Abstract
Relapsed/refractory multiple myeloma patients treated with pomalidomide and dexamethasone have an overall response rate (ORR) of ∼30% and median progression-free survival (PFS) of 4–5 months. Previous studies explored addition of weekly cyclophosphamide, but we hypothesized that daily dosing allows for better synergy. We report the open-label, single-center phase II study of pomalidomide, daily cyclophosphamide and weekly dexamethasone (PCD). Thirty-three patients were evaluable for efficacy and underwent 28-day cycles of pomalidomide (4 mg/day, D1-21), cyclophosphamide (50 mg b.i.d., D1-21) and weekly dexamethasone. All were lenalidomide-refractory and 55% were refractory to lenalidomide and proteasome inhibitor. ORR was 73%; median PFS and overall survival were 13.3 months and 57.2 months respectively. Grade 3/4 toxicities were primarily hematologic but manageable with dose reductions. Early disease progression correlated with MYC expression and flow cytometry demonstrates an activated microenvironment post-PCD. Addition of metronomic cyclophosphamide to pomalidomide and dexamethasone is a cost-effective, oral regimen with encouraging PFS.
Introduction
Despite recent improvements, the prognosis of patients with relapsed/refractory multiple myeloma (RRMM) is limited and developing strategies for patients refractory to first-line immunomodulatory drugs (IMiDs) and proteasome inhibitors remains crucial [Citation1]. Pomalidomide is an immunomodulatory compound with tumoricidal and antiangiogenic properties [Citation2]. In two large studies, RRMM patients treated with pomalidomide and dexamethasone achieved an overall response rate (ORR) of ∼30% and median progression-free survival (PFS) of 4–5 months [Citation3,Citation4].
The addition of the alkylating agent cyclophosphamide is known to improve efficacy of immunomodulatory compounds and overcome resistance [Citation5]. Consequently, the triplet regimen (pomalidomide, cyclophosphamide and corticosteroids) has been investigated with various dosing designs, summarized in [Citation6–10]. All used pomalidomide 4 mg daily on day (D) 1-21, except for Larocca et al. who used 2.5 mg daily on D1-28. Oral cyclophosphamide was typically given weekly (D1, 8, 15, ±22) at doses ranging from 300 to 400 mg, although Weisel et al. gave 500 mg/m2 intravenously on D1 and D15. The only other study to date giving cyclophosphamide on a non-weekly schedule was Larocca et al. where 50 mg was given every other day (D1-28). Overall response rates in these studies ranged from approximately 40–75% in patients with a median of 3–4 lines of prior therapy and 70–100% lenalidomide-refractoriness, with an associated PFS of 6.5–10.5 months and median overall survival (OS) 18.3 months to not reached. Grade 3+ neutropenia and thrombocytopenia rates varied and ranged from approximately 40–70%, and 5–20% respectively.
Table 1. Comparison of previous and current triplet pomalidomide, cyclophosphamide and corticosteroid studies.
Metronomic therapy with daily low-dose cyclophosphamide and a one-week respite to allow hematopoietic recovery might be better tolerated in terms of myelosuppression and more efficacious, due to the higher cumulative dose and longer co-administration with pomalidomide, allowing for sustained in-vivo drug synergy. This metronomic regimen has demonstrated efficacy in refractory B cell malignancies, by targeting the tumor vasculature and suppressing the surge of bone marrow-derived endothelial progenitor cells [Citation11,Citation12]. Importantly, metronomic cyclophosphamide is associated with reduced regulatory T cell (Treg) populations and T/NK cell activation in different tumors [Citation13–15], suggesting a mechanistic synergy with both immunomodulatory and antiangiogenic properties of pomalidomide. To date, the other study using metronomic cyclophosphamide dosing [Citation16] did not permit hematologic support (granulocyte stimulating factors (G-CSF) or platelet transfusions), nor a week respite, resulting in a low maximum tolerated dose of pomalidomide (2.5 mg). Moreover, little is known about biomarkers of response and progression with this triplet regimen. Here, we report the open-label, single-center phase II study of pomalidomide with daily low-dose cyclophosphamide and dexamethasone (PCD) and our translational findings (www.clinicaltrials.gov #NCT02176213).
