Abstract
Glasdegib, in combination with low-dose cytarabine (LDAC), is the first smoothened inhibitor approved for treatment of acute myeloid leukemia. Glasdegib plus LDAC is indicated for patients in whom therapy options are limited, e.g. older patients and those ineligible for intensive chemotherapy due to preexisting comorbidities. This review summarizes the recommendations of a panel of hemato-oncologists regarding the selection of patients best suited for treatment with glasdegib plus LDAC and the management during therapy with this combination. The panel considered the impact of concomitant medications and comorbidities during treatment with glasdegib plus LDAC, and discussed common adverse events (AEs) associated with glasdegib plus LDAC. Management strategies for AEs discussed by the panel included dose modifications, supportive care therapies, and prophylactic treatments. Finally, the panel highlighted the importance of patient communication and education regarding the possible AEs that may occur during treatment.
Introduction
Acute myeloid leukemia (AML) is a complex heterogeneous disease [Citation1–4]. Intensive induction/consolidation therapy gives the best chance for cure, but not all patients are candidates [Citation2,Citation5–7]. Selection is mostly subjective assessment based on clinical observations, with no universally accepted or validated tools to determine eligibility. Characteristics commonly considered in clinical practice include age, Eastern Cooperative Oncology Group performance status (ECOG PS), cytogenetic risk, and comorbidities [Citation2,Citation8–17]. In patients aged ≥60, the following variables were associated with complete remission (CR) or early death: age, de novo AML, laboratory parameters, and comorbidities [Citation18].
Evidence varies regarding intensive chemotherapy (IC) in older patients with AML [Citation13,Citation19–29]. Improved outcomes and survival benefits were reported in patients aged ≥60 who received IC regimens versus those who received no treatment; in some reports, this was irrespective of comorbidity burden [Citation19–24,Citation28,Citation29]. Others indicated that, despite high rates of CR, only a carefully selected subset of older patients with AML can be considered for IC [Citation13,Citation25–27].
Traditionally, patients ineligible for IC have been treated with low-dose cytarabine (LDAC) or hypomethylating agents (HMAs) [Citation2,Citation5–7]. However, a clearer understanding of AML pathogenesis has led to new options, with treatment selection based on patient and disease characteristics. Although decisions are sometimes steered by objective criteria (e.g. FMS-like tyrosine kinase 3 (FLT3) inhibitor for patients with FLT3 mutations), guidance is needed regarding patient selection and therapy management. A meeting of expert hemato-oncologists was held to define the use of glasdegib plus LDAC in treatment of older patients with AML, in particular, to define those best suited for this therapy and provide guidance on managing therapy-related adverse events (AEs). The recommendations of this expert panel are described here. An associated manuscript plain language summary can be found in Supplementary Materials.
Setting and methods
On 12 April 2019 in London, UK, nine hemato-oncologists from centers across Europe, Canada, and the USA participated in an expert panel. All had extensive experience in treating AML and the use of glasdegib plus LDAC, glasdegib as monotherapy, and/or glasdegib in combination with other therapies in patients with AML.
The experts discussed their clinical experience with standard and experimental treatments for AML, patient characteristics that influence their decisions, and AE management with glasdegib plus LDAC. These discussions were captured and formed the foundation of this manuscript that underwent critical review by all experts.
Approved treatments and related clinical trial experience
A number of therapies are approved by the US Food and Drug Administration (FDA) and/or European Medicines Agency (EMA) for treatment of patients with AML ineligible for IC ( and ). Decision-making criteria regarding patient eligibility for IC are both subjective (e.g. inclusion/exclusion criteria, clinical trial characteristic) and objective (e.g. label indication, age, mutations) ( and ).
Figure 1. Agents approved for treatment of newly diagnosed patients with AML ineligible for intensive chemotherapy. AML: acute myeloid leukemia; IC: intensive chemotherapy; IDH: isocitrate dehydrogenase; LDAC: low-dose cytarabine.
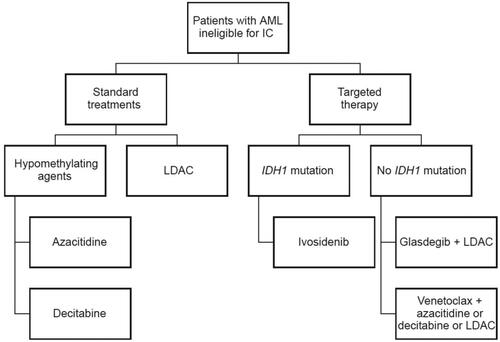
Table 1. Summary of agents approved for treatment of patients with AML ineligible for intensive chemotherapy.
Table 2. Summary of eligibility criteria in key clinical trials in patients with AML who are ineligible for intensive treatment.
Table 3. Summary of baseline characteristics in key clinical trials in patients with AML who are ineligible for intensive treatment.
Standard treatments
Decitabine and azacitidine are standard-of-care therapies for older patients and those ineligible for IC; LDAC is another available alternative treatment [Citation49]. In a randomized study, LDAC 20 mg twice daily (BID) was compared with hydroxyurea in patients primarily aged ≥60 ineligible for chemotherapy. Patients aged <70 were required to have additional comorbidities that precluded chemotherapy [Citation43]. Median age was 74 (range: 51–90). CR was achieved in 18% (LDAC) and 1% (hydroxyurea) and overall survival (OS) was better with LDAC (p=.0009). Common all-causality AEs during course 1 were cardiac (10% and 11%), nausea (6% each), and diarrhea (4% and 10%) with LDAC and hydroxyurea, respectively [Citation43].
