Abstract
Patients with chronic myeloid leukemia (CML) in deep molecular remission may discontinue tyrosine kinase inhibitor (TKI) treatment without relapse. The present study aims to gain insight into the views of CML patients on TKI treatment discontinuation and identify factors that are associated with their willingness to discontinue treatment. A cross-sectional study, among adult Dutch CML patients was conducted to assess willingness and their views on benefits of and concerns about discontinuation. A total of 185 patients participated of whom 76% were willing to discontinue TKI-treatment. Patients considered the absence of side effects the most important benefit whereas fear of disease recurrence was their most prominent concern. Adequate monitoring was the most important prerequisite for TKI-treatment discontinuation. However, ambiguity with respect to perquisites indicate that patients on long-term TKI treatment should be adequately informed both on the possibility to discontinue treatment and on its benefits, risks, and measures that address risks.
Introduction
For about 20 years chronic myeloid leukemia (CML) can be effectively treated with a tyrosine kinase inhibitor (TKI). This group of drugs now includes imatinib, dasatinib, nilotinib, bosutinib, and ponatinib. Five years of TKI treatment has been found to result in a deep molecular response (DMR) in over 50% of patients with the percentage generally increasing with each year of treatment [Citation1,Citation2]. At diagnosis the average age of European patients is about 56 years [Citation3,Citation4]. Although the incidence of CML in Europe is 0.9 − 1.5 cases per 100,000 population [Citation3,Citation4], over the years CML prevalence has substantially increased. In the Netherlands the 20-year prevalence is now well over 2,000 patients (12 per 100,000 population) [Citation4–6]. Moreover, the life expectancy of chronic phase CML patients on successful TKI treatment has become similar to that of the general population [Citation7–9].
TKI treatment was initially believed to be a life-long therapy. However, clinical trial data obtained in patients using the first and second generation TKI’s imatinib, dasatinib and nilotinib show that about 50% of CML patients with a sustained DMR may discontinue TKI treatment without a molecular relapse to maintain a lasting treatment-free remission (TFR). Relapse mostly occurs within the first 3–12 months after discontinuation but relapsed patients generally regain the DMR status shortly after TKI treatment resumption [Citation1,Citation2,Citation10,Citation11]. Clinical study data also indicate that in patients with a failed discontinuation attempt, a second attempt may still be successful [Citation12–14]. Therefore, guidelines for the treatment of CML now include recommendations for TKI-treatment discontinuation [Citation15–17]. In Europe, health care professionals (HCPs) and CML patient associations have also provided specific guidelines for patient counseling and education regarding the discontinuation of TKI-treatment and TFR [Citation18].
Nevertheless, the willingness of patients to discontinue TKI treatment has been found to range from 34% to 83%, showing that not all patients are willing to discontinue treatment [Citation19–25]. Patient-reported reasons to discontinue TKI treatment primarily include relief of side effects, but inconvenience of taking daily medication, planned pregnancy, and treatment costs have also been found to play a role [Citation2,Citation18,Citation21–26]. Patient-reported concerns included the risk of disease recurrence, loss of disease control, fear to experience more side effects after restarting therapy, and the development of disease resistance [Citation2,Citation18–20,Citation22–25]. In the Netherlands, there are as yet no data on the patients’ perspective with regard to TKI-treatment discontinuation. To optimize patient counseling, the aim of the present study was to increase the understanding of the patient perspective on TKI treatment discontinuation and identify factors that were specifically associated with patient willingness to discontinue treatment.
Materials and methods
Study design and setting
A cross-sectional study was conducted between April and August 2018. CML patients were recruited in four hospital outpatient pharmacies, a hematology department and by means of an appeal by the Dutch patient association for people with a hematological malignancy, Hematon. The study was approved by the Medical Ethics Committee of the Amsterdam University Medical Centers.
