Abstract
Data from the large, prospective, multinational, phase 3b JUMP study were analyzed to identify factors predictive of spleen and symptom responses in myelofibrosis patients receiving ruxolitinib. Factors associated with higher spleen response rates included International Prognostic Scoring System (IPSS) low/intermediate-1 risk vs intermediate-2/high risk (43.1% vs 30.6%; adjusted OR [aOR] 0.65 [95% CI 0.44–0.95]), ruxolitinib as first- vs second- or later-line therapy (40.2% vs 31.5%; aOR 0.53 [95% CI 0.38–0.75]), and a ruxolitinib total daily dose at Week 12 of >20 mg/day vs ≤20 mg/day (41.3% vs 30.4%; aOR 0.47 [95% CI 0.33–0.68]). No association was seen between baseline characteristics or total daily dose at Week 12 and symptom response. Ruxolitinib led to higher spleen response rates in patients with lower IPSS risk, and when used earlier in treatment. Higher doses of ruxolitinib were associated with higher spleen response rates, but not with symptom improvement.
INC424 for patients with primary myelofibrosis, post polycythemia myelofibrosis or post-essential thrombocythemia myelofibrosis (JUMP).
2010-024473-39; NCT01493414
Date of registration: 16 December 2011
https://www.clinicaltrialsregister.eu/ctr-search/search?query=2010-024473-39
Trial registration
Introduction
Primary myelofibrosis (PMF) is a rare chronic myeloproliferative neoplasm with an estimated incidence of 0.3 cases per 100,000 persons [Citation1]. A form of myelofibrosis (MF) that is indistinguishable from PMF can occur as part of the natural history of polycythemia vera (post-polycythemia vera myelofibrosis [PPV-MF]) or essential thrombocythemia (post-essential thrombocythemia myelofibrosis [PET-MF]. Clonal myeloproliferation and bone marrow fibrosis associated with MF can lead to clinical manifestations that include splenomegaly and constitutional symptoms [Citation2], which might have an impact on patients’ health-related quality of life (HRQoL). Symptoms include pruritus, night sweats, bone pain, fever, and fatigue [Citation3].
Allogeneic stem cell transplantation is an option for eligible patients; however, it has limited applicability [Citation4]. According to the European Society for Blood and Marrow Transplantation and European LeukemiaNet (EBMT–ELN) working group consensus, patients are potential candidates for allogeneic stem cell transplantation if: (1) they have International Prognostic Scoring System (IPSS), Dynamic IPSS (DIPSS) or DIPSS-plus intermediate-2- or high-risk disease and are age <70 years; or (2) they have intermediate-1-risk disease, are age <65 years, and present with either refractory transfusion-dependent anemia, >2% blasts in the peripheral blood, or adverse cytogenetics [Citation5]. The majority of patients with MF are not candidates for transplantation due to their advanced age, associated comorbidities, or due to a lack of suitable donors. Therefore, for the majority of patients with MF, treatment is generally palliative and aimed at symptom control.
JAK2, CALR, or MPL mutations are present in approximately 90% of patients with MF [Citation2], and the disease is characterized by dysregulation of the Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway [Citation6]. Ruxolitinib is a potent JAK1/JAK2 inhibitor that leads to durable improvements in splenomegaly, symptoms, and HRQoL measures when compared with placebo [Citation7] or best available therapy in patients with intermediate-2-risk or high-risk PMF [Citation8,Citation9]. The phase 2 ROBUST study included patients categorized as having intermediate-1-risk disease, and demonstrated that treatment with ruxolitinib results in clinically meaningful reductions in spleen length and symptoms [Citation10]. Due to the limited number of events at data cutoff and crossover in the randomized trials [Citation11], questions remain as to whether ruxolitinib offers an improvement in overall survival compared with best available therapy [Citation8,Citation9] or placebo [Citation7,Citation12].
The phase 3b JUMP (JAK Inhibitor RUxolitinib in Myelofibrosis Patients; NCT01493414) study was initiated to collect additional safety and efficacy data, and to provide access to ruxolitinib for patients in countries where the drug is not available outside of a clinical trial [Citation13]. The JUMP study included 2233 patients, and is the largest study of ruxolitinib in MF to date. Consistent with results from previous studies, patients in the JUMP study experienced durable improvements in splenomegaly and MF symptoms.