A phase 3 study evaluated decitabine (20 mg/m2 intravenous (IV) on a five-day schedule) versus treatment choice in patients aged ≥65, ECOG PS 0–2, and poor/intermediate-risk cytogenetics [Citation47]. Median OS (mOS) and CR rates were 7.7 months and 15.7% (decitabine) and 5.0 months and 7.4% (treatment choice). Common all-causality AEs (grades 3–4) with decitabine and treatment choice, respectively, were thrombocytopenia (40% and 32%), anemia (34% and 25%), febrile neutropenia (32% and 22%), and neutropenia with decitabine (32%) [Citation47].
In a phase 3 study, patients aged ≥65 with poor/intermediate-risk cytogenetics received azacitidine (75 mg/m2/day) or conventional care regimens [Citation48]. mOS and CR were 10.4 months and 21.9% (azacitidine) and 6.5 months and 21.9% (conventional care). Common all-causality AEs for azacitidine, LDAC, and IC, respectively, were febrile neutropenia (28%, 30.1%, and 31%), neutropenia (26.3%, 24.8%, and 33.3%), and thrombocytopenia (23.7%, 27.5%, and 21.4%) [Citation48].
Targeted therapies
In recent years, a number of targeted therapies have become available, or are in clinical development, for patients ineligible for IC.
The smoothened inhibitor (SMOi) glasdegib 100 mg once daily (QD) is FDA-approved in combination with LDAC 20 mg BID for patients with AML aged ≥75 or ineligible for induction IC [Citation35]. Glasdegib plus LDAC has been granted initial authorization by the EMA to treat newly diagnosed de novo or secondary AML (sAML) in adult patients who are not candidates for standard induction chemotherapy AML [Citation36]. Approval of glasdegib was based on the results from the pivotal phase 2 BRIGHT MDS&AML 1003 trial [Citation40,Citation50–52] (). In BRIGHT AML 1003, survival probability for glasdegib plus LDAC versus LDAC alone, respectively, was 39.4% and 8.4% at 1 year, and 19.0% and 2.8% at 2 years [Citation50]. In a quality-adjusted time without symptoms of disease progression or toxicities (Q-TWiST) analysis of BRIGHT AML 1003, patients receiving glasdegib plus LDAC had longer time in relatively ‘good’ health compared with those receiving LDAC alone [Citation53]. OS was similar, and CR rate was slightly lower, for LDAC alone in the BRIGHT MDS&AML 1003 study compared with the previous LDAC study, indicating that the LDAC control arm in BRIGHT MDS&AML 1003 is representative of the AML population [Citation43]. In BRIGHT MDS&AML 1012, 17% (MDS) and 20% (AML) achieved CR and mOS was not reached with glasdegib (100 mg QD) plus azacitidine (75 mg/m2/day) in either population [Citation54,Citation55]. Glasdegib 100 mg QD is also being investigated in combination with decitabine 20 mg/m2 IV on a 5- or 10-day schedule in older patients with poor-risk AML (NCT04051996). Several other clinical trials of glasdegib as monotherapy or combination therapy in AML have completed or are underway, in particular, a phase 3 trial of glasdegib/placebo plus 7 + 3 or glasdegib/placebo plus azacitidine in untreated AML (NCT03416179/BRIGHT AML 1019), and a number of phase 2 trials in various patient populations with AML (NCT03390296, NCT03226418, NCT01841333), are ongoing.
Table 4. Summary of results from patients ineligible for IC in BRIGHT MDS&AML 1003 (including unpublished data) [Citation40,Citation50–52].
The BCL-2 inhibitor venetoclax plus HMAs or LDAC is FDA-approved for treatment of newly diagnosed AML in patients aged ≥75 or ineligible for induction IC [Citation39]. A phase 1B study evaluated venetoclax (400, 800, or 1200 mg QD) plus HMAs in patients aged ≥65 ineligible for standard induction chemotherapy due to age ≥75, comorbidities (e.g. cardiac disease, prior anthracycline use, sAML), or high probability of treatment-related mortality [Citation42]. mOS was 17.5 months, and 37% achieved CR. Common all-causality AEs (grades 3–4) were febrile neutropenia (43%), decreased white blood cell count (31%), and anemia (25%) [Citation42]. Neutropenia occurred among 40% of patients who experienced AEs leading to venetoclax dose interruption. Additionally, 33% of patients with neutropenia delayed cycle 2 treatment to allow absolute neutrophil count recovery [Citation42]. Another phase 1B/2 study assessed venetoclax 600 mg QD plus LDAC 20 mg/m2/day in patients aged ≥60 ineligible for IC due to comorbidity or other factors [Citation41]: ECOG PS 0–2 was required for patients aged ≥75; ECOG PS 0–3 for patients aged 60–74; an additional comorbidity for those with ECOG PS 0–1 [Citation41]. mOS was 10.1 months, and 26% achieved CR. Dose interruptions due to AEs occurred in 55% of patients and included delayed neutrophil (n= 8) and platelet recovery (n= 10). Dose reductions due to AEs (7%) were mostly due to thrombocytopenia. Common all-causality AEs were nausea (70%), diarrhea (49%), and hypokalemia (48%) [Citation41]. Interim results from a phase 3 study of venetoclax plus LDAC versus LDAC alone, respectively, reported mOS of 7.2 and 4.1 months and CR of 27.3% and 7.4%, a difference that was not statistically significant [Citation56].
