles of ixazomib plus thalidomide and dexamethasone for relapsing/refractory disease followed by ixazomib maintenance therapy. In addition, as information regarding possible differences in QoL between myeloma patients and the general population is not available, we compared several dimensions of QoL between our patients with RRMM with that observed in the normal population of similar age from the same area.
Patients and controls from the general population
Ninety patients with relapsed/refractory MM had been enrolled (median age: 67 years, [International Staging Status (ISS) stage I: 41%, II: 33%, III: 26%, Eastern Cooperative Oncology Group (ECOG) performance status 0–1: 95%, 2: 5%]. Treatment consisted of eight cycles of ixazomib, 4 mg, d 1, 8, and 15, q 28 days; thalidomide, 100 mg/d; and dexamethasone: 40 mg, d 1, 8, and 15. Patients aged ≥75 years had a 50% dose reduction of thalidomide to 50 mg/d and of dexamethasone to 20 mg, and of ixazomib to 3 mg. Maintenance treatment consisted of ixazomib, 4 mg, days 1, 8, and 15 of a 28-day cycle, and was given for one year. Treatment results of this open-label multi-center single arm phase II study have been summarized and will be published in the British Journal of Cancer [Citation13]. The trial protocol was approved by each study site’s Independent Ethics Committee or Institutional Review Board, and the study was conducted in accordance with the Declaration of Helsinki. Two-thousand-three-hundred-thirty-seven individuals aged 60–69 years of the general population from 11 European-countries served as controls [Citation14].
Methods
Health-related quality of life was assessed by the European Organization of Research and Treatment of Cancer (EORTC) questionnaires EORTC QLQ-C30 [Citation15] and the Myeloma EORTC QLQ-MY20 module [Citation16]. Comorbidity was assessed by the clinical care team using the activities of daily living (ADL) [Citation17], and the instrumental activities of daily living (IADL) [Citation18] instruments, and Charlson comorbidity index [Citation19]. The questionnaires were distributed and filled out by patients in paper format prior to study treatment at baseline, and subsequently on day 1 of each treatment cycle, at end of induction therapy, at start of maintenance treatment, and monthly thereafter until the end of month 12, or at time of treatment discontinuation or progression. This study focused on nine pre-selected and clinically relevant QoL domains: Three from the QLQ-MY20 (disease symptoms, side effects of treatment and neuropathy); and six from the QLQ-C30 (health-related QoL, physical, cognitive and social functioning, fatigue, and pain). QLQ-MY20 and QLQ-C30 scales were scored in accordance with their published guidelines [Citation20]. Results were transformed to a standardized 0–100 final ‘scale score.’ For the functional scales (health-related QoL, physical, cognitive, and social functioning), higher scores indicate better QoL, whereas for the symptom scales (fatigue, pain, neuropathy, disease symptoms, and side effects of treatment), lower scores indicate less or no symptoms. As a guide for interpretation, an arbitrary difference of ≥10 points in a scale was considered as the minimal important difference (MID) required to suggest clinical relevance [Citation21]. This cutoff is intended to produce robust results. In other recent myeloma trials (ASPIRE [Citation22], ENDEAVOR [Citation12]), a difference of ≥5 points has been used as cutoff, while in the FIRST study [Citation23], differences from 5 to 12 points have been used for individual domains.
Compliance rates at each scheduled assessment were calculated as the number of compliant patients divided by the number of patients still on protocol treatment at that assessment. Analyses were performed on intention-to-treat patients; data cutoff was 31 March 2019. Due to the phase II design of the study, and HRQoL being only a secondary endpoint not reflected in the sample size calculation, all analyses and results have to be considered explorative. The constantly decreasing number of available observations is inevitable in a highly palliative setting, with missingness clearly not at random. Therefore, no imputation or modeling of missing values was performed; all presented results were based on available data only. Differences were assessed by t-test (two-sample t-test for the comparison with the general population and paired t-test for the comparison within the course of therapy). Wilcoxon tests were used to evaluate differences in the QoL dimensions between patients who started maintenance therapy and those who discontinued earlier. For the comparison of time to progression (TTP) and time to deterioration of different HRQoL domains, and the assessment of the prognostic impact of QoL domains on long-term outcome, a Kaplan–Meier estimation [Citation24] was used. For the comparison of PFS and OS, in patients with poorer or better health related QoL and physical functioning, patients were grouped according to lower or equal or above median scores at baseline and results were compared using Kaplan–Meier estimations [Citation24] and log rank test [Citation25] as appropriate. All p-values are two-sided without any adjusting for multiple testing. Thus, the corresponding term ‘significant’ in case of p ≤ .05 has to be considered explorative without reference to pre-specified hypotheses. The selection of nine QoL domains as most relevant was fixed prospectively, based on the experience from the group’s previous trials, before any statistical analyses were performed.
