Abstract
Angioimmunoblastic T-cell lymphoma (AITL) is a histological subtype of peripheral T-cell lymphoma associated with a poor prognosis. This post-hoc pooled analysis aims to provide insight about the efficacy of pralatrexate monotherapy in a subset of twenty-nine patients with relapsed or refractory (r/r) AITL drawn from two prospective registration trials completed in China and Japan. After a median of two prior lines of therapy, an overall response rate of 52% (15/29 patients; 95% CI 0.34, 0.70) was demonstrated. The estimated median duration of response, progression free survival (PFS) and overall survival (OS) were 6.4 months (196 days), 5.0 months (151 days), and 18.0 months (548 days), respectively. Grades 1–3 mucositis was observed in twenty-three patients (79.3%); and hemato-toxicity in twenty-six (89.7%) patients. Results of this analysis corroborate with data from two previously reported US retrospective cohorts, supporting the potential benefits of pralatrexate monotherapy in patients with r/r AITL.
Introduction
Approximately 20% of peripheral T-cell lymphoma (PTCL) cases are angioimmunoblastic T-cell lymphoma (AITL) [Citation1], making AITL the second most common type of PTCL after PTCL-NOS (not otherwise specified) [Citation2]. First-line therapy is most commonly CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or a similar regimen [Citation3]. However, the 5-year failure-free survival rate for AITL patients is as low as 18%; 82% of patients relapse within 5 years or have disease that is refractory to first-line therapy [Citation4]. Prognosis for individuals with relapsed or refractory AITL (r/r AITL) is poor, with a median overall survival (OS) of just 15 months after the first instance of relapse or progression [Citation5]. Moreover, disease control becomes increasingly difficult with each disease recurrence as evidenced by an OS of 8.3 months after second progression/relapse [Citation5]. Therefore, development and assessment of salvage agents are critical.
Current assessments of the efficacy and safety of available treatments for r/r AITL are generally based on very small patient numbers [Citation6–8]. In many instances, efficacy or safety results were reported for the PTCL population as a whole rather than the specific subtype of AITL [Citation9–11]. Moreover, patients with r/r PTCL have historically been treated with chemotherapy regimens approved for the treatment of B-cell lymphomas [Citation12,Citation13]. Indeed, due to the paucity of studies prospectively comparing treatments for r/r AITL, little is known about the relative efficacies of available agents.
Based on findings from the phase II, pivotal PROPEL trial, pralatrexate was the first single agent that was approved in most areas of the world and initially by the United States Food and Drug Administration (FDA) for r/r PTCL [Citation7]. While the majority of the 111 PTCL patients enrolled in PROPEL had unspecified disease (59 patients, 53%), most subtypes were represented, including 13 patients (12%) with AITL. In evaluable patients, the objective overall response rate (ORR) associated with pralatrexate in the PTCL population was 29% (32/109); in the AITL subset the ORR was 8% (1/13) [Citation7]. Of note, patients in the PROPEL trial were heavily pretreated, with a median of 3 prior treatments (range 1–12).
Since PROPEL, two similar prospective studies examining the efficacy and safety of pralatrexate have been conducted as part of their local regulatory requirements: one in China reported by Hong et al. [Citation14] and the other in Japan reported by Maruyama et al. [Citation15]. The trial conducted in China showed an ORR of 52% in 71 patients with r/r PTCL and an ORR of 55% in 20 patients with r/r AITL treated with pralatrexate [Citation14]. Results from the multicenter phase I/II trial in Japan showed an ORR of 45% in 20 patients with r/r PTCL and an ORR of 44% with pralatrexate treatment in 9 patients with r/r AITL [Citation15]. Here, we present a post-hoc subset analysis of patients with r/r AITL, using data pooled from these two studies.