Recurrent IDH mutations, found in ≈20% of AML cases, are associated with older age, intermediate-risk cytogenetics, and other mutations [Citation6,Citation57,Citation58]. The IDH1 inhibitor ivosidenib is also approved for newly diagnosed patients who are older or ineligible for IC, and patients with R/R disease [Citation37,Citation38]. A phase 1 study investigated ivosidenib in patients aged ≥18 with IDH1-mutated AML; the trial included a cohort of patients who were aged ≥75 or who were ineligible for IC due to comorbidities [Citation37,Citation38,Citation45]. mOS in the primary population was 8.8 months and 21.6% achieved CR [Citation45]. Common all-causality AEs were diarrhea (33.3%), leukocytosis (30.2%), and nausea (29.5%) [Citation45].
The antibody–drug conjugate gemtuzumab ozogamicin (GO) and the IDH2 inhibitor enasidenib are not yet approved by the FDA and EMA for newly diagnosed patients with AML who are ineligible for IC [Citation59–62]; however, they have been investigated in these patients in clinical trials. The phase 3 EORTC-GIMEMA study evaluated GO (6 mg/m2 on day 1, and 3 mg/m2 on day 8) versus best supportive care in elderly patients ineligible for IC [Citation44]. Patients were aged >75 or 61–75 with a World Health Organization (WHO) performance score >2 or otherwise ineligible for IC. mOS was 4.9 (GO) and 3.6 (best supportive care) months; 8.1% of patients receiving GO achieved CR. Common all-causality non-hematologic AEs with GO and best supportive care, respectively, were liver (51.3% and 45.6%), fatigue (45.9% and 60.5%), and infection (44.1% and 42.1%) [Citation44]. In patients aged ≥18 with previously untreated IDH2-mutated AML ineligible for standard treatments (criteria not specified; at the discretion of the investigator), mOS was 11.3 months and 18% achieved CR with enasidenib [Citation46]. Common all-causality AEs were fatigue (44%), decreased appetite (41%), nausea (38%), and constipation (38%) [Citation46].
Summary
Although similarities were observed across key clinical studies (comorbidities were an important criterion for determining ineligibility for IC; older patients tended to present with intermediate or adverse cytogenetic risk at baseline), there were large differences in inclusion/exclusion criteria and definitions for ineligibility for chemotherapy (where this was defined). Therefore, baseline characteristics varied greatly across key studies. This heterogeneity in patient populations makes cross-study comparisons inadequate and inadvisable as a guide for treatment selection in older patients with AML.
Considerations for glasdegib treatment selection
Dysregulation of the Hedgehog signaling pathway, and its component, SMO, play an important role in AML pathogenesis and the persistence of leukemic stem cell (LSC) populations [Citation63–66]. Based on the known mechanism of action of SMOi, glasdegib may eradicate early LSC progenitor populations by reducing LSC dormancy and promoting the differentiation and cell cycle progression of LSCs [Citation67,Citation68].
Glasdegib plus LDAC use is dependent on clinical and patient factors, comorbidities, and concomitant medications (). Results from BRIGHT MDS&AML 1003 that have helped inform the use of glasdegib plus LDAC are presented in .
Table 5. Summary of considerations for glasdegib use.
Baseline risk factors and disease characteristics
Age is important but should not be the only selection criterion, except perhaps in the upper range [Citation13,Citation15,Citation17]. However, increased age is generally associated with poorer outcomes. Older patients may have poorer ECOG PS and general health and present with specific comorbidities that can impact treatment tolerability [Citation2,Citation8,Citation13,Citation15–17]. Concerns surrounding use of IC in older patients with AML stems from the risk of prolonged myelosuppression and high mortality [Citation13,Citation19–29]. In this context, glasdegib plus LDAC can be considered a first-line treatment for patients aged ≥75 [Citation35].
Although glasdegib plus LDAC is approved for patients ineligible for IC, no standard guidelines exist to determine IC eligibility. The BRIGHT MDS&AML 1003 study pre-specified the criteria used to consider a patient to be ineligible for IC, making it more objective than most other studies. Available evaluation tools include the hematopoietic cell transplantation-specific or Charlson comorbidity indexes [Citation9,Citation13,Citation15,Citation17], and models incorporating multiple characteristics [Citation9,Citation13,Citation17,Citation69–72]. Irrespective of the guidelines used, lower-intensity treatments such as glasdegib plus LDAC or venetoclax plus HMAs can be considered for patients with AML ineligible for IC due to existing comorbidities.
Patients with sAML tend to have a poor prognosis, with reduced CR rates and OS [Citation73–76]. BRIGHT MDS&AML 1003 demonstrated glasdegib plus LDAC efficacy in older patients with sAML; CPX-351 is another option, but should be administered only to patients who are eligible for IC and able to withstand prolonged myelosuppression.
Although glasdegib plus LDAC may be effective in patients with therapy-targetable mutations, treatments based on FLT3 or IDH inhibitors should be given priority consideration when mutations of those genes are identified.
Comorbidities
In a renal impairment study, participants with moderate or severe impairment had similar pharmacokinetic (PK) parameters following a single glasdegib 100-mg dose [Citation77]. Coupled with the known safety profile of glasdegib [Citation40,Citation50], this suggests lower starting doses (<100 mg) may not be required in renal impairment. Glasdegib is largely eliminated through hepatic metabolism [Citation78]. In a population PK analysis, glasdegib PK was unaffected by mild hepatic impairment [Citation79]. In a hepatic impairment study, moderate or severe (Child-Pugh class B or C) impairment did not have a clinically meaningful effect on glasdegib exposure following a single 100-mg dose, although long-term data are warranted [Citation80]. Together with previous studies, these data suggest dose modifications are not required in hepatic impairment [Citation80].