Although most patients with MF benefit from ruxolitinib treatment, response may vary among individual patients; in addition, factors associated with response are not well defined. The JUMP study provides data from a large patient population with MF that can be used to analyze and identify clinical factors that might be associated with response to ruxolitinib. Here, using data collected during the JUMP study, we assess the association of patient and disease characteristics and ruxolitinib dose levels with spleen response and symptom improvement.
Methods
Study design and patients
Details of the JUMP study have been published previously [Citation13]. In brief, the JUMP study is a single-arm, open-label, phase 3b, expanded-access study conducted globally in patients with MF (). Eligible patients were age ≥18 years, had a diagnosis of primary or secondary MF according to the World Health Organization criteria [Citation14,Citation15], and a baseline platelet count of ≥50 × 109/L. After a protocol amendment (amendment 2, September 2012), IPSS risk status was added as an eligibility criterion. Following the amendment, patients with IPSS intermediate-2- or high-risk MF [Citation16], with or without splenomegaly, and those with IPSS intermediate-1-risk MF with a palpable spleen (≥5 cm in length; measured at the left costal margin) were eligible.
Figure 1. Study design. aIncluded by amendment to the protocol. bPatients were treated for up to 24 months after the last patient’s first visit (December 23, 2014), unless discontinuation criteria were met. BID: twice daily; Int: intermediate; PET-MF: post-essential thrombocythemia myelofibrosis; PLT: platelet; PMF: primary myelofibrosis: PPV-MF: post-polycythemia vera myelofibrosis.

Ruxolitinib starting dose was based on the baseline platelet count: ruxolitinib 5 mg twice daily was given to patients with a platelet count of ≥50 to <100 × 109/L; ruxolitinib 15 mg twice daily was given to patients with a platelet count of ≥100 to ≤200 × 109/L; and ruxolitinib 20 mg twice daily was given to patients with a platelet count of >200 × 109/L. Dose increases were allowed for insufficient efficacy if neutrophil and platelet counts were adequate, and dose decreases or interruptions per a protocol-specified scheme were mandatory for safety reasons.
The primary endpoint was the safety and tolerability of ruxolitinib. Additional endpoints included the proportion of patients with a ≥50% reduction in palpable spleen length, and change from baseline in patient-reported outcome measures assessing fatigue (Functional Assessment of Chronic Illness Therapy – Fatigue [FACIT-F] scale) and symptoms (Functional Assessment of Cancer Therapy – Lymphoma [FACT-Lym] total score).
The study was approved by the institutional review board at each participating institution and conducted in accordance with applicable local regulations and the principles of the Declaration of Helsinki. All patients provided written informed consent before study entry.
Study assessments
In post hoc analyses, spleen response was evaluated according to the International Working Group – Myeloproliferative Neoplasms Research and Treatment (IWG–MRT) criteria [Citation17]. Response was defined as having a nonpalpable spleen at Week 24 (in patients with a palpable spleen of 5 to 10 cm in length at baseline) or a decrease in spleen length of ≥50% at Week 24 (in patients with a palpable spleen of >10 cm at baseline). Patients with a palpable spleen of <5 cm at baseline were not eligible for spleen response analyses.
Patients who discontinued treatment prematurely because of adverse events, progressive disease, or death were considered non-responders. Patients were considered transfusion-dependent if they received ≥6 units of transfusions within the 12-week period prior to ruxolitinib treatment initiation.
Symptom responses were evaluated using the FACT-Lym total score [Citation18,Citation19] and the FACIT-F scale [Citation20]. For the current analysis, DIPSS risk status was determined using baseline patient and disease characteristics for the 1844 of the 2233 enrolled patients who had information available for all 5 baseline parameters (white blood cell [WBC] count, age, hemoglobin [Hb], constitutional symptoms, and blasts). IPSS risk status was determined at the time of diagnosis. However, since the requirement for an IPSS assessment was instituted by a protocol amendment (amendment 2, September 2012) after 50% of the patients had already been recruited, some patients are missing IPSS information. DIPSS scores were calculated based on baseline values of Hb, WBC count, blasts, age, and the presence of constitutional symptoms [Citation21].