Methods
Clinical trials
Based on limited data in different populations and need for additional regulatory approved therapies for PTCL, two trials were undertaken, one in China and the other in Japan, to investigate the efficacy and safety of pralatrexate in Asian patients. The trial conducted in China (FOT12-CN-301) was a confirmatory, phase 3, single-arm, open-label study in patients ≥ 18 years old that were recruited from 15 hospitals in China [Citation14]. Patients were enrolled to the trial from September 2015 to November 2016 (ClinicalTrials.gov identifier: NCT03349333). The trial conducted in Japan (PDX-JP1) was an open-label, single-arm, phase I/II study in patients ≥ 20 years old that were recruited from 12 centers in Japan [Citation15]. Patients were enrolled to the trial from March 2014 to September 2015 (ClinicalTrials.gov identifier: NCT02013362). For both studies, all patients gave written informed consent. Both studies were conducted in accordance with the Declaration of Helsinki as well as the Ordinance on Good Clinical Practice. At each participating institution, the studies were approved by the Institutional Review Board and/or Ethics Committee.
Patients, treatment, and assessments
This combined analysis of patients treated with single-agent pralatrexate is focused on r/r AITL, defined as having failed at least one prior line of therapy, with no upper limit on the number of prior therapies. The methodology used by the two prospective trials (as previously reported) was similar to the PROPEL trial: studies were conducted in similar patient populations, used the same treatment regimen, and reported on similar outcome measures [Citation14,Citation15]. PTCL diagnosis for each patient was confirmed by central pathology review. Pralatrexate 30 mg/m2 was administered intravenously once weekly for 6 weeks in 7-week cycles and continued for 24 months (Chinese study only) or until disease progression, treatment intolerance, investigator decision, withdrawal of consent; or initiation of new PTCL therapy or a prohibited concomitant drug or nonpharmacologic therapy or pregnancy (Japanese study only). Both trials evaluated the efficacy and safety of pralatrexate treatment. All treatment responses were assessed by central, independent review. Adverse events (AE) of interest for the present pooled analysis included oral mucositis/mucositis/stomatitis and hematological toxicities (anemia, decreased hemoglobin, granulocytopenia, leukopenia, lymphopenia, neutropenia/febrile neutropenia, pancytopenia, and thrombocytopenia). Mucositis is a common adverse event that occurs during pralatrexate treatment and requires proactive management; hematological toxicities are common during treatment for hematologic malignancies; thus, these two types of events were chosen as treatment-related adverse events of interest. If a patient experienced multiple episodes of the same adverse event, the event occurrence with the highest grade was used for analysis. If an adverse event caused a delay of treatment, a dose reduction and a discontinuation of treatment, only the worse outcome (discontinuation) was noted.
Statistical analysis
A post-hoc analysis of pralatrexate efficacy in patients with r/r AITL was performed based on patient level data extracted from both studies [Citation14,Citation15]. Twenty patients from the Chinese study (11 responders) [Citation14] and 9 patients from the Japanese study (4 responders) were included [Citation15].
A random-effects model, which assumes and accounts for heterogeneity in treatment effect sizes across studies, was employed to calculate the aggregate ORR. In a random-effects model, the weight assigned to each study is not only determined by the study sample size, but it is also based on the effect size itself. Therefore, if a study is an outlier but has a high sample size, it will not have as much influence on the aggregate result in a random-effects model as compared to a fixed-effects model. Additionally, the double arcsine transformation was applied to the raw study values prior to implementing the model. This transformation was applied to account for the problem of confidence limits outside the 0 to 1 range that can occur when modeling on the natural scale and the problem of variance instability that can occur when modeling on the natural scale or the logit transformed scale. The double arcsine transformed ORR from the model was then back transformed to a proportion for ease of interpretation.
To investigate the existence of study heterogeneity, a Cochran’s Q-test was performed which provided a p-value to determine if there was evidence of heterogeneity. However, the test has poor power to identify heterogeneity when there are only a few studies included in the meta-analysis. Thus, a more precise measure of heterogeneity (I2), was also calculated to indicate the percentage of variance in a meta-analysis that is attributable to study heterogeneity (from 0 to 100%). Time to event analysis for duration of response (DoR), PFS, and OS were carried out using the Kaplan–Meier method. DoR was derived based on responders and it was measured from first response date to the date of progressive disease (PD) or death, whichever came first. PFS and OS were derived based on the safety population for the Chinese study and the full analysis set for the Japanese study. PFS was measured from first treatment date until PD or death, whichever came first. OS was measured from first treatment date until death.
