Hereditary hematological malignancies are increasingly recognized. Indeed, myeloid neoplasm with germline predisposition was added as a new entity by the 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia, National Comprehensive Cancer Network (NCCN) Guidelines on Myelodysplastic Syndromes (version 2.2017), and 2017 European LeukemiaNet recommendations [Citation1–3]. Evaluation of underlying genetic defects is recommended as part of diagnosing hematological malignancies. Information bearing on germline defects can significantly impact the clinical management of patients, guide treatment recommendations, and inform screening for family members [Citation4]. However, studies in solid tumors have shown that a substantial number of patients with clinically actionable genetic variants are missed by current testing guidelines [Citation5–7]. A study on the referral criteria of patients with myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), and aplastic anemia showed that only 4.5% of these patients were referred for cancer genetic risk assessment [Citation8].
Gene panel-based tumor sequencing has now become standard-of-care testing for cancer patients. Testing tumor cells not only identifies somatic mutations for personalized therapy but may also reveal possible germline abnormalities. Studies on gene sequencing of multiple tumor types found that germline pathogenic/likely pathogenic variants (PVs) were present in 3-19.7% of patients [Citation9]. Unexpectedly, 55-60% of patients with germline PVs detected during tumor testing would have been missed by the genetic testing recommended by current guidelines [Citation10,Citation11]. Drazer et al. demonstrated that 12% of PVs from tumor testing of hematological malignancies were of germline origin [Citation12]. More recently, the American College of Medical Genetics and Genomics provided guidance regarding the reporting of germline variation in patients undergoing tumor testing [Citation13]. Management of potential germline PVs from tumor testing is a current challenge in clinical practice.
For hematological malignancies, tumor-only hotspot gene panels are widely used because matched germline samples (i.e. cultured skin fibroblasts) are not readily available for paired analysis. Most panels cover genes that are clinically significant for both somatic and germline mutations, such as TP53, RUNX1, GATA2, CEBPA, and ETV6. This makes accurately identifying potential germline PVs for follow-up confirmation challenging for clinicians. In this study, we analyzed 930 sequencing test results from 650 patients with hematological malignancies. We also propose a repeat testing approach to narrow candidate germline PVs for follow-up confirmation.
From November 2015 to December 2019, we performed 930 gene panel tests on bone marrow or peripheral blood samples of 650 patients with MDS, AML, and other hematological malignancies. We sequenced 54 genes using the Trusight myeloid kit (lllumina) and the enriched libraries on Illumina NextSeq 500 or 550 instruments. We used a custom bioinformatics pipeline (see details in Supplemental Information) for data analysis. The study was approved by our institutional review board.
From 930 gene panel tests performed on 650 patients with hematological malignancies, we reviewed PVs from nine hereditary cancer-associated genes—CBL, CDKN2A, ETV6, GATA2, NRAS, PTEN, PTPN11, RUNX1, and TP53—and identified 236 PVs reported in the ClinVar database as germline origin from 213 tests in 178 patients. After removing probable somatic variants with a variant allele frequency (VAF) below 30% for single nucleotide variants and 20% for frameshift variants, 75 PVs in 73 (11.8%) patients remained as possible germline PVs (Supplemental Figure S1). These PVs were present in all of the investigated genes except NRAS, with TP53 present most frequently ().
Table 1. Statistics of possible pathogenic variants from 73 patients.
If following the previously reported approach for identifying germline variants from tumor gene panel sequencing, all 75 PVs would be recommended for germline confirmation [Citation9]. We found that repeat testing dramatically reduced the number of variants for follow-up germline confirmation. In the 650 patients studied, 160 (24.6%) underwent gene panel testing 2–9 times for disease monitoring (Supplemental Figure S2). Of the 73 patients with PVs, 28 (38.4%) were tested more than once. Among the 30 PVs in the 28 patients with repeated testing, 23 (76.7%) PVs in the 21 patients were not present in all of the repeat tests, indicating the somatic nature of these variants. Only seven (23.3%) PVs were always present in seven patients (Supplemental Figure 1; ). Our findings are consistent with previous studies as we found that the vast majority of possible germline PVs identified from tumor testing are actually somatic [Citation12].
Compared with germline panel sequencing for hereditary hematological malignancies, the turnaround time for identifying a germline PV from tumor testing is slightly longer. The time elapsed between the initial and the second testing in our cohort was 3 to 1325 days (median 117 days) with 32% patients repeatedly tested within 56 days (Supplemental Figure S3). Germline testing of hematological malignancies normally takes 6-8 weeks and requires cultured skin fibroblasts [Citation14]. Turnaround time delays caused by culture failure and artifacts must be considered as well. Because the number of candidate germline PVs in each patient from tumor testing is very low, only a small amount of DNA is needed for follow-up germline confirmation. Direct skin tissue, hair roots, fingernails, plucked eyebrow, and bone marrow mesenchymal cells are all good sources for targeted analysis using Sanger sequencing for confirmation. These samples are also potential tissue sources for gene panel sequencing if clinically validated. The use of these tissue sources also reduces overall turnaround time.