Methods
Study design
This study was approved by the program for the protection of human subjects and the institutional review board at the Icahn School of Medicine at Mount Sinai. The primary objective was to evaluate ORR. Secondary objectives were to evaluate safety, response duration, OS and PFS, to study potential biomarkers of response, to assess the immune microenvironment and the genomic basis of response.
Patients started with 28-day cycles of pomalidomide 4 mg daily × 21 days; cyclophosphamide 50 mg b.i.d. × 21 days and dexamethasone 40 mg weekly × 3 (20 mg if age ≥75 years). Dose reduction for hematologic toxicity began with cyclophosphamide and alternated with pomalidomide (unless concurrent reduction of both agents was considered necessary by the treating physician). If the regimen was not tolerated after decreasing the dose for both agents twice, study treatment was discontinued.
Study population
Patients with RRMM and at least two prior lines of therapy, refractory to lenalidomide, with measurable disease, adequate performance status and organ function (creatinine <3 mg/dL, normal hepatic function, normal electrolytes) were eligible. Hematologic inclusion criteria were permissive (per µL absolute neutrophil count >1,000; platelets ≥50,000 or ≥30,000 if bone marrow (BM) plasma cell % < or ≥50% respectively). G-CSF and platelet support were permitted during screening and study treatment if needed. Patients were excluded if they had active infections, > grade (G) 2 peripheral neuropathy or hypersensitivity to thalidomide or lenalidomide. ORR was calculated using IMWG criteria PFS and OS were calculated by Kaplan-Meier analysis. Safety was assessed using Common Toxicity Criteria Adverse Event criteria.
Correlative methods
Correlative studies were performed on BM aspirates and peripheral blood (PB) collected at screening, at cycle 3 day 15 (C3D15), and at the time of progression. Detailed descriptions of immunohistochemistry, RNA-sequencing and workflow, multiparameter flow cytometry and Olink multiplex assay are included in the Supplemental Methods. RNA-sequencing data are publicly available at GEO under accession number GSE138717.
Results
Thirty-three patients completed cycle 1 of all three agents and were considered evaluable for efficacy and safety. The characteristics of these patients are summarized in . The median age was 65 years, with 24% of patients over the age of 70. Patients received a median of 3 prior lines of treatment, including autologous stem cell transplant (ASCT) in 85%. High-risk cytogenetic features (del17p13, t(4;14) and/or 1q21 gain) were present in 48% of patients. All patients were lenalidomide-refractory and 55% were refractory to both lenalidomide and proteasome inhibitor.
Table 2. Baseline patient characteristics and responses to treatment.
As shown in , there were 2 stringent complete responses (sCR), 2 complete responses (CR), 7 very good partial responses (VGPR), 13 partial responses (PR), 3 minimal responses (MR), 5 stable disease (SD) and 1 progressive disease (PD) for an ORR of 73% and a CBR (i.e. MR or greater for >2 months) of 82%. The median PFS was 13.3 months and median OS was 57.2 months ().
Figure 1. Kaplan-Meier progression free survival (A) and overall survival (B) curves for patients treated with pomalidomide, cyclophosphamide and dexamethasone (n = 33).
