WHO classification of hematologic malignancies is based on a combination of: (1) clinical presentation; (2) morphologic findings; (3) immunophenotype of abnormal cells; and (4) presence of recurrent genetic abnormalities [Citation1]. As our understanding of disease pathogenesis advances, genetic abnormalities appear to play an ever larger role in disease classification. Same genetic abnormalities can be detected in various hematologic neoplasms, depending on the lineage and maturation stage of the cell affected, as well as additional genetic and epigenetic factors. For example, CCND1/IGH translocation (and cyclin D1 overexpression) is a defining feature of mantle cell lymphoma, but is also frequently seen in plasma cell neoplasms; BRAF V600E mutation drives proliferation in both hairy cell leukemia and Langerhans cell histiocytosis.
Combination of MYC and BCL2 (and/or BCL6) rearrangements is frequently seen in high grade B-cell lymphomas, and is associated with an aggressive clinical course and poor response to therapy [Citation2,Citation3]. Recently, this combination of genetic abnormalities was also described in follicular lymphoma with conventional clinical and morphologic features [Citation4–6]. In multiple myeloma, MYC dysregulation due to rearrangement of the MYC locus is a well-documented feature, particularly associated with disease progression [Citation7–10]. In contrast, although myeloma cells frequently have high BCL2 expression [Citation11], translocations of the BCL2 locus appear extremely rarely in published myeloma literature [Citation12,Citation13]. Here we report a patient with non-secretory multiple myeloma harboring the combination of MYC/IGH and IGH/BCL2 rearrangements.
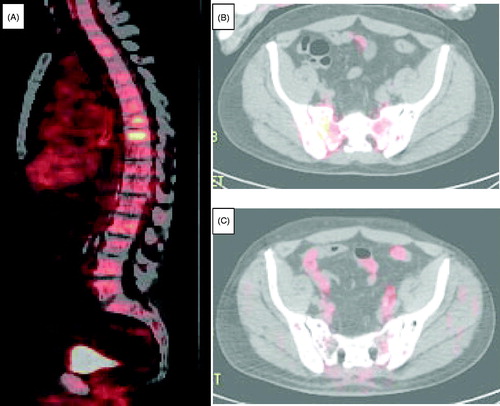
A 67-year-old man, with one year history of mild leukopenia and thrombocytopenia of unclear etiology, presented with severe bone pain involving his back and hips. He had no other significant past medical problems. A CT scan followed by a PET/CT scan showed widespread lytic bone lesions (). An MRI bone survey showed extensive abnormal marrow signal of all visualized osseous structures suggestive of a diffuse infiltrative neoplastic process. No lymphadenopathy was found on examination or seen by imaging studies.
Figure 1. PET/CT scans of the involved bones. (A) Spine at the time of diagnosis. (B) Pelvis at the time of diagnosis. (C) Pelvis after 7 months of myeloma-directed therapy.
His CBC was as follows: Hb 8.6 g/dL (down from 13.3 g/dL five months prior), WBC 3830/μL, platelets 1,03,000/μL. No monoclonal protein was detected by serum studies. SPEP with immunofixation studies were negative and serum free light chain ratio was normal. He had normal kidney and liver function. Calcium level was normal.
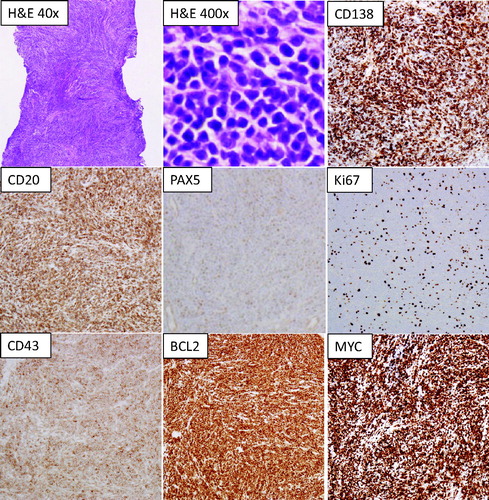
Initial bone marrow aspiration and biopsy showed marrow entirely replaced by a monotonous malignant neoplasm with extensive tumor necrosis, and was essentially non-diagnostic. This was followed by a targeted biopsy of the right iliac lytic bone lesion. This targeted biopsy showed bone marrow replacement by plasmacytoid cells, with prominent fibrosis (). The combined flow cytometry and immunohistochemical phenotype of the neoplastic cells showed strong positivity for CD38, CD79a, MYC, and BCL2; and variable positivity for CD20, CD43, CD138, PAX5, MUM1, BOB1, and Ki67 (10%). CD45 was very dim to negative by flow cytometry, and negative by immunohistochemistry. The neoplastic cells were negative for CD5, CD10, CD19, CD56, CD117, BCL6, LMO2, OCT2, SOX11, cyclin D1, IgD, IgA, IgG, IgM, kappa, lambda, and EBV by in-situ hybridization for EBER.
Figure 2. Morphologic and immunohistochemical characterization of the neoplastic cells. Unless otherwise indicated, the images are taken using 100x magnification.
FISH studies were performed using a combination of lymphoma and myeloma panels. Positive findings included IGH/BCL2 fusion, MYC/IGH fusion, and monosomy 13, all in 90-100% of the cells. No abnormalities were seen using probes for chromosomes 3, 5, 7, and 15; no abnormalities of 1q region, no FGFR3/IGH fusion, TP53 deletion, MALT1 rearrangement, or BCL6 rearrangement were detected. Molecular genetic study showed absence of MYD88 L265P mutation by PCR.