Results
Patient population
Baseline patient characteristics and exposure to prior treatment in the intent-to-treat (ITT) group are shown in . QoL data were available in 89 of 90 ITT patients at baseline and in 41 of the 43 patients who started maintenance therapy and in 13 patients at the planned completion of maintenance treatment. Details of the patient flow during the study are shown in Supplementary Figure 1. Median follow-up was 19.1 months.
Table 1. Patient characteristics.
Comparison of QoL between patients and individuals from the general population
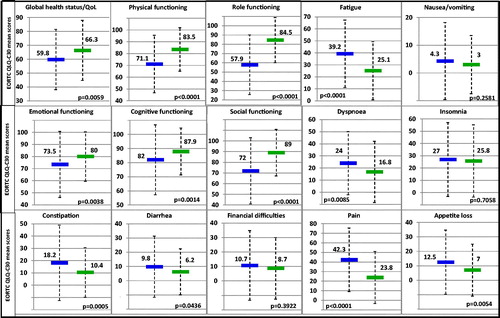
A significant impairment in several dimensions of QoL was noted when the baseline data of the patients were compared with the general population of similar age and same geographic region (). Among those, physical (−12.4, p < .0001), role (−26.6, p < .0001), social function (−17, p < .0001), fatigue (+14.1, p < .0001), and pain (+18.5, p < .0001) were all significantly worse in the patient cohort and exceeded the MID. Significant differences without meeting the threshold for clinical relevance were noted for global health status/QoL (−6.5, p = .0059), emotional (−6.5, p = .0038), cognitive function (−5.9, p = .0014), dyspnea (+7.2, p = .0085), appetite loss (+5.5, p = .0054), constipation (+7.8, p = .0005), and diarrhea (+3.6, p = .0436). No difference between patients and the general population was noted for nausea/vomiting, insomnia, and financial burden.
Compliance
Compliance rates were consistently high throughout the study period. At each predefined time point, more than 95% of patients still on therapy completed the questionnaires as requested.
Induction therapy with ixazomib-thalidomide-dexamethasone
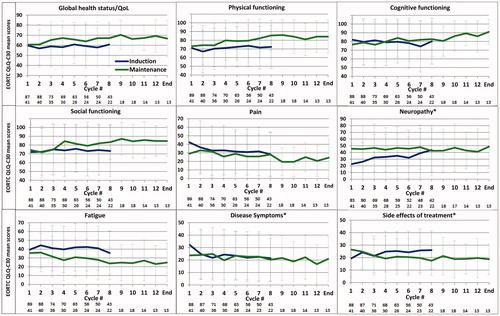
Induction therapy was started in 90 patients and 43 completed all eight cycles. Thirty-three of 47 discontinued due to disease progression (70.2%), 5 due to investigator decision (10.6%), 3 developed unacceptable adverse events (6.4%), and 2 each (4.3%) stopped induction treatment due to death, patient’s decision, and insufficient response, respectively. During induction therapy, most of the relevant dimensions in QoL, like global health-status/QoL, physical, cognitive, and social function, and fatigue remained stable, while a clinically relevant reduction in pain was noted during cycles 5–8, and at the end of induction therapy. The reduction was significant for cycle 5 (−10.8, p = .0124) and cycle 8 (−14.0, p = .0331). The mean score of disease symptoms reached the MID at cycle 3 (−10.7, p = .0001), cycle 6 (−10.6, p = .0035), and at cycle 8 (−10.9, p = .0852). Patient-reported neuropathy was present in 41% of patients at time of enrollment into the trial (mean score 22.5). Polyneuropathy (PNP) worsened significantly and clinically meaningfully shortly after starting therapy at cycles 4, 5, 7, 8, and at end of induction treatment, where the highest difference was noted (+23.0, p = .0094) ().