In patients ineligible for IC, cytopenias occurred more frequently with glasdegib plus LDAC versus LDAC but were not accompanied by increased rate of sepsis or bleeding [Citation51]. It is thought that higher absolute rates of cytopenia were due to longer treatment duration with glasdegib plus LDAC compared with LDAC [Citation40]. With cytopenia rates adjusted to exposure, transfusion requirements were lower in patients treated with glasdegib plus LDAC. Glasdegib plus azacitidine did not substantially increase hematologic toxicities, cytopenic complications, or AEs related to cytopenias versus azacitidine [Citation54]. As a result of the prolonged myelosuppression reported with HMAs plus venetoclax, glasdegib plus LDAC can be a treatment option when the treating physician considers the patient to be at higher risk of prolonged cytopenias, or when there might be limited access to transfusions or emergency care for neutropenia-related infections. Additionally, glasdegib plus LDAC is an alternative for patients ineligible for venetoclax due to risk of severe, long-lasting myelosuppression or previous toxicities with venetoclax.
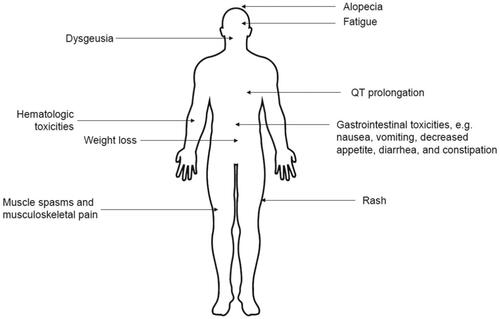
Prior to initiating treatment in older patients or those unfit for IC, evaluate medical history and comorbidities regarding AEs commonly associated with SMOi, including alopecia, muscle spasms, musculoskeletal pain, and gastrointestinal AEs [Citation35,Citation81–84]. Patients should be educated on AE signs, symptoms, and appropriate management strategies. Prophylactic or supportive-care therapies should be initiated with glasdegib plus LDAC treatment. As an oral medication, glasdegib does not require in-clinic administration and may be preferred for frail patients, particularly when transfusions or IV administration will affect quality of life (QoL).
Concomitant medications
A full review of concomitant medications is essential to identify potential drug–drug interactions with glasdegib and modify treatment plans appropriately before initiating therapy.
Patients undergoing treatment for AML are at increased risk of fungal infections; antifungal agents are routinely used to manage this or as prophylaxis [Citation85]. Azoles, the most commonly administered antifungals [Citation85], inhibit cytochrome P450 (CYP) 3A4, and glasdegib is largely metabolized by the CYP system. In a healthy participant study, coadministration of glasdegib with ketoconazole elicited 140% and 40% increases in glasdegib plasma exposure and peak concentration, respectively [Citation78,Citation86]. In BRIGHT MDS&AML 1003, comparisons between patients who received CYP3A4 inhibitors versus those who did not were limited due to differences in exposure; however, rates of AEs and grade 3–4 AEs were 93.3% versus 100%, and 90% versus 82.5%, respectively (unpublished data). The glasdegib product label advises use of alternatives to strong CYP3A inhibitors [Citation35]. However, if coadministration is required, modify doses and monitor patients for AEs. The benefit of antifungals outweighs the risks; monitor the corrected QT interval (QTc) after 1, 2, and 4 weeks when azoles are coadministered with glasdegib.
Glasdegib exposure and plasma concentrations in healthy participants are ≈70% and 35% lower, respectively, when coadministered with the CYP3A4 inducer rifampin [Citation87]. Avoid concomitant use of glasdegib with strong CYP3A4 inducers (e.g. rifampin, bosentan, dexamethasone, carbamazepine, and phenytoin) [Citation35]; dexamethasone should not be used as an antiemetic in patients receiving glasdegib. If coadministration with moderate CYP3A4 inducers is required, modify doses and monitor patients for AEs [Citation35].
Avoid coadministration of glasdegib with QT-prolonging agents (e.g. antiarrythmics, antimalarials, and macrolides). If coadministration is necessary, monitor patients for QT prolongation [Citation35]; monitor potassium and magnesium closely and correct abnormalities.
Two studies in healthy participants demonstrated that glasdegib can be administered with proton pump inhibitors, irrespective of food intake [Citation88,Citation89], which simplifies dosing recommendations and may facilitate adherence. Allopurinol, furosemide, and paracetamol may also be coadministered with glasdegib [Citation40].
Response monitoring
Patients should not be removed from glasdegib plus LDAC treatment due to lack of CR alone. Improvement (e.g. in transfusion requirements) in the absence of CR is compatible with, but not confirmatory for, glasdegib’s action on LSCs rather than as a cytotoxic agent [Citation35]. The panel recommended, in the absence of AML progression, patients receive ≥6 treatment cycles per product label, even if CR is not observed by cycle 2–3, particularly if other clinical benefits are seen.
Managing AEs associated with glasdegib plus LDAC
The most common AEs with glasdegib ( and ) are related to the mechanism of action of SMOi [Citation90–95], although frequency and severity varies due to different PK properties. Most can be managed with dose modifications and/or temporary interruptions; however, alternative strategies are available () [Citation35,Citation81–84]. In general, complete blood counts, electrolytes, and renal and hepatic function should be assessed prior to initiating treatment and at least weekly for the first month. Electrolytes and renal function should be monitored monthly throughout treatment [Citation35].
Table 6. Summary of the management of the most common AEs associated with glasdegib [Citation35,Citation81–84].