Patient recruitment
CML patients (aged ≥18 years) treated for at least three months with a TKI were invited to participate. Patients were sent an information letter (and a reminder after 2 weeks) containing a direct web link to the questionnaire. The web link was also available on the Hematon website. Upon request, a hard copy was available for all patients. Data from the online version of the questionnaire were converted into an SPSS database. Data from the hard copy version were manually processed and checked by a second investigator. Consent was obtained from all participants.
Questionnaire
The questionnaire contained 36 self-composed questions on patient and treatment characteristics, patient-reported benefits, concerns, and prerequisites of TKI treatment discontinuation, and patient willingness to discontinue treatment. The questionnaire also contained the validated 5-item Medication Adherence Report Scale (MARS-5) [Citation27] (see Appendix 1). The questionnaire was pre-tested by a panel consisting of both HCPs and CML patients and adjusted if necessary.
Patient and treatment characteristics included age, gender, education, household, paid employment, (non-)academic hospital, date of diagnosis, current and previous TKI use, whether or not currently on treatment, quality of life (QoL), side effects, whether or not being a member of a patient association (Hematon or CMyLife) and awareness of TKI-treatment discontinuation trials. QoL was scored on a 0–10 scale (0 very bad, to 10 very well).
Patients on TKI treatment were queried about side-effects in the past three months, and their impact on daily life. Patients who had discontinued treatment were queried about side effects experienced before discontinuation. Questions regarding the severity of side effects were answered using a 5-point Likert scale (1 not at all, to 5 very much). The MARS-5 questionnaire consists of five statements about both intentional and unintentional non-adherence behavior. Scored on a 5-point scale, the total score varies between 5 and 25. Patients in our study were considered optimally adherent if the total score was 25, whereas a patient was considered sub-optimally adherent if the score was <25 [Citation28].
Questions on perceived benefits and concerns of, and prerequisites that would allow TKI treatment discontinuation were to be answered by checking (multiple) statements. In addition, patients were asked to identify the most important benefit and concern, and prerequisite for TKI treatment discontinuation. They could also provide additional information in open-text fields.
Patient willingness to discontinue TKI treatment was assessed both by questions on the attractiveness and plausibility of the idea of discontinuation and on the considerations and willingness with regard to actual discontinuation. Patients were also asked whether the possibility to discontinue treatment would influence their opinion about the importance of TKI treatment. A 5-point Likert scale was used to answer the questions on willingness. Patients were finally asked to indicate their willingness to discontinue TKI treatment in the hypothetical case that a certain relapse risk level (20%, 40%, 60%, or 80%) would exist upon discontinuation. Questions were answered by using a 5-point Likert scale (1 not at all, to 5 very much).
Statistical analyses
Data were analyzed using SPSS version 22.0 for Windows (IBM Corp, Armonk, NY, USA). Data were described as frequencies (percentages) for categorical variables, means and standard deviation (SD) for normally distributed continuous variables, and medians and interquartile range for skewed continuous variables. Logistic regression analysis was used to identify factors that could be associated with CML patients’ willingness to discontinue TKI-treatment. Patients on TKI-treatment were included in the regression analyses. The main outcome, ‘willingness to discontinue TKI-treatment’, was also dichotomized into ‘not willing’ and ‘willing’ to discontinue TKI-treatment. ‘Absolutely not willing’, ‘not willing’, and ‘neutral’ were considered ‘not willing’ whereas ‘willing’ and ‘absolutely willing’ were considered as ‘willing’. Patient and treatment characteristics were included in the model. First, univariable logistic regression analyses were used to identify factors related to the main outcome. Second, all factors from this analysis with p ≤ 0.157 (Akaike Information Criterion) were included in a multivariable logistic regression model [Citation29]. A backward elimination procedure was performed, where all variables were included and at each step the variable with the highest p value was removed from the model until variables remained with p ≤ 0.05. Variables with a non-linear relationship with the outcome were dichotomized: age <55 vs. ≥55 years, lower education vs. higher education, adherent (MARS-5 = 25) vs. non-adherent (MARS-5 < 25). An exploratory analysis was performed with a different dichotomization where neutral was considered willing to discontinue TKI-treatment, similar results were obtained (data not shown). A two-sided p value of ≤0.05 was considered significant.