Maintenance therapy with ixazomib
Forty-three of the 76 eligible patients (56.6%) started maintenance therapy and 13 of them (17.1%) completed all 12 cycles. Reasons for discontinuation were disease progression in 26, PNP in 1, and patient’s decision in 3 patients. Patients who started maintenance therapy did not differ at baseline in the scores of the important domains (health-related QoL, physical functioning, fatigue, pain, neuropathy, disease symptoms, and cognitive function; differences were not significant, and data not shown) from patients who stopped therapy before the planned enrollment in the ixazomib maintenance phase. Patients who discontinued before the end of induction therapy in general scored lower at time of last QoL assessment compared to those who were started on maintenance (assessment at start of maintenance), but a clinical relevant significant difference was noted for fatigue (−15.7, p = .021) and neuropathy (+17.6, p = .019), only ().
Table 2. Mean scores and standard deviation of parameters of QoL in patients at start of maintenance therapy and in those who discontinued treatment earlier.
During maintenance treatment (), scores improved for most dimensions assessed, specifically for health-related QoL and physical functioning (reaching the MID at cycles 8–10, cycle 12, and at end of maintenance and was significant for cycle 9 only (+12.9, p = .0151). For cognitive functioning, a clinically relevant difference was observed at cycle 11 and end of maintenance (+12.9 and +14.6, not significant), while for social functioning scores ≥10 were noted at cycles 4, 9, 11, 12, and at end of maintenance, but differences were not significant. Regarding the symptom scales, clinically relevant improvement was observed for fatigue at cycles 8–10 and 12 and at end of maintenance [significant at cycle 8 only (−11.8, p = .0284)], while scores for neuropathy, pain, disease symptoms, and side effects of treatment remained relatively stable during maintenance therapy ().
Time to deterioration of global health status/QoL, physical functioning, progressive disease, and to reduction in pain and disease symptoms
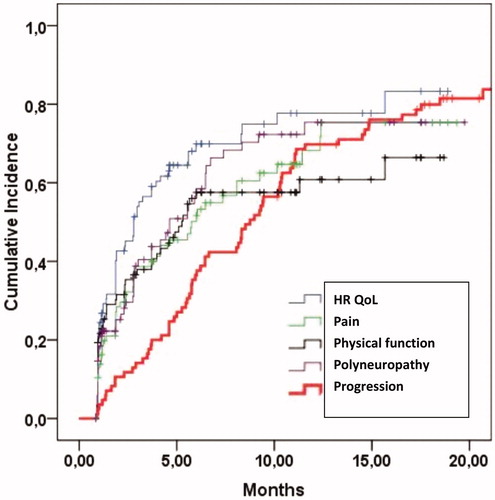
Time to deterioration of the health-related QoL and neuropathy by MID ≥10 was shorter (median 2.8 and 4.6 months, respectively) compared to time to objective signs of disease progression [median: 8.8 months (), and time to deterioration of physical functioning and pain was also shorter than time to progression (median 5.3 and 5.8 months, respectively].
Variables associated with PFS and OS
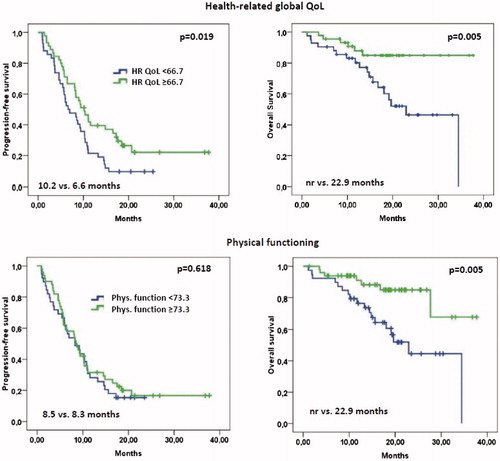
Global health-status/QoL and physical functioning were associated with survival (). PFS and OS were significantly longer in patients with a health-status/QoL score of 66.7, which was the median value at baseline, or greater compared to those with lower scores (median: 10.2 vs 6.6 months, p = .019, and not reached vs 22.9 months, p = .005, respectively). Similarly, when the median score of 77.3 of physical functioning was used as cutoff, OS was found to be significantly longer in those with higher scores (not reached vs. 22.9 months, p = .005), but for PFS no difference between both groups was noted (median 8.5 vs 8.3 months, p = .618).
Discussion
Traditionally, physicians’ primary treatment goal was improvement in response rate, quality of response, PFS, and OS. With patients living longer, increasing patient involvement in decision-making, and increased focus on QoL data by regulatory authorities [Citation26,Citation27], patient-reported outcome data have become an essential source of information, both in clinical trials and in clinical practice [Citation28]. This study highlights the significant impairment of most dimensions of QoL in our patients compared to the general population, shows improvement and deterioration of specific QoL items during treatment with IxaThalDex, improvement in almost all items during ixazomib maintenance therapy, and a decrease of specific QoL items already a few months before the criteria for progressive disease were met.