Results
Patient characteristics
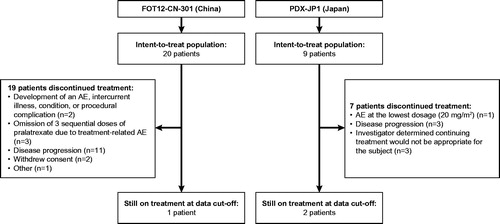
Patient characteristics for each study are described in . There were 29 patients included in the pooled analysis: 20 patients from the Chinese study and 9 from the Japanese study. Of these 29 patients, 15 were female and 14 were male. The median age was 62 years, with a range of 42–83. The median number of previous therapies was 2, with a range of 1–8.
Table 1. Patient characteristics and efficacy.
Patient disposition is described in . Of these 29 patients, reasons for discontinuation included development of an AE, intercurrent illness, condition, procedural complication or an intolerable AE at the lowest dose (20 mg/m2; n = 3), determination by an investigator that continuing treatment would not be appropriate (n = 3), omission of 3 sequential doses of pralatrexate due to a treatment-related AE (n = 3), disease progression (n = 14), withdrawal of consent (n = 2), or other reasons (n = 1). At the data cutoff (21 July 2017 for the Chinese study and 28 December 2015 for the Japanese study), 1 patient from the Chinese study and 2 patients from the Japanese study were still on treatment.
Efficacy
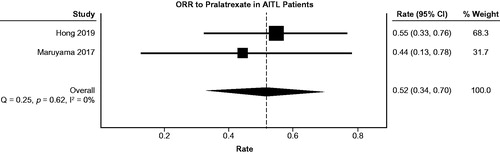
Several measures of efficacy were obtained. A pooled analysis revealed an ORR of 52% (95% confidence interval (CI): 0.34–0.70) in a population of 29 patients with AITL who received at least one dose of study medication (). The p-value from the Q-test was 0.62 and the I2 was 0% (95% CI: 0 to 0%). The non-significant p-value indicates that there was not a significant difference in results between the 2 studies. Any differences between study results are therefore most likely due to chance, rather than an actual difference in treatment effect.
Figure 2. A pooled analysis showed an ORR of 52% (95% confidence interval: 0.34, 0.70) in a population of 29 patients with AITL. The size of each square in the forest plot is proportional to the weight assigned to each study in the model. The diamond symbolizes the aggregate ORR and 95% CI. The p-value from the Q-test was .62 and the I2 was 0% (95% CI = 0 to 0%).
Other measures of efficacy are listed in . Out of 29 patients, 4 achieved a complete response (CR; although 3 were unconfirmed), 11 achieved a partial response (PR), 3 had stable disease (SD), 8 had PD, and 3 patients were not evaluable for response (NE). For responding patients, the median time to response (TtR) was 1.4 months (43 days with a range of 11–244 days). The median time on treatment was 1.87 months (57 days with a range of 1–540 days).
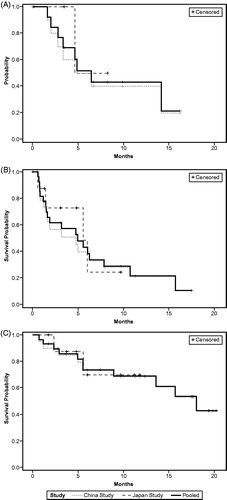
The estimated median DoR in 15 responders was 6.4 months (196 days, 95% CI 3.3 months-not reached; ). Two responders from the Japanese trial were still in response at the time of data cutoff. The estimated median PFS was 5.0 months (151 days, 95% CI 1.6–7.9 months; ), and estimated median OS was 18.0 months (548 days, 95% CI 13.6 months-not reached; ). Eighteen patients were still alive at the end of data collection (including two patients who withdrew from the study). Of these censored patients, the median follow-up time was 11.28 months (343 days, range 52–614 days).