Results
Patient and treatment characteristics
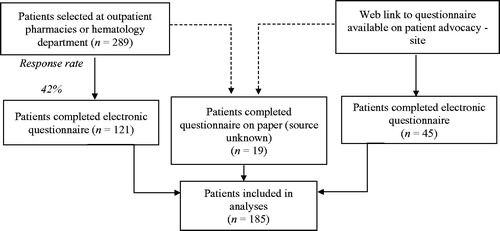
presents an overview of patient recruitment. A total of 185 patients participated in the study. shows the patient and treatment characteristics. About 40% of the patients was younger than 55 years of age. Over 20% of the patients had discontinued TKI treatment. The median treatment duration of these patients (i.e. years since CML diagnosis) was considerably longer than of patients who were on treatment (9.5 vs. 5.5 years). Imatinib was still the TKI most used (36%). The remaining patients were treated with dasatinib (33%) or nilotinib (25%). About 55% of all patients had switched TKIs during treatment. Patients who discontinued TKI treatment mostly had used imatinib (35%) or nilotinib (35%). The majority of patients (65%) reported to be optimally adherent.
Table 1. Patient and treatment characteristics (n = 185).
About 50% of the patients on treatment experienced side effects. Sixty percent of these patients reported that they had considerable impact on their lives. In the smaller group of patients who had discontinued TKI treatment about 70% of the patients reported to have experienced side effects. In patients on treatment almost as many patients had a high or low QoL. In contrast, 70% of the patients who had discontinued TKI treatment reported to have a high QoL. Most patients had heard about discontinuation of TKI-treatment studies (89%). The patients reported their physician (89%) as the primary source for being informed about the possibility to discontinue TKI treatment, followed by the patient association (47%), and on the internet (22%).
Patient views on TKI treatment discontinuation
Patient views on the benefits and concerns of, and prerequisites for TKI treatment discontinuation are shown in . Four major (>10%) benefits of TKI discontinuation were reported of which not experiencing side effects was the most frequently reported benefit (75%) followed by no longer having to think about taking the medication (50%), the feeling of being cured (33%), and not having to think about the illness (19%). Not experiencing side effects was also considered the most important benefit.
Table 2. Patient reported benefits, concerns, and prerequisites for discontinuing TKI-treatment.
Patients reported a larger number (8) of major concerns. Those most frequently (>30%) reported were it feels unsafe (37%), fear of aggressive relapse (36%) and regret on the decision to stop at a relapse (30%). Fear of an aggressive molecular relapse was considered the most important concern.
Patients also reported a variety of prerequisites for TKI discontinuation. Those most frequently reported were the ability to successfully resume treatment (70%), being assured of frequent monitoring (61%), the view that the probability of a relapse would be small (48%), the availability of a new (different) TKI in the case of relapse (38%) and the view that symptoms should not be more serious in the case of relapse (32%). Patients considered being assured of frequent monitoring was the most important prerequisite.
Willingness to discontinue TKI-treatment
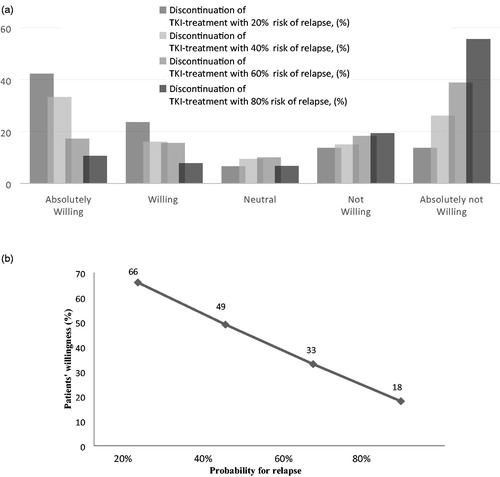
About a third of patients under current TKI treatment (N = 151) had considered to discontinue TKI treatment in the period before the survey. For 75% of these patients, discontinuation was an attractive prospect while 36% also thought it to be a plausible treatment option. Finally, most patients (76%) indicated they were willing to discontinue TKI-treatment. Moreover, 6% of the patients using a TKI, reported that awareness of the possibility to discontinue TKI treatment positively influenced their perception of the importance of TKI treatment. With an increasing risk level of relapse (20%, 40%, 60%, and 80%) the willingness of patients to discontinue TKI treatment decreased from 66 to 18% ().