Although the impact of myeloma on QoL may vary greatly between individual patients depending on the stage, aggressiveness of the disease, treatment, and the biologic fitness of the affected patients, many dimensions of physical QoL are severely impaired, particularly when compared to the general population. The greatest impairment was noted for role, social, and physical function, followed by fatigue and pain. Other dimensions, such as health-related QoL, emotional well-being, cognitive function, dyspnea, appetite loss, and constipation, were also markedly impaired, but differences did not reach the MID (Δ ≥10), indicating men’s ability to adapt to difficult life situations. The baseline scores obtained in our patients are similar to those reported for the ENDEAVOR study comparing carfilzomib-dexamethasone with bortezomib-dexamethasone [Citation12], the TOURMALINE MM-1 trial comparing ixazomib-lenalidomide-dexamethasone with lenalidomide-dexamethasone [Citation8], the ASPIRE study comparing carfilzomib-lenalidomide-dexamethasone with lenalidomide-dexamethasone [Citation29], but more favorable compared to the patient populations enrolled in the first-line treatment studies FIRST [Citation23] and VISTA [Citation30]. It remains unclear whether this difference in baseline QoL scores between older and recent trials is a random observation, or the result of a more careful selection of study patients for the more recent studies, or differences in disease status.
Induction therapy with IxaThalDex resulted in the intended reduction of pain, but was associated with increasing neuropathy, while most other dimensions of QoL assessed, notably health-related QoL, physical functioning, and emotional well-being, remained rather stable during exposure to the treatment regimen. The 43 patients who started maintenance therapy had significantly less fatigue and somewhat less pain but significantly more neuropathy compared to those who discontinued treatment earlier. Other differences in terms of better health-related QoL, physical functioning, and fewer disease symptoms were noted, but these differences did not meet the required MID. Nevertheless, these results indicate that, like in most trials with a maintenance arm, patients must have responded to previous therapy, making them more likely to tolerate treatment longer and to benefit from prolonged therapy. Recognizing this phenomenon is important when interpreting the improvement in several dimensions of QoL at start of, and during maintenance therapy, which likely reflects a combination of both, the selection of patients with a priori better QoL, and the discontinuation of thalidomide and dexamethasone as side effects prone therapy. It also highlights excellent tolerance of ixazomib, particularly when given as single agent maintenance therapy, which was not associated with diarrhea or other relevant gastrointestinal toxicity.
The compliance rate was always above 95% as long as the patients were treated according to the protocol. Patient-reported assessments are the gold standard for QoL studies and results sometimes are discordant to physician’s assessments, who often underrate the severity of symptoms such as pain; rarely, as noted in the Tourmaline-1 study, contrary findings are made, where patients did not report more nausea and vomiting during ixazomib maintenance but care givers did. Of note, assessments were no longer performed after patients discontinued therapy either because of progression of disease, or because of any other reason, which is one of the limitations of this study. Other limitations may be due to the open label design of the study which is inherent in phase II trials, and due to the limited number of patients that completed maintenance therapy, which in part is unavoidable in patients with RRMM due to the increasing proportion of treatment failures during patient’s course of their disease. For rating of neuropathy only question 43 of the QLQ MY-20 was used, which is less informative than a neuropathy-specific questionnaire and has not been validated as such. Also, one should keep in mind that the EORTC instruments do not specifically capture satisfaction with sexual function, an item which is of importance to a proportion of patients with excellent control of their disease [Citation31,Citation32]. Furthermore, other instruments such as the FACT-MM PRO [Citation33] or the FACT-G could have been used, but we selected the EORTC QLQ C30 and QOL-MY20 questionnaire for this study as the latter tools have uniformly been used in all of the recent MM trials [Citation9,Citation12,Citation13,Citation28,Citation29,Citation23] with QoL assessments, a fact which facilitates comparison of results.