Safety
For this analysis, the treatment-related adverse events (TRAEs) of interest were oral mucositis/stomatitis and hematological toxicities (including anemia, decreased hemoglobin, granulocytopenia, leukopenia, lymphopenia, neutropenia/febrile neutropenia, pancytopenia, and thrombocytopenia). Twenty-three out of the 29 patients (79.3%) experienced oral mucositis/mucositis/stomatitis (). While there were no Grade 4 events reported, 8/29 (27.6%) patients and 9/29 (31.0%) patients experienced a Grade 2 and a Grade 3 event, respectively. Although not statistically evaluated, rates of TRAEs of interest were similar between Chinese and Japanese patient populations.
Table 2. Safety (mucositis/oral mucositis/stomatitis and hematological toxicities as treatment-related adverse events of interest).
Discussion
The ORR of 52% observed in this pooled analysis of 29 patients with AITL is in contrast to the 8% ORR reported in the PROPEL trial (n = 13), suggesting that pralatrexate appears effective as a second-line treatment for AITL. This discrepancy recalls the familiar notion of interpreting results with caution, especially when working with a limited sample size in a rare disease population. However, the ORR of pralatrexate observed in this pooled analysis is comparable to that of other single agents that have been approved for use in r/r PTCL in China, Japan, and the United States () [Citation6,Citation16–24]. These ORRs range from 17% to 54%, with equally small populations in most of the studies. Moreover, evidence corroborating pralatrexate single-agent efficacy in r/r AITL has begun to emerge from regions outside the United States, where pralatrexate is gaining regulatory approval. For example, data from an Australian retrospective study of 8 patients with r/r AITL treated with pralatrexate yielded an ORR of 38% [Citation25]. And results of a multicenter study of pralatrexate in 21 Taiwanese patients with r/r PTCL and a median of one prior line of therapy showed a 71% ORR in the AITL population (5/7 patients) [Citation26].
Table 3. Efficacy of single agent treatment options for r/r AITL. NA indicates that this data is not available for the subgroup of r/r AITL patients (may be only available for the r/r PTCL group as a whole).
We strove to avoid typical limitations of pooled analyses (e.g. study heterogeneity, methodology and data extraction errors) by including a homogenous target patient population for which patient level data were available. It is quite challenging to put the results of this pooled analysis of single-agent pralatrexate efficacy into context with combination chemotherapy efficacy for r/r AITL because many of the older studies evaluating combination chemotherapy were retrospective in nature or included a heterogeneous lymphoma population. The few studies evaluating combination chemotherapy in a strict r/r AITL or PTCL population (with small sample sizes) showed comparable ORRs. A small retrospective study of a modified ESHAP regimen (etoposide, methylprednisolone, high-dose cytarabine, and carboplatin) in 9 patients with r/r AITL reported an ORR of 44% [Citation27]. A study of the GDP regimen (gemcitabine, dexamethasone, cisplatin) in 25 r/r PTCL patients (4 with AITL) reported an ORR of 72% in the combined patient population, but did not report ORR for AITL patients specifically [Citation10]. Similarly, a study of the ICE (ifosfamide, carboplatin, etoposide) regimen in 15 r/r PTCL patients (5 with AITL) reported an ORR of 20% in the combined patient population [Citation11]. This same study also reported an ORR of 69% for the DexaBEAM (dexamethasone, carmustine, etoposide, cytarabine, melphalan) regimen in 16 patients with r/r PTCL (2 with AITL).