Factors associated with the willingness to discontinue TKI-treatment
shows the univariable and multivariable analysis of factors potentially associated with the willingness to discontinue TKI-treatment. According to the multivariable analysis having paid employment (OR 2.48, 95% CI 1.11–5.52), and being aware of discontinuation studies (OR 4.05, 95% CI 1.48–11.10) were positively associated with the willingness to discontinue TKI-treatment.
Table 3. Factors associated with patients' willingness to discontinue TKI-treatment.
Discussion
Patients eligible for TKI treatment discontinuation suffer from side effects resulting from their TKI use. In the case of patients who discontinued TKI treatment side effects might have been related to the occurrence of a withdrawal syndrome [Citation1,Citation2]. Although being considered mostly low grade, these effects cause considerable discomfort and affect a patients’ QoL [Citation1,Citation2,Citation8,Citation18]. Similar to other studies [Citation20–25], in the present survey patients rated the absence of side effects the most important benefit of TKI treatment discontinuation. The remaining major benefits are clearly related to the idea that successful discontinuation would be some way back to a life without CML: no more worries about the disease, and the inconvenience of correctly taking medication. However, notably in view of the considerable relapse rate adequate counseling should prevent patients attempting a TFR from thinking that they are cured.
As in other studies, the potential negative consequences of TKI treatment discontinuation were primarily related to emotional stress producing feelings of uncertainty, anxiety, and regret that may be brought about by treatment discontinuation or its failure [Citation19,Citation20,Citation22,Citation24,Citation25]. Indeed, relapse and the subsequent resumption of TKI treatment have been found to increase anxiety and depression [Citation30]. In view of the still considerable rate of relapse, these kind of concerns are also a major reason for certain patients not to discontinue TKI treatment. This, however, may cause some patients to be treated for longer than necessary [Citation19,Citation22,Citation23].
Ambiguity in the views of CML patients is also reflected in the prerequisites for treatment discontinuation that, in addition to a stable DMR status as the main clinical condition, must be met before patients would be willing to discontinue TKI treatment. In line with guideline recommendations [Citation15–17] patients should find the required assurance in frequent monitoring, and the ability to successfully resume TKI treatment [Citation2,Citation18].
At the time of our study (2018), the results of several studies on treatment discontinuation with TKI were already well known and, given its importance for the treatment of CML as such, the process of guideline development was well underway. It is therefore not surprising that most patients knew about the possibility of TKI treatment discontinuation. Yet, it appeared that a significant number of these patients was inadequately informed about this new treatment option and its possible consequences. Nearly 50% of the patients only would discontinue TKI treatment if the chance of a relapse was small while 20% only would do so if the period between discontinuation and eventual relapse would be long. Significant numbers of patients also feared that in the case of relapse they would be unable to take their previous medication, lose control of their disease, experience a withdrawal syndrome, or that symptoms would be more serious. These statements show that patient information regarding TKI treatment discontinuation had not always been adequate. Given the impact of this treatment option on a patients’ life and its potential consequences, it is of utmost importance that inadequacies in patient counseling be addressed [Citation18]. In addition to mentioning the obvious benefits of TKI treatment discontinuation, patients should be particularly reassured because the majority of the concerns that were translated into certain prerequisites do not actually exist as the clinical study data clearly show [Citation1,Citation2].