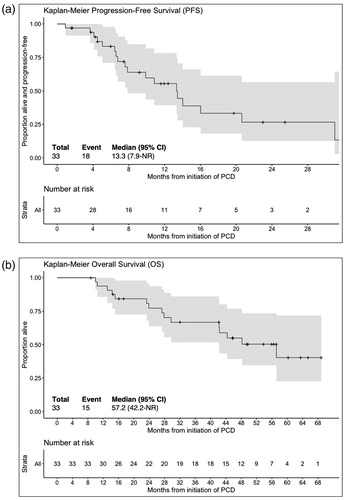
Two additional patients did not complete cycle 1: one withdrew from the study because of personal preference and another patient was taken off the study per treating physician’s discretion. All 35 patients were included in the safety evaluation. Grade 3/4 toxicities were primarily hematologic, with neutropenia in 31 (89%), thrombocytopenia in 8 (23%) and anemia in 6 (17%) but were generally not associated with sequelae of infections or bleeding and manageable with G-CSF administration and/or dose reductions. Twenty-nine patients (83%) required at least one dose of G-CSF. Febrile neutropenia was seen in only five (14%) patients. The rate of neutropenia is relatively higher than reported in earlier studies (), however, at baseline 10 (29%) patients had grade 1-2 neutropenia and also, 20 (57%) had grade 1-3 thrombocytopenia. Furthermore, 7 (21%) had baseline marrow with more than 50% plasma cell infiltration. Grade 3/4 infectious adverse were observed in 16 (46%) patients: 9 pneumonias, 4 viral upper respiratory infections, 1 toxoplasma encephalitis, 1 urinary tract infection and 1 skin infection. Other adverse effects potentially attributable to treatment were rash (n = 4), insomnia (4), peripheral neuropathy (3), fatigue (3), hypertension (3), weight gain (3), syncope (2), fall (2), hip pain (2) and 1 of each of the following: pneumonitis, systemic inflammatory response syndrome, venous thromboembolism, myelodysplastic syndrome, hyperglycemia, hematuria, muscle weakness, extremity edema, mood alteration, and mucositis. Neutropenia led to dose reduction of cyclophosphamide in 23 (70%) and pomalidomide in 9 (27%) patients. Cyclophosphamide and pomalidomide were reduced for other reasons (fatigue, hematuria, rash, neuropathy) in 3 (9%) and 7 (20%) patients, respectively. Dexamethasone dose reduction was necessary in 11 (33%) of patients.
The study protocol was modified after interim analysis showed higher soluble programmed death-ligand 1 (sPD-L1) in the BM plasma in patients with shorter PFS and an increase of sPD-L1 and PD-1 positive marrow-infiltrating lymphocytes at progression (Figure S6). These biomarkers have been correlated with a higher efficacy of immune checkpoint inhibitors [Citation17]. Consequently, treatment with a PD-L1 inhibitor (durvalumab) at the time of progression was allowed. Two patients were treated with durvalumab monotherapy after progression on PCD. However, one developed PD and the other achieved MR within 1 month but came off study due to autoimmune myocarditis, after which the addition of durvalumab was removed from the protocol.
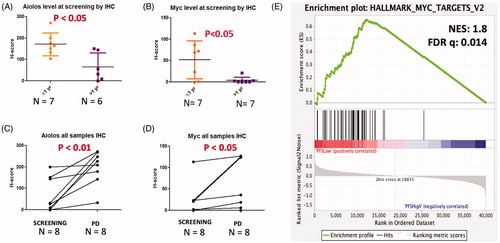
RNA-sequencing and multiparameter flow cytometry were performed on bone marrow aspirates and blood at screening, during therapy, and at progression to understand the mechanisms of response (Supplemental Methods). We found at the time of screening, increased MYC expression (both at the protein and RNA level) and increased protein levels of Aiolos to be associated with poor PFS (<1 year) (, Figure S1(A)). Gene set enrichment analysis confirmed the aberrant expression of MYC target genes in patients with short PFS ().
Figure 2. Increased protein expression of Aiolos and Myc and Myc target gene enrichment at screening are associated with poor PFS and PD. (A,B) Quantitative analysis of Aiolos and MYC H-score values in short vs. long PFS subjects (1-year cutoff). (C,D) The level of Aiolos and MYC in patient’s CD138+ plasma cells at screening and at disease progression. (E) GSEA identifies MYC Hallmark Targets V2 as most significantly enriched gene set in patients with PFS <1 year.