Statistical analyses
Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were derived by fitting a logistic regression with response as a binomial independent variable and the patient subgroups as binomial covariates. Baseline characteristics included as covariates were sex (male vs female), IPSS risk status (intermediate-2/high risk vs low/intermediate-1 risk), spleen length (≥10 vs <10 cm), transfusion dependence (dependence vs independence), Hb level (<10 vs ≥10 g/dL), platelet count (<100 vs ≥100 × 109/L), time since MF diagnosis (>2 vs ≤ 2 years), age (>65 vs ≤ 65 years), WBC count (>25 vs ≤ 25 × 109/L), MF subtype (primary vs secondary MF), DIPSS risk status (low/intermediate-1 vs intermediate-2/high), prior treatment (ruxolitinib as first-line vs as a later-line therapy), and blasts (≥1% vs <1%). The titrated total daily dose of ruxolitinib at 12 weeks (≤ 20 mg/day vs >20 mg/day) was also assessed.
Analyses were performed on the total patient population, and on subpopulations after stratification by DIPSS risk status.
Overall survival by spleen response in the low/intermediate-1 and intermediate-2/high DIPSS risk groups was assessed using Kaplan–Meier methods, with a good spleen response defined as described in Study Assessments.
Results
Patients
This analysis includes results for 2233 patients who were treated in the JUMP study from 2011 to 2017 [Citation13] at 279 clinical sites across 26 countries in Europe (82.0% [n = 1831]), Latin America (8.5% [n = 190]), North America (2.4% [n = 53]), and other regions (7.1% [n = 159]).
Median patient age was 67 years, mean time from initial diagnosis was 51.7 months, and 59.4% of patients had PMF (). In almost half of patients (49.1%), IPSS risk status information was missing; 0.1% (2 patients) were defined as low risk, 16.3% were defined as low/intermediate-1 risk, and 34.6% were defined as intermediate-2/high risk per IPSS stratification. DIPSS stratification was determined for the 1844 patients (82.6% of the total group) who had information available for all 5 required parameters: 60 of these 1844 patients (3.3%) were classified as low risk; Hb level <10 g/dL and platelet count <100 × 109/L were reported in 38.5% and 6.2% of patients, respectively; 7.1% of patients were considered transfusion-dependent. More patients had received prior treatment (59.3%) than were treatment-naive (40.7%), and most patients (65.9%) had a palpable spleen of ≥10 cm in length. The mean FACT-Lym total score at baseline was 113.9, and the mean FACIT-F score was 32.7.
Table 1. Patient baseline characteristics.
Drug exposure
Most patients (93.1% [n = 2078]) started ruxolitinib treatment at >20 mg/day, while 6.9% (n = 155) started treatment at ≤ 20 mg/day. Median duration of ruxolitinib exposure was 12.4 months (range <0.1 to 59.7), and the mean daily dose of ruxolitinib was 28.7 mg. Patients with an Hb level >10 g/dL and/or a platelet count >100 × 109/L more frequently received doses of ruxolitinib >20 mg/day when compared with patients who had an Hb level <10 g/dL and a platelet count <100 × 109/L (). A total of 1138 patients (51.0%) had >1 year, 674 patients (30.0%) had >2 years, and 289 patients (13.0%) had >3 years of exposure to ruxolitinib.
Table 2. Comparison of baseline Hb levels and platelet counts with ruxolitinib dose at Week 12.
Characteristics associated with spleen response
Most patients experienced a reduction in spleen size while receiving ruxolitinib. Of 2049 patients assessed, the mean change from baseline in palpable spleen length was −68.3% (median −75.0% [range −100.0% to 133.3%]). At Week 24, 34.3% of evaluable patients (672 of 1960) achieved a spleen response by IWG–MRT criteria.
In the univariate analysis, baseline factors associated with higher spleen response rates were IPSS low/intermediate-1 risk status, Hb level ≥10 g/dL, platelet count ≥100 × 109/L, time since MF diagnosis of ≤2 years, ruxolitinib as first-line treatment, blasts <1%, and ruxolitinib dose >20 mg/day. The predictive power of receipt of prior treatment (vs no prior treatment) was restricted to patients in the DIPSS intermediate-2/high-risk group (37.3% vs 26.8%; adjusted OR [aOR] 0.62 [95% CI 0.45–0.84]).
In the multivariate analysis, IPSS low/intermediate-1 risk, ruxolitinib as first-line treatment, and ruxolitinib dose >20 mg/day at Week 12 were significant predictors of spleen response (). Ruxolitinib as first-line treatment (vs as second or later line of therapy) was also a significant predictor of spleen response irrespective of DIPSS risk status (low/intermediate-1 risk: 45.2% vs 38.0%; aOR 0.59 [95% CI 0.37–0.93]; intermediate-2/high risk: 37.3% vs 26.8%; aOR 0.46 [95% CI 0.27–0.79]).