This study provides information on another important issue in MM, which may be informative for other cancers as well, namely on the presumed coupling between PFS and good QoL. Generally, physicians anticipate that patients enjoy a good QoL as long as they maintain their response status without meeting the criteria of progression. This dogma is not supported by the data obtained in this study, which show a clinically relevant deterioration in the scores of certain dimensions of quality of life such as global health-status/QoL, physical functioning, fatigue, and pain 2–3 months before progression of the underlying malignant disease (). In our previous analysis of the QoL data of the ENDEAVOR trial [Citation12], we observed similar findings indicating that this is a general phenomenon, which reduces the benefits of the time without progression by roughly 2–3 months. The most likely explanation is the rigidness of the criteria for myeloma progression, which do not include the clinical term ‘symptomatic progression’ [Citation34], but instead require a 25% increase in paraprotein and/or meeting other parameters, which usually are observed with some delay from the time the malignant clone starts to regrow again. Obviously, evolving relapse may induce symptoms way before the arbitrarily defined criteria of progression are met. This discrepancy is more frequently seen in heavily pretreated patients with an increasing uncoupling between M-protein secretion and tumor proliferation, because of increasing downregulation of XBP-1 resulting in increasing de-differentiation of myeloma cells and impairment of their protein synthesis capacity [Citation35]. Consequently, patient-reported outcomes need to be assessed in parallel to clinical metrics in order to allow a more precise appreciation of the impact of any therapeutic regimen.
Lastly, our results support previous findings of an association between QoL at baseline and survival in myeloma [Citation36,Citation37] and in other cancers [Citation38]. Better health-related QoL was associated with increased PFS, and both higher health-related QoL and physical functioning were associated with prolonged survival. Taken together, our results highlight that PRO assessments are an essential requirement for understanding patient’s individual needs and impairments, for prognostication, and for elucidating the impact of therapy on the various dimensions of QoL.
Author contributions
H. L., W. P., S. T. K., A. E., M. S., D. L., R. H., E. G., A. P., K. W., D. N., H. E., W. W., H. R., L. P., T. J., K. K., T. M., R. G., and N. Z. treated patients within the study, S. N. provided Clinical Lymphoma, Myeloma & Leukemia January 2019 data from individuals of the general population; A. H. provided statistical expertise. A. M. analyzed data. H. L. wrote a first draft of the manuscript. All authors participated in the manuscript development and approved the final version.
GLAL-2019-0649-File008.tif
Download TIFF Image (19.3 KB)Acknowledgments
The authors thank Dr. Daniela Wolkersdorfer for her support in conducting the trial within the AGMT (Arbeitsgemeinschaft Medikamentöse Tumortherapie).
Disclosure statement
H. L. received research funding from Takeda, Amgen; Speaker’s Bureau from Takeda, Amgen, Janssen, BMS, Celgene; and consultancy fees from PharmaMar. S. K. received consultancy fees from Takeda. A. H. received Honoraria from Roche. E. G. received Honoraria from Takeda, Janssen, Amgen, BMS and Advisory Board from Takeda, Janssen, Amgen, Novartis. A. P. received Honoraria and Advisory Board from Takeda, Celgene. K. W. received Honoraria from Novartis, Janssen, Celgene, Amgen, Onyx, Takeda, BMS and consultancy fees from Janssen, Celgene, Amgen, BMS, Takeda, Onyx. H. E. received Speaker’s Bureau, Advisory Board from Celgene, Janssen, Amgen, BMS, Novartis and consultancy fees, Honoraria from Celgene, Janssen, BMS, Amgen. W. W. received research funding from Amgen, BMS, Celgene, Janssen, Novartis, Roche, Takeda and Advisory Board/Consultancy fees from Amgen, BMS, Celgene, Janssen, Novartis, Pfizer, Roche, Sandoz, Takeda. TM received Honoraria from Takeda. NZ received honoraria from Takeda, Celgene, Janssen, Amgen. The remaining authors declare no competing financial interests.
Additional information
Funding
References
- Landgren O, Iskander K. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med. 2017;281(4):365–382.
- Stephens RJ, Hopwood P, Girling DJ, et al. Randomized trials with quality of life endpoints: are doctors' ratings of patients' physical symptoms interchangeable with patients' self-ratings? Qual Life Res. 1997;6(3):225–236.
- Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22(17):3485–3490.
- Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713–1721.
- Sonneveld P, Verelst SG, Lewis P, et al. Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia. 2013;27(10):1959–1969.
- Macquart-Moulin G, Viens P, Bouscary M-L, et al. Discordance between physicians' estimations and breast cancer patients' self-assessment of side-effects of chemotherapy: an issue for quality of care. Br J Cancer. 1997;76(12):1640–1645.
- Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–1634.
- Leleu X, Masszi T, Bahlis NJ, et al. Patient-reported health-related quality of life from the phase III TOURMALINE-MM1 study of ixazomib-lenalidomide-dexamethasone versus placebo-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Am J Hematol. 2018;93(8):985.
- Dimopoulos MA, Gay F, Schjesvold F, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019;393(10168):253–264.