Flow cytometric data demonstrated a shift toward a more activated effector-memory T cell phenotype upon treatment at C3D15 with a decrease in naïve and terminally differentiated T cells both in PB and BM (Figure S2). We observed a significant increase of CD4 + OX40+ T cells (% of CD4+ T cells) in PB and a comparable upward trend in BM. A similar, albeit less pronounced increase at C3D15 was observed for CD4 + PD-1+ and CD4 + LAG3+ cells in the blood (Figure S3). We also noted a significant increase in regulatory T cells (Tregs) (Figure S4). The findings in other populations are summarized in Supplementary Results (Figure S5). The immunophenotypic changes were not significantly correlated with duration or depth of response.
Discussion
The addition of twice daily metronomic cyclophosphamide to pomalidomide and dexamethasone resulted in a very encouraging 73% ORR, 13.3 months PFS and 57.2 months OS, comparing favorably to similar treatment regimens studied before (). This is remarkable given the high-risk profile compared to other studies: nearly half the patients had high-risk cytogenetic features, all were lenalidomide-refractory and half were also refractory to proteasome inhibitors. The median age and number of treatment lines in our cohort were similar to those summarized in (with the exception of Lee et al. [Citation8] who studied PCD in the elderly and Garderet et al. [Citation7] who studied the role of PCD in the context of first relapse). The rates of refractoriness to lenalidomide and/or proteasome inhibitors were more varied between the cohorts in , which might contribute to some of the reported differences in outcome. A direct comparison between clinical trials should be interpreted with caution, however, due to heterogeneity in patient population and study design.
The regimen was generally well tolerated, with mainly hematologic toxicities. The neutropenia rates are higher than in many other pomalidomide-based regimens, including the previously reported studies with similar regimens in . The higher cytopenia rates may be in in part due to permissive hematologic inclusion criteria and significant baseline cytopenias. However, due to the encouraging PFS, patients in this study also remained on PCD approximately 3-7 months longer than the other regimens, which increases the time for adverse event capture. The cytopenias were typically not associated with infections or bleeding and manageable with G-CSF or dose reductions. However, given that more than half of the patients required dose reduction of cyclophosphamide, a starting cyclophosphamide dose of 50 mg daily is likely to be better tolerated in patients with heavy marrow involvement and/or baseline cytopenias. In those without these concerns, 50 mg b.i.d. may be reasonable initially but once an adequately deep response is achieved, reduction to once daily should be considered to limit toxicity.
Earlier studies on pomalidomide and dexamethasone in MM have shown conflicting results regarding the prognostic significance of CRBN and its targets [Citation14,Citation18–20]. In this study, increased expression of MYC and increased protein levels of Aiolos before treatment were associated with poor PFS (<1 year). These observations are consistent with MYC upregulation in patients with shorter PFS driven at least in part by upstream proteins, e.g. Ikaros/Aiolos. Previous studies have shown that dysregulation of the IRF4/MYC pathways is associated with lenalidomide resistance [Citation21–24], supporting our findings. Our data suggest that MYC expression at baseline could predict long-term PCD efficacy and should be taken into consideration when planning future treatment protocols. It also raises the hypothesis that more powerful immunomodulatory compounds or targeted inhibition of MYC may exert synergistic anti-malignant activity in RRMM.
Immunomodulatory compounds have been associated with a wide array of immunomodulatory effects, many of which have been demonstrated only in vitro [Citation2]. We observed a shift toward an activated T cell compartment, albeit with significant increase of Tregs between screening and C3D15. Notably, there was no association between PFS and Treg increase. Our findings suggest that PCD acts in part through promotion of an activated microenvironment that is partially reversed upon progression. Although these findings suggest a role for checkpoint inhibition, we observed myocarditis in one of two patients receiving durvalumab at progression. The role of strategies aimed at secondary checkpoints (e.g. TIGIT) might be a more viable strategy to enhance efficacy and durability of the PCD regimen, to be pursued in future clinical trials with scrupulous safety monitoring, given the concerns over potential adverse effects [Citation25].