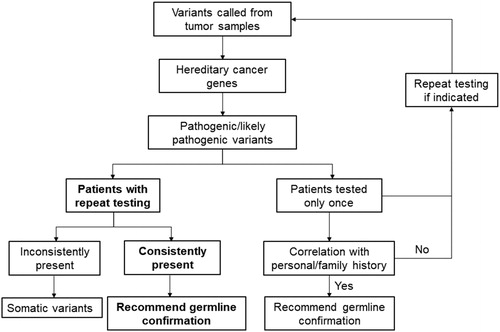
The PVs consistently present in repeat testing are in GATA2 (1 out of 4), RUNX1 (3 out of 8), and TP53 (3 out of 11) (, Supplemental Table 1), and have been reported as germline PVs by clinical laboratories in ClinVar. Five of these PVs have been reviewed by the ClinGen expert panel (Supplemental Table S1). All seven variants have also been reported as somatic in hematological malignancies in the Catalogue of Somatic Mutations in Cancer (COSMIC) database; accordingly, the variants may be somatic. Notably, the VAFs of the GATA2 variant in the patient P1 were constantly high in initial and repeat testing (89.6% and 98.6%, Supplemental Table S1). This patient was diagnosed with AML at 21 years old and had recurrent infection, lower leg edema, and monosomy 7 in bone marrow. The patient’s clinical history highly suggested MonoMAC syndrome, a germline predisposition to MDS and AML caused by GATA2 defect [Citation15]. As the blast cells in both bone marrow specimens are approximately 90%, the high VAFs may have been caused by high tumor burden and the loss of heterozygosity. Confirmation using a germline tissue would be necessary to achieve definitive diagnosis in this patient. To distinguish a variant origin, family history may or may not be helpful because of incomplete penetrance, de novo mutations, small family size, or incomplete family history. Follow-up evaluation of the 7 PVs on a germline specimen is warranted if the clinical scenario is appropriate. For the 45 patients without repeat testing, we would recommend germline confirmation for the patients with indicated personal and family history, and repeat tumor testing for the rest of the patients if clinically applicable (Supplemental Figure S1).
Tumor-only mutation hotspot gene panels have the potential to identify patients without typical presentation of germline predispositions, or in whom germline PVs may be missed. However, in our study, with repeat testing, only 7 of 650 (1.1%) patients were identified as candidates for follow-up germline confirmation. To improve the utility of tumor-only sequencing for possible germline variant screening, the following steps can be implemented in gene panel analyses. (1) Include genes for hereditary hematological malignancies, such as ANKRD26, RTEL1, SRP72, TERC, DDX41, SAMD9, SAMD9L among others [Citation15]; (2) Include all implicated exons and introns; (3) Include copy number variation analysis to detect large deletions and duplications; (4) Perform close follow-up of the patients with repeat tumor sequencing when clinically indicated.
In summary, although genetic testing is the first choice for patients with typical presentation of hematological malignancy predisposition, tumor-only sequencing is an alternative method by which to prioritize possible germline defects for patients without typical presentations. A comprehensive gene panel tailored to detect both somatic and germline defects is recommended. We demonstrate that repeat testing is a powerful tool to reduce the number of candidate germline variants for confirmation. We propose this effective approach to prioritize possible germline PVs for follow-up confirmation in tumor testing of hematological malignancies, which integrates our current knowledge of germline predisposition in cancer, repeat testing, VAF, and personal/family history ().
Authorship contributions
Z.L. conceived and designed the project; J.S., S.S, and P.C. performed bioinformatics analysis; K.P., S.B, and H.H. performed experiments and/or data analyses; J.T. and R.O. performed data analyses; J.S and Z.L. performed data analysis and wrote the manuscript.
GLAL-2020-0904-File004.docx
Download MS Word (110.4 KB)GLAL-2020-0904-File002.docx
Download MS Word (107.8 KB)Acknowledgments
We thank Dr. David Bernard for useful comments, and Sasha Pejerrey and Adrienne Winston for editorial assistance.
Disclosure statement
All authors declare no competing interests related to the work described.
Additional information
Funding
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(1):60–87.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Quinn E, Nichols KE. Cancer predisposition syndromes associated with myeloid malignancy. Semin Hematol. 2017;54(2):115–122.
- Neben CL, Zimmer AD, Stedden W, et al. Multi-gene panel testing of 23,179 individuals for hereditary cancer risk identifies pathogenic variant carriers missed by current genetic testing guidelines. J Mol Diagn. 2019;Jul21(4):646–657.
- Ademuyiwa FO, Salyer P, Ma Y, et al. Assessing the effectiveness of the National Comprehensive Cancer Network genetic testing guidelines in identifying African American breast cancer patients with deleterious genetic mutations. Breast Cancer Res Treat. 2019;178(1):151–159.
- LaDuca H, Polley EC, Yussuf A, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2020;22(2):407–415.
- Clifford M, Bannon S, Bednar EM, et al. Clinical applicability of proposed algorithm for identifying individuals at risk for hereditary hematologic malignancies. Leuk Lymphoma. 2019;60(12):3020–3027.
- Trottier AM, Cavalcante de Andrade Silva M, Li Z, et al. Somatic mutation panels: time to clear their names. Cancer Genet. 2019;235–236:84–92.
- Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA. 2017;18(9):825–835.
- Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373(24):2336–2346.
- Drazer MW, Kadri S, Sukhanova M, et al. Prognostic tumor sequencing panels frequently identify germ line variants associated with hereditary hematopoietic malignancies. Blood Adv. 2018;2(2):146–150.
- Li MM, Chao E, Esplin ED, et al. Points to consider for reporting of germline variation in patients undergoing tumor testing: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2020;22(7):1142–1148.
- Guidugli L, Johnson AK, Alkorta-Aranburu G, et al. Clinical utility of gene panel-based testing for hereditary myelodysplastic syndrome/acute leukemia predisposition syndromes. Leukemia. 2017;31(5):1226–1229.
- Godley LA, Shimamura A. Genetic predisposition to hematologic malignancies: management and surveillance. Blood. 2017;130(4):424–432.