Common non-hematologic AEs observed during glasdegib treatment include alopecia, dysgeusia, fatigue, gastrointestinal AEs, muscle spasms, and rash () [Citation40]. It is important to inform patients of the possibility of these AEs and that they are common with SMOi treatment. Additionally, a full review of the patient’s medical history, comorbidities, underlying deficiencies, and concomitant medications should be completed before initiating treatment to identify contributory factors for AEs. Pharmacologic/supportive care therapies or nonpharmacologic management strategies should be considered, and existing treatments may need to be modified, either prophylactically or in the event of an AE [Citation81–84]. Patients should be advised to maintain healthy physical activity, and nutritional and sleeping habits. Guidance should be provided on any behavioral changes that can minimize the risk of certain AEs [Citation81–84]. If necessary, AEs can be managed by reducing or interrupting the glasdegib and/or LDAC dose [Citation35].
For non-hematologic grade 3 AEs, glasdegib and/or LDAC should be interrupted until symptoms become mild or return to baseline [Citation35]. Glasdegib can then be resumed at the same dose level or reduced to 50 mg. If toxicity recurs once, the dose should be reduced (if not done previously), and treatment should be discontinued upon second recurrence. If the AE is glasdegib related, LDAC may be continued, or vice versa [Citation35]. Treatment should be discontinued in the event of non-hematologic grade 4 AEs [Citation35].
Although glasdegib is associated with anemia and thrombocytopenia [Citation40], these conditions are often present at baseline and causality is difficult to assess in the setting of active leukemia. Patients should be monitored regularly for myelosuppression and be advised of the potential for hematologic AEs. Ensuring that patients report symptoms (e.g. bruising easily, unexpected bleeding, blood in urine or stools, fever, extreme fatigue) can help identify AEs early in the treatment process. As detailed in the product label, glasdegib plus LDAC should be permanently discontinued with platelets <10 × 109/L and neutrophil count <0.5 × 109/L for >42 days in the absence of persistent disease [Citation35]. Transfusions, granulocyte colony-stimulating factor, and antibacterial prophylaxis should be used per local guidelines.
The possibility of febrile neutropenia and associated complications increases with age, poor WHO performance score, and comorbidities [Citation96]. Prophylactic antimicrobial treatment should be considered in at-risk patients and patients should be advised to report symptoms promptly (e.g. increased body temperature, chills, and sweating) [Citation96]. For management, granulocyte colony-stimulating agents should be considered, particularly with difficult-to-control infections. In the event of neutropenic fever, patients should report immediately to the clinic or emergency center. Prompt assessment, identification, and treatment with antimicrobial therapy is important (e.g. IV broad spectrum antibiotics ≤1 h of occurrence), as well as ongoing monitoring of response, with therapy plan modifications as appropriate [Citation96].
QTc prolongation is uncommon but needs awareness. In addition to monitoring electrolyte levels (particularly magnesium and potassium) and electrocardiograms (ECGs) throughout treatment, evaluating patients for comorbidities and QT-prolonging concomitant medications is important. Further details on managing specific QTc interval events are shown in [Citation35]. A pooled analysis of glasdegib trials (N= 412) revealed no events of torsades de pointes (unpublished data).
Other AEs listed on the product label are dyspnea, edema, and hemorrhage (each ≥20% in product label). It is important to discuss the possibility of AEs with patients prior to treatment initiation, evaluate comorbidities and concomitant medications that may lead to increased risk of AEs, and ensure patients are given the necessary information on AE signs and symptoms. Weight loss (<20% in BRIGHT MDS&AML 1003) is multifactorial and may result from other AEs or leukemia itself. As some patients may view weight loss as desirable rather than an AE, emphasize the importance of reporting any changes in body weight [Citation35,Citation81–84].
Summary
Glasdegib is the first SMOi approved for treatment of AML and targets the LSC population that can persist following standard chemotherapy. Treatment selection is multifactorial and includes patient age, comorbidities, concomitant medications, and risk factors. In contrast with other therapies, glasdegib 100 mg QD plus LDAC 20 mg BID can be considered for older patients (≥75) and patients with poorer risk profiles and prognostic scores, ineligible for IC, with sAML, or who received prior HMAs for MDS. As an oral medication, glasdegib does not require in-clinic administration. Additionally, glasdegib plus LDAC can be administered to patients with renal or hepatic impairment and severe cardiac disease. Prior to treatment initiation, a full evaluation of medical history, concomitant medications, and comorbidities should be performed. Patients should be educated on common AEs and mitigation strategies and regularly monitored for AEs during treatment. Key management strategies for common treatment-related AEs are dose modifications and interruptions. Effective AE management can lead to improved patient outcomes, QoL, and medication adherence.
Supplemental Material
Download PDF (1.4 MB)Acknowledgements
The authors would like to thank B. Douglas Smith for his contributions to the advisory board discussions. Medical writing support, under the direction of the authors, was provided by Anne Marie McGonigal, PhD, of Engage Scientific Solutions, and funded by Pfizer.
Disclosure statement
Jorge E. Cortes: consultancy from Pfizer, Novartis, Takeda, Jazz, Biopath Holdings, BiolineRx; grants from Pfizer, Novartis, Takeda, Jazz, BMS, Forma Therapeutics, Amphivena, Merus; other support, e.g. travel to meetings, from Pfizer.
Anna Candoni: board membership, consultancy and speaker's bureau from Gilead, Novartis, Pfizer, Celgene, Janssen, Incyte, MSD.
Richard E. Clark: consultancy from Pfizer, Jazz, Abbvie, Novartis, BMS; grants from Novartis, BMS; speaker's bureau from Pfizer; other support, e.g. travel to meetings, from Pfizer.