Figure 2. Factors predicting higher spleen response rates in multivariate analysis. ORs and the corresponding 95% CIs were derived by fitting a logistic regression with response as a binomial independent variable and the patient subgroups as binomial covariates. Factors associated with a higher spleen response rate are shown in bold font. Patient characteristics favored by the OR are labeled with a dot (•). BL: baseline; CI: confidence interval; DIPSS: Dynamic International Prognostic Scoring System; IPSS: International Prognostic Scoring System; LCM: left costal margin; MF: myelofibrosis; OR: odds ratio; WBC: white blood cell.
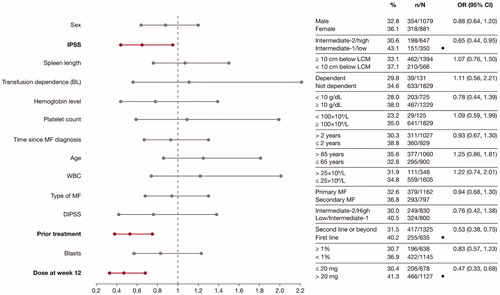
Higher spleen response rates correlated with a higher ruxolitinib dose in both univariate and multivariate analyses. Patients who received a titrated dose of ruxolitinib of >20 mg/day at Week 12 had higher spleen response rates than those who received ≤20 mg/day (41.3% vs 30.4%; aOR 0.47 [95% CI 0.33–0.68] by multivariate analysis) (). Spleen response rates according to ruxolitinib dose were consistent across both DIPSS risk groups (low/intermediate-1 risk: 46.7% with >20 mg/day vs 35.6% with ≤20 mg/day; aOR 0.44 [95% CI 0.27–0.70]; intermediate-2/high risk: 37.4% with >20 mg/day vs 26.4% with ≤ 20 mg/day; aOR 0.5 [95% CI 0.28–0.88]).
Characteristics associated with symptom improvement
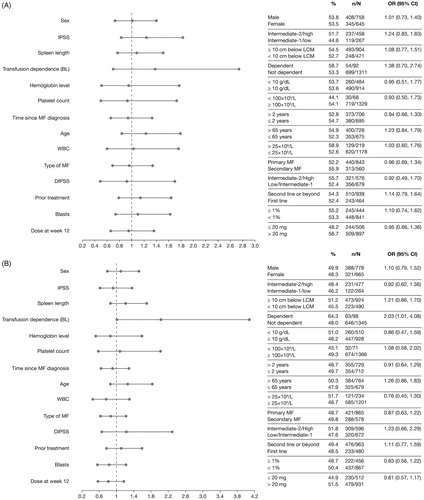
In the univariate analysis, only a titrated total daily dose of ruxolitinib at >20 mg/day at Week 12 of therapy was correlated with achieving a symptom response based on the FACT-Lym total score (48.2% with ≤ 20 mg/day vs 56.7% with >20 mg/day; OR 0.71 [95% CI: 0.57–0.88]) and the FACIT-F scale (44.9% with ≤ 20 mg/day vs 51.5% with >20 mg/day; OR 0.77 [95% CI 0.62–0.96]).
In the multivariate analysis, no associations between baseline characteristics or titrated dose at Week 12 and symptom response were seen (), even after stratification by DIPSS risk status (low/intermediate-1 risk vs intermediate risk-2/high risk).
Figure 3. Factors predicting improvements in HRQoL and symptoms, assessed by improvements in FACT–Lym total score (A) and FACIT-F score (B) in multivariate analyses. ORs and the corresponding 95% CIs were derived by fitting a logistic regression with response as a binomial independent variable and the patient subgroups as binomial covariates. Factors associated with a higher spleen response rate are shown in bold font. Patient characteristics favored by the OR are labeled with a dot (•). BL: baseline; CI: confidence interval; DIPSS: Dynamic International Prognostic Scoring System; FACT-Lym: Functional Assessment of Cancer Therapy-Lymphoma; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue; HRQoL: health-related quality of life; IPSS: International Prognostic Scoring System; LCM: left costal margin; MF: myelofibrosis; OR: odds ratio; WBC: white blood cell.