- Johnson DC, Corthals SL, Walker BA, et al. Genetic factors underlying the risk of thalidomide-related neuropathy in patients with multiple myeloma. J Clin Oncol. 2011;29(7):797–804.
- Royle K-L, Gregory WM, Cairns DA, et al. Quality of life during and following sequential treatment of previously untreated patients with multiple myeloma: findings of the Medical Research Council Myeloma IX Randomised Study. Br J Haematol. 2018;182(6):816–829.
- Ludwig H, Moreau P, Dimopoulos MA, et al. Health-related quality of life in the ENDEAVOR study: carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J. 2019;9(3):23.
- Ludwig H, Poenisch W, Knop St, et al. Ixazomib-thalidomide-dexamethasone for induction therapy followed by Ixazomib maintenance treatment in patients with relapsed/refractory multiple myeloma. Br J Cancer. 2019; in print.
- Nolte S, Liegl G, Petersen MA, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer. 2019;107:153–163.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Cocks K, Cohen D, Wisløff F, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43(11):1670–1678.
- Katz S, Ford AB, Moskowits RW, et al. Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914–919.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Fayers P, Aaronson NK, Bjordal K, et al. The EORTC QLO-C30 Scoring manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001.
- Osoba D, Bezjak A, Brundage M, et al. Analysis and interpretation of health-related quality-of-life data from clinical trials: basic approach of The National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer. 2005;41(2):280–287.
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152.
- Delforge M, Minuk L, Eisenmann J-C, et al. Health-related quality-of-life in patients with newly diagnosed multiple myeloma in the FIRST trial: lenalidomide plus low-dose dexamethasone versus melphalan, prednisone, thalidomide. Haematologica. 2015;100(6):826–833.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481.
- Bland JM, Altman DG. The logrank test. BMJ. 2004;328(7447):1073.
- Basch E, Geoghegan C, Coons SJ, et al. Patient-reported outcomes in Cancer Drug Development and US Regulatory Review: perspectives from industry, the food and drug administration, and the patient. JAMA Oncol. 2015;1(3):375–379.
- Gnanasakthy A, Barrett A, Evans E, et al. A review of patient-reported outcomes labeling for oncology drugs approved by the FDA and the EMA (2012-2016). Value Health. 2019;22(2):203–209.
- Maguire R, Fox PA, McCann L, et al. The eSMART study protocol: a randomised controlled trial to evaluate electronic symptom management using the advanced symptom management system (ASyMS) remote technology for patients with cancer. BMJ Open. 2017;7(5):e015016.
- Stewart AK, Dimopoulos MA, Masszi T, et al. Health-related quality-of-life results from the open-label, randomized, phase III ASPIRE trial evaluating carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in patients with relapsed multiple myeloma. J Clin Oncol. 2016;34(32):3921–3930.
- Delforge M, Dhawan R, Robinson D, et al. Health-related quality of life in elderly, newly diagnosed multiple myeloma patients treated with VMP vs. MP: results from the VISTA trial. Eur J Haematol. 2012;89(1):16–27.
- Catamero D, Noonan K, Richards T, et al. Distress, fatigue, and sexuality: understanding and treating concerns and symptoms in patients with multiple myeloma. CJON. 2017;21(5):7–18.
- Osborne TR, Ramsenthaler C, de Wolf-Linder S, et al. Understanding what matters most to people with multiple myeloma: a qualitative study of views on quality of life. BMC Cancer. 2014;14:496.
- Wagner LI, Robinson D, Weiss M, et al. Content development for the Functional Assessment of Cancer Therapy-Multiple Myeloma (FACT-MM): use of qualitative and quantitative methods for scale construction. J Pain Symptom Manage. 2012;43(6):1094–1104.
- Dancey JE, Dodd LE, Ford R, et al. Recommendations for the assessment of progression in randomised cancer treatment trials. Eur J Cancer. 2009;45(2):281–289.
- Carrasco DR, Sukhdeo K, Protopopova M, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11(4):349–360.
- Strasser-Weippl K, Ludwig H. Psychosocial QOL is an independent predictor of overall survival in newly diagnosed patients with multiple myeloma. Eur J Haematol. 2008;81(5):374–379.
- Viala M, Bhakar AL, de la Loge C, et al. Patient-reported outcomes helped predict survival in multiple myeloma using partial least squares analysis. J Clin Epidemiol. 2007;60(7):670–679.
- Gotay CC, Kawamoto CT, Bottomley A, et al. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363.