Michael Heuser: consultancy from Pfizer, Bayer Pharma AG, Novartis, Prime Oncology, Abbvie, Daiichi Sankyo; grants from Pfizer, Astellas, Bayer Pharma AG, Daiichi Sankyo, BergenBio, Karyopharm, Novartis, Roche.
Brian Leber: board membership, consultancy, expert testimony, speaker's bureau, other support, e.g. travel to meetings, from Pfizer.
Pau Montesinos: consultancy, grants, speaker's bureau from Pfizer.
Paresh Vyas: consultancy, other support, e.g. travel to meetings, from Pfizer; grants from BMS/Celgene, Novartis, Merck; speaker's bureau from AbbVie, BMS/Celgene, Novartis, Pfizer, Jazz, Astellas, Daiichie Sankyo; royalties to institution from BD.
Amer M. Zeidan: consultancy from Celgene/BMS, Abbvie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Novartis, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme; grants from Celgene/BMS, Abbvie, Astex, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Novartis, Aprea, ADC Therapeutics.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an International Expert Panel. Blood. 2017;129(4):424–447.
- Gu R, Yang X, Wei H. Molecular landscape and targeted therapy of acute myeloid leukemia. Biomark Res. 2018;6:32.
- Martignoles JA, Delhommeau F, Hirsch P. Genetic hierarchy of acute myeloid leukemia: from clonal hematopoiesis to molecular residual disease. Int J Mol Sci. 2018;19(12):3850.
- Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–61.
- Bohl SR, Bullinger L, Rücker FG. New targeted agents in acute myeloid leukemia: new hope on the rise. Int J Mol Sci. 2019;20(8):1983.
- Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593–606.
- NCCN Practice Guidelines in Oncology: acute myeloid leukemia [Internet]. National Comprehensive Cancer Network; 2019; [cited 2019 Mar 26]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/aml_blocks.pdf
- Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997–999.
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(6):721–749.
- Brandwein JM, Zhu N, Kumar R, et al. Treatment of older patients with acute myeloid leukemia (AML): revised Canadian consensus guidelines. Am J Blood Res. 2017;7(4):30–40.
- Finn L, Dalovisio A, Foran J. Older patients with acute myeloid leukemia: treatment challenges and future directions. Ochsner J. 2017;17(4):398–404.
- Sanford D, Ravandi F. Management of newly diagnosed acute myeloid leukemia in the elderly: current strategies and future directions. Drugs Aging. 2015;32(12):983–997.
- Kantarjian H. Acute myeloid leukemia-major progress over four decades and glimpses into the future. Am J Hematol. 2016;91(1):131–145.
- Pettit K, Odenike O. Defining and treating older adults with acute myeloid leukemia who are ineligible for intensive therapies. Front Oncol. 2015;5:280.
- NCCN Practice Guidelines in Oncology: older adult oncology [Internet]. National Comprehensive Cancer Network; 2019; [cited 2019 Jun 25]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf
- Klepin HD, Estey E, Kadia T. More versus less therapy for older adults with acute myeloid leukemia: new perspectives on an old debate. Am Soc Clin Oncol Educ Book. 2019;39:421–432.
- Krug U, Rollig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet. 2010;376(9757):2000–2008.
- Medeiros BC, Satram-Hoang S, Hurst D, et al. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94(7):1127–1138.
- Juliusson G. Older patients with acute myeloid leukemia benefit from intensive chemotherapy: an update from the Swedish acute leukemia registry. Clin Lymphoma Myeloma Leuk. 2011;11:S54–S59.
- Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179–4187.
- Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916–1924.
- Sorror ML, Storer BE, Elsawy M, et al. Intensive versus non-intensive induction therapy for patients (pts) with newly diagnosed acute myeloid leukemia (AML) using two different novel prognostic models. Blood. 2016;128(22):216.
- Bell JA, Galaznik A, Farrelly E, et al. A retrospective study evaluating treatment patterns and survival outcomes in elderly patients with acute myeloid leukemia treated in the United States with either 7 + 3 or a hypomethylating agent. Leuk Res. 2019;78:45–51.
- Prebet T, Boissel N, Reutenauer S, et al. Acute myeloid leukemia with translocation (8;21) or inversion (16) in elderly patients treated with conventional chemotherapy: a collaborative study of the French CBF-AML intergroup. J Clin Oncol. 2009;27(28):4747–4753.
- Ross K, Gillespie-Twardy AL, Agha M, et al. Intensive chemotherapy in patients aged 70 years or older newly diagnosed with acute myeloid leukemia. Oncol Res. 2015;22(2):85–92.
- Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–4429.
- Kim I, Koh Y, Yoon SS, et al. Fludarabine, cytarabine, and attenuated-dose idarubicin (m-FLAI) combination therapy for elderly acute myeloid leukemia patients. Am J Hematol. 2013;88(1):10–15.
- Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–1248.