Overall survival by DIPSS risk group stratification
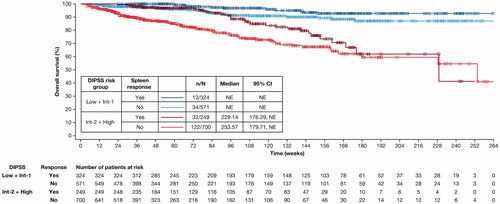
When analyzed by DIPSS risk status, the Kaplan–Meier analysis of median overall survival was not reached in patients in the DIPSS low/intermediate-1 risk group. The Kaplan–Meier analysis of median overall survival in patients in the DIPSS intermediate-2/high risk group and who achieved a spleen response at Week 24 was 229.1 weeks (vs 253.6 weeks in those who did not respond) (). Interpretation of this result must be approached with caution given the small sample sizes; 32 patients achieved spleen response and 122 patients did not.
Figure 4. Kaplan–Meier estimate of overall survival of spleen responders vs non-responders at Week 24 stratified by DIPSS risk status. Spleen response was defined as having a non-palpable spleen at Week 24 (in patients with a palpable spleen 5–10 cm below the left costal margin at baseline) or a decrease in spleen length by ≥50% at Week 24 (in patients with a palpable spleen >10 cm at baseline). Patients with missing spleen data were considered non-responders. CI: confidence interval; DIPSS: Dynamic International Prognostic Scoring System; Int: intermediate; NE: not evaluable.
Discussion
This secondary analysis of the JUMP study identified less advanced disease, higher doses of ruxolitinib, and ruxolitinib as the first-line therapy as independent factors associated with higher spleen response rates. The study included a large population that comprised diverse MF patient subgroups, including patients with DIPSS low and intermediate-1 risk, and those with lower platelet counts. Although clinically meaningful improvements in symptoms were seen as early as 4 weeks after starting ruxolitinib in the JUMP study [Citation13], symptom improvement (as assessed using the FACT-Lym total score and the FACIT-F scale) was not associated with any of the clinical or demographic factors assessed in the multivariate analyses. These results are in line with the main findings of the JUMP study, showing that symptom response rates were not affected by the ruxolitinib starting dose, and were similar when evaluated according to MF subtype [Citation13].
A previous subgroup analysis of the COMFORT-I trial (NCT00952289) did not show any baseline factors associated with spleen response to ruxolitinib [Citation22]. The COMFORT trials primarily focused on patients with higher-risk MF (intermediate-2 risk and high risk), and most of the patients had already failed one or more lines of prior treatments. The study population of the JUMP study is more diverse, and further enhances our understanding of the use of ruxolitinib in a real-life setting. The current study allowed for upward or downward titrations of the ruxolitinib dose based on platelet counts at baseline and during follow-up. We found that in patients with a good hematologic reserve (Hb level >10 g/dL and platelet count >100 × 109/L), the likelihood of maintaining a higher dose of ruxolitinib at Week 12 is high. However, in patients with a lower hematologic reserve (Hb level <10 g/dL and platelet count <100 × 109/L), the likelihood of maintaining a higher dose at Week 12 is low. Therefore, patients with a low hematologic reserve may benefit from starting at a relatively lower dose of ruxolitinib, which should be gradually titrated upwards if blood counts allow. Our finding that doses of ruxolitinib >20 mg/day led to higher spleen response rates suggests that patients should be titrated to the highest tolerated dose of ruxolitinib to achieve a maximal response.
Our study shows that ruxolitinib provides higher spleen response rates when given as first-line therapy, as opposed to being used as a later-line therapy. This outcome is also supported by results from the COMFORT-II study (NCT00934544), in which no meaningful responses were observed with best available therapy when compared with ruxolitinib [Citation9]. Furthermore, this analysis extends the findings of the JUMP [Citation13] and ROBUST [Citation10] trials, showing efficacy of ruxolitinib with higher spleen response rates in patients with low or intermediate-1 risk MF.