Pomalidomide and dexamethasone have been used effectively in triplet regimens with other new agents in RRMM [Citation26–32]. Compared to cyclophosphamide, however, these are less cost-effective and not readily available within all healthcare systems. Our study confirms the efficacy of PCD, identifies MYC as a biomarker for inferior outcome and sheds light on how this drug combination alters the immune phenotype in the tumor micro-environment as well as in the blood. Although caution must be taken when comparing clinical trials, the PFS is amongst the highest published to date with an all oral, cost-effective regimen that is not significantly impacted by renal impairment.
Author contributions
AC provided investigation, conceptualization, methodology, analysis, resources, original draft, supervision review and edits of the manuscript. HJC, OVO, and SP provided analysis, investigation, resources, review and edits of the manuscript. AL, VL, DTM, SKS and OVO provided data analysis, creation of tables and figures for the correlative studies. NV provided formal analysis of the data, provision of study materials, research data management and creation of tables and figures, review and edits of the manuscript, and preparation of the data presentation. DM, JR, CI, KL, EF, ISM, JT, DV, EC, KZ, LL, GS, SC, MW, WEP and, AT provided research and investigation process, resources, review and edits of the manuscript, and visualization of the project. SJ, SP provided conceptualization, methodology, research and investigation process, review and edits of the manuscript, supervision, and funding acquisition of the project. All coauthors were provided final approval of the published version.
GLAL-2020-0536-File004.docx
Download MS Word (71.3 KB)Disclosures statement
The following authors have served as paid consultants from the identified companies: AC for Novartis, Celgene; SJ for Novartis, Celgene. SP has research support from Celgene and Karyopharm. SC, MW, WEP, and AT were Celgene employees at the time of the study. The following authors have no conflicts to disclose: OVO, HJC, NV, DM, JR, CI, KL, EF, ISM, JT, DV, EC, KZ, LL, GS, DTM, VL, SKS, AL.
Additional information
Funding
References
- Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443–2448.
- Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24(1):22–32.
- Dimopoulos MA, Palumbo A, Corradini P, et al. Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): a phase 3b study in refractory multiple myeloma. Blood. 2016;128(4):497–503.
- Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. The Lancet Oncology. 2013;14(11):1055–1066.
- Nijhof IS, Franssen LE, Levin MD, et al. Phase 1/2 study of lenalidomide combined with low-dose cyclophosphamide and prednisone in lenalidomide-refractory multiple myeloma. Blood. 2016;128(19):2297–2306.
- Baz RC, Martin TG, Lin HY, et al. Randomized multicenter phase 2 study of pomalidomide, cyclophosphamide, and dexamethasone in relapsed refractory myeloma. Blood. 2016;127(21):2561–2568.
- Garderet L, Kuhnowski F, Berge B, et al. Pomalidomide, cyclophosphamide, and dexamethasone for relapsed multiple myeloma. Blood. 2018;132(24):2555–2563.
- Lee HS, Kim K, Kim SJ, et al.; the Korean Multiple Myeloma Working Party (KMMWP). Pomalidomide, cyclophosphamide, and dexamethasone for elderly patients with relapsed and refractory multiple myeloma: a study of the Korean Multiple Myeloma Working Party (KMMWP-164 study. Am J Hematol. 2020;95(4):413–421.
- Trudel S, Tessoulin B, Jullien M, et al. Pomalidomide, cyclophosphamide, and dexamethasone for relapsed/refractory multiple myeloma patients in a real-life setting: a single-center retrospective study. Ann Hematol. 2019;98(6):1441–1447.
- Weisel KC, Scheid C, Zago M, et al. Addition of cyclophosphamide on insufficient response to pomalidomide and dexamethasone: results of the phase II PERSPECTIVE Multiple Myeloma trial. Blood Cancer J. 2019;9(4):45.
- Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7(8):455–465.