In addition to the favorable ORR observed in this pooled analysis, the estimated median OS of 18.0 months was notable and corroborates with other recent evidence. A case-match control analysis of the PROPEL study found that r/r AITL patients treated with pralatrexate had an estimated OS of 9.77 months compared to 5.5 months for the matched control historical population (HR 0.448; 95% CI 0.175–1.142) [Citation22]. One retrospective analysis assessed the impact of pralatrexate or romidepsin on the outcome of patients with AITL (n = 141) or PTCL-NOS (n = 180) [Citation5]. In a multivariate analysis assessing risk factors after first progression or relapse, treatment with pralatrexate at any time during therapy was found to be associated with a significantly longer OS after first relapse or progression in AITL patients (HR 0.39; 95% CI 0.16–0.97; p = .044).
The majority of other trials of single agents do not report DoR, PFS, or OS in an r/r AITL patient population (see ). In these studies, reported median DoR ranged from 5.5 months [Citation6] to 23 months [Citation24], although this second value reflected DoR from a single responding patient. Reported median PFS ranged from 4.8 months to 6.7 months [Citation6,Citation17]. To our knowledge, no other single agents besides pralatrexate have reported OS data for r/r AITL. It is challenging to compare the efficacy of pralatrexate with combination chemotherapy due to the lack of data in r/r AITL patients for this latter therapy type. A study of a modified ESHAP regimen in 9 patients with r/r AITL did not report DoR but did report a median PFS of 4 months and a median OS of 33.1 months [Citation27]. A study of the GDP regimen in 25 r/r PTCL patients (4 with AITL) did not report DoR or any data for r/r AITL patients specifically, but reported a median PFS of 9.3 months; the median OS was not reached with a median follow-up duration of 27.1 months [Citation10]. A study of the ICE regimen reported a median PFS of 2 months and a median OS of 29.8 months in the r/r PTCL patient population [Citation11]. For the DexaBEAM regimen, the median PFS was 6.4 months and the median OS was 22.8 months in the combined patient population [Citation11].
In addition to highlighting the efficacy of pralatrexate in r/r AITL in general, our findings appear to suggest improved performance of pralatrexate in earlier lines of treatment for r/r PTCL. Indeed, evidence in three different r/r AITL populations shows improved ORR with fewer median number of prior therapies: with 1 prior line, 71.4% ORR [Citation26]; with 2 prior lines, 52% ORR (current analysis), and with 3 prior lines, 8% ORR (PROPEL) [Citation7]. These results corroborate with diminishing OS advantage after first, second, or third progression/relapse (15, 8.3, and 6 months, respectively) in a retrospective analysis of AITL patients who received pralatrexate or romidepsin [Citation5].
Results of this pooled analysis suggest the efficacy of pralatrexate in r/r AITL may be better than previously thought. After consideration of these data, it is reasonable that pralatrexate should be considered as a viable treatment option for r/r AITL patients, especially for the second-line or third-line setting. Future research should prospectively compare with the efficacy of different approved agents in randomized clinical trials.
Acknowledgments
The authors would like to thank Tomoko Murase and Rieko Ichihara, of Mundipharma Japan for their assistance in statistical support for this manuscript. Medical writing support was provided by Phillips Gilmore Oncology Communications and CoCre8ed Consulting.
Disclosure statement
JZ: Nothing to disclose. EMY: Employee of Mundipharma. YM: Has received grants and honoraria from Takeda and Kyowa Hakko Kirin Co; and has also received honoraria from Chugai, Novartis, Otsuka, Astellas, AsahiKASEI, Sumitomo Dainippon, Mochida, Bristol-Myers Squibb, Pfizer, Nippon Shinyaku, Janssen, Celgene, Eisai, Mundipharma, and Meiji Seika. KT: Has received grants and personal fees from Mundipharma, Eisai, Takeda, Kyowa Hakko Kirin, Celgene, Chugai Pharma, and Ono Pharmaceutical; has also received personal fees from Zenyaku Kogyo, HUYA Bioscience International, Yakult, Daiichi Sankyo, Bristol-Myers Squibb, Meiji Seika Kaisha, Solasia Pharma, and Verastem; and has received grant support from Abbvie and Janssen.
Additional information
Funding
References
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31(2):240–246.
- de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br J Haematol. 2010;148(5):673–689.