As observed in several other studies [Citation19,Citation22,Citation23,Citation25], a relatively high proportion of CML patients wanted to discontinue TKI-treatment. In the multivariable analysis awareness of this new treatment option was also positively associated with the willingness to discontinue treatment. Moreover, the importance of awareness was demonstrated by the finding that the post-survey rate of the willingness to discontinue treatment was much higher than the percentage of patients who reported to have considered TKI treatment discontinuation prior to the survey. However, as pointed out above the information on discontinuation should be accurate and complete. Not being aware of the possibility of TKI treatment discontinuation and not being informed about its consequences indeed has been related to lower rates of patients willing to discontinue TKI treatment [Citation20,Citation21,Citation24]. In this respect, inadequate information about TKI treatment discontinuation may cause patients to overestimate its clinical consequences as resulting in reluctance to discontinue treatment [Citation25].
Increased awareness of the possibility to discontinue TKI treatment positively influenced the view of some patients on the importance of TKI treatment. On the other hand, about one-third of the patients was sub-optimally adherent. Optimal adherence to TKI treatment is paramount to achieving an optimal molecular response, while its depth is related to treatment duration [Citation15–17,Citation31]. Despite these requirements, awareness of the possibility of successful TKI treatment discontinuation and knowledge that in the event of relapse treatment can be successfully resumed, will encourage patients who have been treated for a long time to interrupt TKI treatment for shorter or longer periods [Citation8,Citation32–35]. HCPs counseling patients on long-term TKI treatment must be aware of this paradox which also urgently requires more clarity. Moreover, the approach to adequately contend with non-adherence must be different from that of the first decade of TKI treatment when maintaining adherence was considered a life-saving necessity.
As previously observed [Citation19,Citation22], in the present study the willingness to discontinue TKI treatment was inversely related to risk of relapse. Remarkably, even in the case of a high risk of relapse (80%), a small but substantial number of patients still wanted to discontinue TKI treatment. As noted previously [Citation21–25,Citation36], both the desire of patients to discontinue treatment and the offering of discontinuation by HCPs seems to primarily relate to the discomfort caused by TKI treatment and its side effects and the consequences thereof such as non-adherence and loss of QoL. However, given the still limited likelihood of a persistent TFR, it is not unlikely that assurance of successful treatment resumption now plays an important, but above all a barrier-lowering, role in the decision to discontinue TKI treatment [Citation1,Citation2,Citation18,Citation22].
In addition to awareness of the possibility of TKI treatment discontinuation, in the multivariable analysis the factor paid employment was also associated with the willingness of patients to discontinue treatment. Since in most European countries including the Netherlands, CML treatment is fully reimbursed, this statement does not relate to the costs of the treatment itself. Rather, it appears to be indicative of the overall burden that the disease and its treatment place on CML patients. Especially in patients who are not yet receiving old age benefits, particularly those between 55 and 65–70 years of age, the lack of income from work can be a burden. Indeed, side effects of TKI treatment, fatigue in particular, as well as stress and mental issues are known to impact on patient activities of daily living and prevent them to work [Citation8,Citation32]. It has also been found that patients who discontinued treatment felt a great sense of relief and freedom [Citation37]. It is therefore possible that (younger) patients in paid work are more hindered by their medication than those who do not work. This would strengthen the desire to get rid of these concerns and thereby increase their willingness to discontinue TKI treatment [Citation8,Citation21,Citation23].
Strengths and limitations
A strength of the study is that patients were invited to participate through various channels including a hematology department, a number of hospital outpatient pharmacies and the website of the Dutch patient association for hematological malignancies. The population is therefore representative for the Dutch CML patient population.
A limitation is that the total response rate to the survey is unknown as the result of the broad distribution of the questionnaire through a web link. This may have introduced selection bias. However, the response rate of the patients who were invited by the hospitals was at least 42%. Moreover, since the questionnaires were answered anonymously, there were no data on non-responders. Another limitation is that a substantial part of the questionnaire was not validated. The questionnaire, however, was pre-tested in a panel of professionals and patients and adjusted accordingly. Moreover, the authors have extensive experience in implementing patient surveys on the basis of patient interviews and questionnaires.