- Ruan J, Martin P, Coleman M, et al. Durable responses with the metronomic rituximab and thalidomide plus prednisone, etoposide, procarbazine, and cyclophosphamide regimen in elderly patients with recurrent mantle cell lymphoma. Cancer. 2010;116(11):2655–2664.
- Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4 + CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648.
- Sehgal K, Das R, Zhang L, et al. Clinical and pharmacodynamic analysis of pomalidomide dosing strategies in myeloma: impact of immune activation and cereblon targets. Blood. 2015;125(26):4042–4051.
- Hughes E, Scurr M, Campbell E, et al. T-cell modulation by cyclophosphamide for tumour therapy. Immunology. 2018;154(1):62–68.
- Larocca A, Montefusco V, Bringhen S, et al. Pomalidomide, cyclophosphamide, and prednisone for relapsed/refractory multiple myeloma: a multicenter phase 1/2 open-label study. Blood. 2013;122(16):2799–2806.
- Rosenblatt J, Avigan D. Targeting the PD-1/PD-L1 axis in multiple myeloma: a dream or a reality?. Blood. 2017;129(3):275–279.
- Schuster SR, Kortuem KM, Zhu YX, et al. The clinical significance of cereblon expression in multiple myeloma. Leuk Res. 2014;38(1):23–28.
- Gandhi AK, Mendy D, Waldman M, et al. Measuring cereblon as a biomarker of response or resistance to lenalidomide and pomalidomide requires use of standardized reagents and understanding of gene complexity. Br J Haematol. 2014;164(2):233–244.
- Qian X, Dimopoulos MA, Amatangelo M, et al. Cereblon gene expression and correlation with clinical outcomes in patients with relapsed/refractory multiple myeloma treated with pomalidomide: an analysis of STRATUS. Leuk Lymphoma. 2019;60(2):462–470.
- Amatangelo MD, Neri P, Ortiz M, et al. Resistance to Lenalidomide in Multiple Myeloma Is Associated with a Switch in Gene Expression Profile. Blood. 2015;126(23):1789–1789.
- Franssen LE, Nijhof IS, Couto S, et al. Cereblon loss and up-regulation of c-Myc are associated with lenalidomide resistance in multiple myeloma patients. Haematologica. 2018;103(8):e368–e371.
- Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006;108(6):2020–2028.
- Zhu YX, Shi C-X, Bruins LA, et al. Identification of lenalidomide resistance pathways in myeloma and targeted resensitization using cereblon replacement, inhibition of STAT3 or targeting of IRF4. Blood Cancer J. 2019;9(2):19.
- Krauss AC, Mulkey F, Shen Y-L, et al. FDA analysis of pembrolizumab trials in multiple myeloma: Immune related adverse events (irAEs) and response. J Clin Oncol. 2018;36(15_suppl):8008–8008.
- Badros A, Hyjek E, Ma N, et al. Pembrolizumab, pomalidomide, and low-dose dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;130(10):1189–1197.
- Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–981.
- Mark TM, Forsberg PA, Rossi AC, et al. Phase 2 study of clarithromycin, pomalidomide, and dexamethasone in relapsed or refractory multiple myeloma. Blood Adv. 2019;3(4):603–611.
- Mateos M-V, Blacklock H, Schjesvold F, et al.; KEYNOTE-183 Investigators. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6(9):e459–e469.
- Mikhael J, Richardson PG, Usmani SZ, et al. Final results of a phase Ib study of isatuximab (ISA) plus pomalidomide (Pom) and dexamethasone (dex) in relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2018;36(15_suppl):8038–8038.
- Shah JJ, Stadtmauer EA, Abonour R, et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood. 2015;126(20):2284–2290.
- Siegel DSD, Schiller GJ, Samaras CJ, et al. Pomalidomide (POM) + low-dose dexamethasone (LoDEX) + daratumumab (DARA) in relapsed and/or refractory multiple myeloma (RRMM) after lenalidomide (LEN)-based treatment (Tx) failure. J Clin Oncol. 2018;36(15_suppl):8027–8027.