The initial diagnosis favored B-cell lineage lymphoma with marked plasmacytic differentiation, and the patient was treated with 6 cycles of BR (bendamustine 90 mg/m2 on days 1, 2 with rituximab at 375 mg/m2 on day 1 every 4 weeks), followed by maintenance rituximab (every 2 months). Shortly after initiation of chemotherapy his symptoms significantly improved and eventually he became pain free. However, three months after completing BR chemotherapy he developed recurrent severe bone pain. A PET/CT scan showed progression of bone lesions. Repeat monoclonal protein studies (including SPEP and UPEP with immunofixation, serum and urine free light chains) were negative.
A repeat bone marrow biopsy showed plasmacytoid cell infiltrate with morphologic and immunophenotypic features similar to the previous specimen. Chromosome study showed a complex karyotype in 3 of 9 metaphases, and included t(14;18)(q32;q21), monosomy of chromosomes 1, 13, 16, and 22; an unbalanced translocation between 1q and 22q; additional material of unknown origins on 6q, 8q; and a marker chromosome {42,X,-Y,-1,add(6)(q11.2),add(8)(q24.1),-13,add(14)(q32),t(14;18)(q32;q21),-16,-22,der(22)t(1;22)(q23;q13),+mar[3]/46,XY[6]}. Repeated FISH studies on CD138-enriched plasma cells confirmed the presence of IGH/BCL2 and MYC/IGH fusions, as well as monosomy 13 and gain of chromosome 1q. The patient’s diagnosis was revised to indicate an ambiguity in the level of differentiation, and a high likelihood that the neoplastic process represents a cytogenetically unusual true plasma cell neoplasm (non-secretory multiple myeloma).
Treatment with anti-myeloma agents was initiated (carfilzomib 70 mg/m2 IV, cyclophosphamide 300 mg/m2 IV and dexamethasone 40 mg IV, all given weekly x 3 weeks of every 4 weeks). After 2 cycles of the myeloma-directed therapy, patient achieved an excellent clinical response with resolution of his pain. After 4 months restaging PET/CT scan showed that diffuse bone lesions have decreased in metabolic activity, with majority demonstrating only background activity. A repeat PET/CT scan at the 7 month time point showed no radiologic evidence of the disease (). Bone marrow aspiration and biopsy at the 8 month time point was negative for residual disease, including absence of clonal immunoglobulin gene rearrangement by PCR analysis.
The morphology and phenotype of the neoplastic cells (CD138 + CD19-CD45-CD10-BCL6-LMO2-) favor classification of this process as a non-secretory multiple myeloma, rather than follicular lymphoma. Non-secretory behavior is consistent with both IgH alleles being involved in translocation events, to MYC and BCL2 loci, respectively. This case illustrates three points: (1) there are rare cases of lymphoid neoplasms which are difficult to box into existing WHO classification categories, due to mixed morphologic, phenotypic, and/or genetic features; (2) true plasma cell neoplasms can rarely have IGH/BCL2 rearrangement, as described previously [Citation12,Citation13]; and (3) combination of MYC/IGH and IGH/BCL2 is not synonymous with high grade B-cell lymphoma.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2017.
- Snuderl M, Kolman OK, Chen, YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol. 2010;34(3):327–340.
- Tomita N, Tokunaka M, Nakamura N, et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica. 2009;94(7) :935–943.
- Miao Y, Hu S, Lu X, et al. Double-hit follicular lymphoma with MYC and BCL2 translocations: a study of 7 cases with a review of literature. Hum Pathol. 2016;58:72–77.
- Miyaoka M, Kikuti YY, Carreras J, et al. Clinicopathological and genomic analysis of double-hit follicular lymphoma: comparison with high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements. Mod Pathol. 2018;31(2):313–326.
- Ziemba JB, Wolf Z, Weinstock M, et al. Double-hit and triple-hit follicular lymphoma. Am J Clin Pathol. 2020.
- Avet-Loiseau H, Gerson F, Magrangeas F, Intergroupe Francophone du Myélome, et al. Rearrangements of the c-myc oncogene are present in 15% of primary human multiple myeloma tumors. Blood. 2001;98(10):3082–3086.
- Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. Oncogene. 2001; 20(40):5611–5622.
- Misund K, Keane N, Stein CK, MMRF CoMMpass Network, et al. MYC dysregulation in the progression of multiple myeloma. Leukemia. 2020;34(1):322–326.
- Shou Y, Martelli ML, Gabrea A, et al. Diverse karyotypic abnormalities of the c-myc locus associated with c-myc dysregulation and tumor progression in multiple myeloma. Proc Natl Acad Sci Usa. 2000;97(1):228–233.
- Gong J-N, Khong T, Segal D, et al. Hierarchy for targeting prosurvival BCL2 family proteins in multiple myeloma: pivotal role of MCL1. Blood. 2016;128(14):1834–1844.
- Kalff A, Khong T, Wall M, et al. A rare case of IGH/MYC and IGH/BCL2 double hit primary plasma cell leukemia. Haematologica. 2015;100(2):e60–e62.
- Nishida K, Tamura A, Nakazawa N, et al. The Ig heavy chain gene is frequently involved in chromosomal translocations in multiple myeloma and plasma cell leukemia as detected by in situ hybridization. Blood. 1997;90(2):526–534.