- Lunning MA, Vose JM. T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129(9):1095–1102.
- Vose J, Armitage J, Weisenburger D, International T-Cell Lymphoma Project. International peripheral T-Cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130.
- Chihara D, Fanale MA, Miranda RN, et al. The survival outcome of patients with relapsed/refractory peripheral T-cell lymphoma-not otherwise specified and angioimmunoblastic T-cell lymphoma. Br J Haematol. 2017;176(5):750–758.
- Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123(20):3095–3100.
- O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182–1189.
- Toumishey E, Prasad A, Dueck G, et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer. 2015;121(5):716–723.
- Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103(8):2920–2924.
- Park B-B, Kim WS, Suh C, et al. Salvage chemotherapy of gemcitabine, dexamethasone, and cisplatin (GDP) for patients with relapsed or refractory peripheral T-cell lymphomas: a consortium for improving survival of lymphoma (CISL) trial. Ann Hematol. 2015;94(11):1845–1851.
- Mikesch J-H, Kuhlmann M, Demant A, et al. DexaBEAM versus ICE salvage regimen prior to autologous transplantation for relapsed or refractory aggressive peripheral T cell lymphoma: a retrospective evaluation of parallel patient cohorts of one center. Ann Hematol. 2013;92(8):1041–1048.
- Schmitz N, Prange E, Haferlach T, et al. High-dose chemotherapy and autologous bone marrow transplantation in relapsing angioimmunoblastic lymphadenopathy with dysproteinemia (AILD). Bone Marrow Transplant. 1991;8(6):503–506.
- Lindahl J, Kimby E, Björkstrand B, et al. High-dose chemotherapy and APSCT as a potential cure for relapsing hemolysing AILD. Leuk Res. 2001;25(3):267–270.
- Hong X, Song Y, Huang H, et al. Pralatrexate in Chinese patients with relapsed or refractory peripheral T-cell lymphoma: a single-arm, multicenter study. Targ Oncol. 2019;14(2):149–158.
- Maruyama D, Nagai H, Maeda Y, et al. Phase I/II study of pralatrexate in Japanese patients with relapsed or refractory peripheral T-cell lymphoma. Cancer Sci. 2017;108(10):2061–2068.
- O'Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33(23):2492–2499.
- Shi Y, Jia B, Xu W, et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10(1):69.
- Shi Y, Dong M, Hong X, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26(8):1766–1771.
- Maruyama D, Tsukasaki K, Uchida T, et al. Multicenter phase 1/2 study of forodesine in patients with relapsed peripheral T cell lymphoma. Ann Hematol. 2019;98(1):131–142.
- Zinzani PL, Karlin L, Radford J, et al. European phase II study of mogamulizumab, an anti-CCR4 monoclonal antibody, in relapsed/refractory peripheral T-cell lymphoma. Haematologica. 2016;101(10):e407–e410.
- Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-CC chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32(11):1157–1163.
- O’Connor O, Marchi E, Volinn W, et al. Strategy for assessing new drug value in orphan diseases: an international case match control analysis of the PROPEL study. JNCI Cancer Spectr. 2018;2(4):pky038.
- Coiffier B, Pro B, Prince HM, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7(1):11.
- Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117(22):5827–5834.
- Admojo L, Van Der Weyden C, Gao C, et al. A retrospective analysis of pralatrexate efficacy and tolerability in Australia. Presented at Blood 2018 Annual Scientific Meeting; 2018 Oct 21–24, 2018; Brisbane Convention & Exhibition Centre, Australia.
- Wang M-C, Ko B-S, Chiou T-J, et al. Interim update from a multi-center study of pralatrexate in Asian patients with relapsed or refractory (R/R) peripheral T-cell lymphoma (PTCL). Presented at the 24th European Hematology Association Congress. 2019 Jun 15; Amsterdam, Netherlands.
- Kogure Y, Yoshimi A, Ueda K, et al. Modified ESHAP regimen for relapsed/refractory T cell lymphoma: a retrospective analysis. Ann Hematol. 2015;94(6):989–994.