Conclusion
In the present study most patients with CML wanted to discontinue TKI-treatment. Discomfort caused by TKI side effects and the desire to get rid of the disease and its treatment were important reasons for the patients’ willingness to discontinue treatment. On the other hand, fear of disease recurrence and uncertainty about the effects brought about by discontinuation, relapse and TKI treatment resumption were dominant concerns associated with treatment discontinuation. Ambiguities in the prerequisites which patients believe should be met when TKI treatment is discontinued, indicate that they were inadequately informed about TKI treatment discontinuation. Its availability as a recommended treatment option not only requires that patients on long-term TKI treatment are made aware of its existence but also that they have to be adequately informed on its benefits, risks and measures that address risks and improve their QoL. In this manner, the process of shared decision making regarding the discontinuation of TKI-treatment can be optimized.
GLAL-2020-0856-File008.docx
Download MS Word (46.8 KB)Acknowledgments
The authors thank the patients who completed the questionnaire, the hospital outpatient pharmacies for their cooperation and the Hematon patient association for sharing the web link to the questionnaire with their members. The authors thank the patients who pre-tested the questionnaire.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Clark RE. Tyrosine kinase inhibitor therapy discontinuation for patients with chronic myeloid leukaemia in clinical practice. Curr Hematol Malig Rep. 2019;14(6):507–514.
- Cortes J, Rea D, Lipton JH. Treatment-free remission with first- and second-generation tyrosine kinase inhibitors. Am J Hematol. 2019;94(3):346–357.
- Hoffmann VS, Baccarani M, Hasford J, et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29(6):1336–1343.
- Thielen N, Visser O, Ossenkoppele G, et al. Chronic myeloid leukemia in the Netherlands: a population-based study on incidence, treatment, and survival in 3585 patients from 1989 to 2012. Eur J Haematol. 2016;97(2):145–154.
- Janssen JJWM, Boekhorst PAW, Posthuma EFM, et al. Richtlijn chronisch myeloide leukemie [Guidelines for chronic myeloid leukemia] [Internet]. The Netherlands: 2018. [cited 2020 Jun 29]. Available from: https://hematologienederland.nl/wp-content/uploads/2019/07/hovon-cml-richtlijn-14-04-2018_geautoriseerd.pdf.
- Integraal Kankercentrum Nederland. Dataselectie [data selection] [Internet]. The Netherlands: 2020. [cited 2020 Jun 23]. Available from: https://www.iknl.nl/.Dutch.
- Bower H, Björkholm M, Dickman PW, et al. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851–2857.
- Hewison A, Atkin K, McCaughan D, et al. Experiences of living with chronic myeloid leukaemia and adhering to tyrosine kinase inhibitors: a thematic synthesis of qualitative studies. Eur J Oncol Nurs. 2020;45:101730.
- Sasaki K, Strom SS, O'Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2(5):e186–e193.
- Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747–757.
- Shah NP, García-Gutiérrez V, Jiménez-Velasco A, et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: the DASFREE study. Leuk Lymphoma. 2020;61(3):650–659.
- Legros L, Nicolini FE, Etienne G, French Intergroup for Chronic Myeloid Leukemias, et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123(22):4403–4410.
- Fava C, Rege-Cambrin G, Dogliotti I, et al. Observational study of chronic myeloid leukemia Italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica. 2019;104(8):1589–1596.
- Legros L, Nicolini FE, Etienne G, et al. The TKI-free duration after a first discontinuation attempt that failed in CP CML patients is a predictive factor of TKI-free remission after a second attempt [abstract]. Blood. 2019;134(Supplement_1):28–28.
- Hochhaus A, Saussele S, Rosti G, et al. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv41–iv51.
- Radich JP, Deininger M, Abboud CN, et al. Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(9):1108–1135.
- Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–984.
- Saglio G, Sharf G, Almeida A, et al. Considerations for treatment-free remission in patients with chronic myeloid leukemia: a joint patient-physician perspective. Clin Lymphoma Myeloma Leuk. 2018;18(6):375–379.
- Sanford D, Kyle R, Lazo–Langner A, et al. Patient preferences for stopping tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Oncol. 2014;21(2):e241–e249.
- Flynn KE, Myers JM, D'Souza A, et al. Exploring patient decision making regarding discontinuation of tyrosine kinase inhibitors for chronic myeloid leukemia. Oncologist. 2019;24(9):1253–1258.
- Lou J, Huang J, Wang Z, et al. Chronic myeloid leukemia patients and treatment-free remission attitudes: a multicenter survey. Patient Prefer Adherence. 2018;12:1025–1032.
- Goldberg S, Hamarman S. Patients with chronic myelogenous leukemia may not want to discontinue tyrosine kinase inhibitor therapy [abstract. Blood. 2015;126(23):1584–1584.
- Jiang Q, Liu Z-C, Zhang S-X, et al. Young age and high cost are associated with future preference for stopping tyrosine kinase inhibitor therapy in Chinese with chronic myeloid leukemia. J Cancer Res Clin Oncol. 2016;142(7):1539–1547.
- Boquimpani CM, Szczudlo T, Mendelson E, et al. Attitudes and perceptions of patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) toward treatment-free remission (TFR) [abstract]. Blood. 2014;124(21):4547–4547.
- Villemagne Sanchez LA, O'Callaghan C, Gough K, et al. Patient perceptions of treatment-free remission in chronic myeloid leukemia. Leuk Lymphoma. 2018;59(2):406–415.
- Sharf G, Marin C, Bradley JA, et al. Treatment-free remission in chronic myeloid leukemia: the patient perspective and areas of unmet needs. Leukemia. 2020;34(8):2102–2112.
- Chan AHY, Horne R, Hankins M, et al. The medication adherence report scale (MARS‐5): a measurement tool for eliciting patients' reports of non‐adherence. Br J Clin Pharmacol. 2020;86(7):1281–1288.
- Timmers L, Boons CCLM, Kropff F, et al. Adherence and patients’ experiences with the use of oral anticancer agents. Acta Oncol. 2014;53(2):259–267.
- Royston P, Moons KGM, Altman DG, et al. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604.
- Sogawa R, Kimura S, Yakabe R, et al. Anxiety and depression associated with tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia. Int J Clin Oncol. 2018;23(5):974–979.
- Marin D, Bazeos A, Mahon F-X, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–2388.
- Boons CC, Harbers L, Timmers L, et al. Needs for information and reasons for (non) adherence in chronic myeloid leukaemia: Be aware of social activities disturbing daily routines. Eur J Haematol. 2018;101(5):643–653.
- Eliasson L, Clifford S, Barber N, et al. Exploring chronic myeloid leukemia patients' reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35(5):626–630.
- Langabeer SE, Faryal R, O'Dwyer M, et al. Patient-initiated discontinuation of tyrosine kinase inhibitor for chronic myeloid leukemia. Case Rep Hematol. 2020;2020:9571691. doi: 10.1155/2020/9571691.
- Wu S, Chee D, Ugalde A, et al. Lack of congruence between patients' and health professionals' perspectives of adherence to imatinib therapy in treatment of chronic myeloid leukemia: a qualitative study. Palliat Support Care. 2015;13(2):255–263.
- Devos T, Verhoef G, Steel E, et al. Interruption or discontinuation of tyrosine kinase inhibitor treatment in chronic myeloid leukaemia: a retrospective cohort study (SPARKLE) in Belgium. Acta Haematol. 2019;142(4):197–207.
- Mortensen GL, Mourek J. Drivers and barriers to medication adherence in patients with chronic myeloid leukaemia: a qualitative study. J Hematol Oncol. 2017;3(1):1–15.