- Highlights of prescribing information: Vidaza (azacitidine) [Internet]. US Food and Drug Administration; 2008; [cited 2019 Sep 11]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050794s011lbl.pdf
- Summary of product characteristics: Vidaza (azacitidine) [Internet]. European Medicines Agency; 2013; [cited 2019 Sep 12]. Available from: https://www.ema.europa.eu/en/documents/product-information/vidaza-epar-product-information_en.pdf
- Approval letter: cytarabine injection [Internet]. US Food and Drug Administration; 1999; [cited 2019 Sep 16]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/075383.PDF
- Highlights of prescribing information: Dacogen (decitabine) [Internet]. US Food and Drug Administration; 2018; [cited 2019 Sep 11]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021790s021lbl.pdf
- Summary of product characteristics: Dacogen (decitabine) [Internet]. European Medicines Agency; 2019; [cited 2019 Sep 12]. Available from: https://www.ema.europa.eu/en/documents/product-information/dacogen-epar-product-information_en.pdf
- Highlights of prescribing information: Daurismo (glasdegib) [Internet]. US Food and Drug Administration; 2020; [cited 2020 Mar 27]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210656s002s004lbl.pdf
- Summary of opinion (initial authorisation): Daurismo (glasdegib) [Internet]. European Medicines Agency; 2020; [cited 2020 May 06]. Available from: https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-daurismo_en.pdf
- Highlights of prescribing information: Tibsovo (ivosidenib) [Internet]. US Food and Drug Administration; 2018; [cited 2019 Sep 13]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211192s001lbl.pdf
- Public summary of opinion on orphan designation: ivosidenib [Internet]. European Medicines Agency; 2017; [cited 2019 Mar 22]. Available from: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/16/1802-public-summary-opinion-orphan-designation-ivosidenib-treatment-acute-myeloid-leukaemia_en.pdf
- Highlights of prescribing information: Venclexta (venetoclax) [Internet]. US Food and Drug Administration; 2018 [cited 2019 Mar 19]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208573s009lbl.pdf
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–389.
- Wei AH, Strickland SA Jr., Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–1284.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
- Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114–1124.
- Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972–979.
- DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–2398.
- Pollyea DA, Tallman MS, de Botton S, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33(11):2575–2584.
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677.
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299.
- Heuser M, Ofran Y, Boissel N, et al. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(6):697–712.
- Heuser M, Fiedler W, Sekeres MA, et al. Clinical benefit of glasdegib plus low-dose cytarabine in patients with de novo and secondary acute myeloid leukemia: long-term analysis of a phase 2 randomized trial. Clin Lymphoma Myeloma Leuk. 2019;19:S231.
- Papayannidis C, Smith BD, Heuser M, et al. Low-dose cytarabine with or without glasdegib in newly diagnosed patients with acute myeloid leukemia: long-term analysis of a phase 2 randomized trial. Clin Lymphoma Myeloma Leuk. 2019;19:S228–S229.
- Cortes J, Heidel FH, Fiedler W, et al. Glasdegib improved overall survival in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) who achieved complete remission (CR) and those who did not achieve CR. Poster session presented at: European Hematology Association; 2018 Jun 14–19; Stockholm, Sweden.
- Kwon Y, Bell TJ, Solem C, et al. Quality-adjusted survival for low-dose cytarabine (LDAC) versus glasdegib + LDAC among newly diagnosed acute myeloid leukemia patients who are not candidates for intensive chemotherapy: a Q-TWiST analysis. Blood. 2019;134(Suppl. 1):2610.
- Zeidan A, Schuster MW, Krauter J, et al. Clinical benefit of glasdegib in combination with azacitidine or low-dose cytarabine in patients with acute myeloid leukemia. Abstract at 62nd ASH Annual Meeting & Exposition; 2019 Dec 5–8; San Diego, CA.
- Sekeres MA, Schuster MW, Joris M, et al. A phase 1b study of glasdegib in combination with azacitidine in patients with untreated higher-risk myelodysplastic syndromes, acute myeloid leukemia, and chronic myelomonocytic leukemia. Abstract at 62nd ASH Annual Meeting & Exposition; 2019 Dec 5–8; San Diego, CA.
- AbbVie. AbbVie provides update from phase 3 study evaluating VENCLEXTA® (venetoclax) in combination with low-dose cytarabine in newly-diagnosed patients with acute myeloid leukemia (AML) [Internet]. AbbVie; 2020; [cited 2020 Mar 27]. Available from: https://news.abbvie.com/news/press-releases/abbvie-provides-update-from-phase-3-study-evaluating-venclexta-venetoclax-in-combination-with-low-dose-cytarabine-in-newly-diagnosed-patients-with-acute-myeloid-leukemia-aml.htm
- Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol. 2017;18(3):17.
- Papayannidis C, Sartor C, Marconi G, et al. Acute myeloid leukemia mutations: therapeutic implications. Int J Mol Sci. 2019;20(11):2721.
- Highlights of prescribing information: Mylotarg (gemtuzumab ozogamicin) [Internet]. US Food and Drug Administration; 2017; [cited 2019 Mar 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761060lbl.pdf
- Summary of product characteristics: Mylotarg (gemtuzumab ozogamicin) [Internet]. European Medicines Agency; 2019; [cited 2019 Mar 22]. Available from: https://www.ema.europa.eu/en/documents/product-information/mylotarg-epar-product-information_en.pdf
- Highlights of prescribing information: Idhifa (enasidenib) [Internet]. US Food and Drug Administration; 2017; [cited 2019 Mar 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209606s000lbl.pdf
- Highlights of prescribing information: Xospata (gilteritinib) [Internet]. US Food and Drug Administration; 2018; [cited 2019 Mar 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211349s000lbl.pdf
- Campbell V, Copland M. Hedgehog signaling in cancer stem cells: a focus on hematological cancers. Stem Cells Cloning. 2015;8:27–38.
- Ok CY, Singh RR, Vega F. Aberrant activation of the hedgehog signaling pathway in malignant hematological neoplasms. Am J Pathol. 2012;180(1):2–11.
- Khan AA, Harrison CN, McLornan DP. Targeting of the Hedgehog pathway in myeloid malignancies: still a worthy chase? Br J Haematol. 2015;170(3):323–335.
- Pollyea DA, Jordan CT. Therapeutic targeting of acute myeloid leukemia stem cells. Blood. 2017;129(12):1627–1635.
- Tauchi T, Okabe S, Katagiri S, et al. Targeting the Hedgehog signaling pathway by glasdegib limits the self-renewal of MDS-derived induced potent stem cells (iPSC). J Cancer Sci Ther. 2017;9(6):479–484.
- Fukushima N, Minami Y, Kakiuchi S, et al. Small-molecule Hedgehog inhibitor attenuates the leukemia-initiation potential of acute myeloid leukemia cells. Cancer Sci. 2016;107(10):1422–1429.
- AML-SCORE [Internet]. Study Alliance Leukemia; 2019; [cited 2019 Sep 13]. Available from: https://www.aml-score.org
- Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93(10):1267–1291.
- Walter RB, Othus M, Borthakur G, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417–4423.
- Walter RB, Othus M, Orlowski KF, et al. Unsatisfactory efficacy in randomized study of reduced-dose CPX-351 for medically less fit adults with newly diagnosed acute myeloid leukemia or other high-grade myeloid neoplasm. Haematologica. 2018;103(3):e106–e109.
- Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best Pract Res Clin Haematol. 2007;20(1):29–37.
- Granfeldt Ostgard LS, Medeiros BC, Sengelov H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641–3649.
- Boddu PC, Kantarjian HM, Ravandi F, et al. Characteristics and outcomes of older patients with secondary acute myeloid leukemia according to treatment approach. Cancer. 2017;123(16):3050–3060.
- Boddu P, Kantarjian HM, Garcia-Manero G, et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv. 2017;1(17):1312–1323.
- Shaik MN, LaBadie RR, Hee B, et al. Evaluation of the impact of renal impairment on the pharmacokinetics of glasdegib [abstract]. Clin Pharmacol Ther. 2020;107(Suppl. S1):S69.
- Lam JL, Vaz A, Hee B, et al. Metabolism, excretion and pharmacokinetics of [14C]glasdegib (PF-04449913) in healthy volunteers following oral administration. Xenobiotica. 2017;47(12):1064–1076.
- Lin S, Shaik N, Martinelli G, et al. Population pharmacokinetics of glasdegib in patients with advanced hematologic malignancies and solid tumors. J Clin Pharmacol. 2019;60(5):605–616.
- Masters JC, LaBadie RR, Salageanu J, et al. Pharmacokinetics (PK) and safety of glasdegib in participants with moderate and severe hepatic impairment: phase 1, open-label, single-dose, parallel-group study [abstract]. Clin Pharmacol Ther. 2020;107(Suppl. S1):S91.
- Lacouture ME, Dreno B, Ascierto PA, et al. Characterization and management of Hedgehog pathway inhibitor-related adverse events in patients with advanced basal cell carcinoma. Oncologist. 2016;21(10):1218–1229.
- Yang X, Dinehart BA. Practical tips for managing Hedgehog pathway inhibitor side effects [Internet]. Practical Dermatology; 2017; [cited 2019 Sep 16]. Available from: http://v2.practicaldermatology.com/pdfs/pd0117_CF_BCC.pdf
- Mohan SV, Chang AL. Management of cutaneous and extracutaneous side effects of smoothened inhibitor therapy for advanced basal cell carcinoma. Clin Cancer Res. 2015;21(12):2677–2683.
- Jacobsen AA, Kydd AR, Strasswimmer J. Practical management of the adverse effects of Hedgehog pathway inhibitor therapy for basal cell carcinoma. J Am Acad Dermatol. 2017;76(4):767–768.
- Maertens JA, Girmenia C, Bruggemann RJ, et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother. 2018;73(12):3221–3230.
- Shaik MN, LaBadie RR, Rudin D, et al. Evaluation of the effect of food and ketoconazole on the pharmacokinetics of the smoothened inhibitor PF-04449913 in healthy volunteers. Cancer Chemother Pharmacol. 2014;74(2):411–418.
- Shaik MN, Hee B, Wei H, et al. Evaluation of the effect of rifampin on the pharmacokinetics of the smoothened inhibitor glasdegib in healthy volunteers. Br J Clin Pharmacol. 2018;84(6):1346–1353.
- Giri N, Lam LH, LaBadie RR, et al. Evaluation of the effect of new formulation, food, or a proton pump inhibitor on the relative bioavailability of the smoothened inhibitor glasdegib (PF-04449913) in healthy volunteers. Cancer Chemother Pharmacol. 2017;80(6):1249–1260.
- Shaik N, Hee B, Wei H, et al. Evaluation of the effects of formulation, food, or a proton-pump inhibitor on the pharmacokinetics of glasdegib (PF-04449913) in healthy volunteers: a randomized phase I study. Cancer Chemother Pharmacol. 2019;83(3):463–472.
- Summary of product characteristics: Odomzo (sonidegib) [Internet]. European Medicines Agency; 2018; [cited 2019 Mar 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/odomzo-epar-product-information_en.pdf
- Summary of product characteristics: Erivedge (vismodegib) [Internet]. European Medicines Agency; 2019; [cited 2019 Mar 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/erivedge-epar-product-information_en.pdf
- Highlights of prescribing information: Erivedge [Internet]. US Food and Drug Administration; 2012; [cited 2019 Mar 18]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203388lbl.pdf
- Highlights of prescribing information: Odomzo [Internet]. US Food and Drug Administration; 2016; [cited 2019 Mar 18]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205266s002lbl.pdf
- Basset-Seguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334–348.
- Cortes JE, Gutzmer R, Kieran MW, et al. Hedgehog signaling inhibitors in solid and hematological cancers. Cancer Treat Rev. 2019;76:41–50.
- Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2016;27(Suppl. 5):v111–v118.