The results from the current analysis are also consistent with those of a previous study conducted at 18 Italian centers and examining factors associated with response to ruxolitinib in patients with MF [Citation23]. The baseline characteristics of this study population were broadly similar to those of the JUMP study population, with the exception of the proportion of patients defined as IPSS intermediate-2/high risk (84.3%; 64.9% in the current study) and the mean time since initial diagnosis (44.4 months; 51.7 months in the current study). Factors associated with a lower probability of spleen response were those related to more advanced disease (IPSS intermediate-2 or high risk, a greater degree of splenomegaly, transfusion dependency), >2 years from initial diagnosis to starting ruxolitinib, and a platelet count of <200 × 109/L, which also corresponded to a lower ruxolitinib starting dose [Citation23].
The limitations of the current analysis include the lack of MF-specific HRQoL data, and the fact that cytogenetic and molecular data were not available, as well as attrition in the study due to the commercial availability of ruxolitinib limiting the length of follow-up time. Therefore, this study is unable to address the question of durability of response in a meaningful way.
This secondary analysis of the JUMP study data identified lower IPSS risk as a significant factor for predicting higher spleen response rates. The findings also demonstrate that treating patients with ruxolitinib earlier in the disease course, using ruxolitinib as first-line therapy, and maintaining ruxolitinib treatment at a higher dose all lead to higher spleen response rates. The predictive factors identified in this study are applicable to routine clinical practice, and might help healthcare professionals optimize the use of ruxolitinib in the management of patients with MF.
Author contributions
V. Gupta, M. Griesshammer, B. Martino, L. Foltz, R. Tavares, H.K. Al-Ali, P. Giraldo, P. Guglielmelli, E. Lomaia, and P. Raanani enrolled patients, performed research, and contributed to data collection, analysis, and interpretation. R. Tiwari performed statistical analyses and contributed to data interpretation. E. Zor, C. Bouard, and C. Paley contributed to data interpretation. All authors critically reviewed each version of the manuscript and approved the final version for submission. The authors confirm that this work has not been published previously.
Ethics approval
The study was approved by the institutional review board at each participating institution and conducted in accordance with applicable local regulations and the principles of the Declaration of Helsinki. All patients provided written informed consent before entry into the study.
Acknowledgments
The authors received writing/editorial support in the preparation of this manuscript provided by James Matthews, PhD, of Excerpta Medica, funded by Novartis Pharmaceuticals Corporation. Parts of this study have been presented at the annual meeting of the European Hematology Association 2018 (poster presentation).
Disclosure statement
V. Gupta has received consultancy fees, honoraria, and research funding from Novartis, has received consultancy fees and research funding from Incyte, and further support from Celgene, Sierra Oncology, and Pfizer.
M. Griesshammer has received consultancy fees and honoraria from and has served on speakers bureaus for AOP Orphan, Baxalta, Gilead, Novartis, and Shire, and has received honoraria from and has served on speakers bureaus for Sanofi.
L. Foltz has received consultancy fees from Pfizer; research funding from Constellation, Incyte, and Promedior; and consultancy fees, honoraria, and research funding from Celgene and Novartis.
R. Tavares has received consultancy fees from Novartis.
H.K. Al-Ali has received consultancy fees, honoraria, and research funding from Novartis and Celgene and a travel grant from AOP Orphan.
E. Lomaia has received a travel grant from Novartis and Pfizer and has served on advisory boards for Novartis and Celgene.
P. Raanani has received consultancy fees and grants from and has served on advisory boards for Novartis, Pfizer, and Ariad (Medison), and has received consultancy fees from and has served on advisory boards for Bristol Myers Squibb.
C. Bouard, C. Paley, R. Tiwari, and E. Zor are employees of Novartis.
B. Martino, P. Giraldo, and P. Guglielmelli declare no competing interests.
Data availability statement
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
Additional information
Funding
References
- Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-2012. Br J Haematol. 2016;174(3):382–396.
- Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(12):1262–1271.
- Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Myeloproliferative neoplasms. Version 2. 2019; [cited 2020 Jun 15]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
- Kröger NM, Deeg JH, Olavarria E, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29(11):2126–2133.
- Tefferi A, Guglielmelli P, Pardanani A, et al. Myelofibrosis treatment algorithm 2018. Blood Cancer J. 2018;8(8):72.
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807.
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798.
- Harrison CN, Vannucchi AM, Kiladjian J-J, on behalf of the COMFORT-II investigators, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701–1707.
- Mead AJ, Milojkovic D, Knapper S, et al. Response to ruxolitinib in patients with intermediate-1-, intermediate-2-, and high-risk myelofibrosis: results of the UK ROBUST trial. Br J Haematol. 2015;170(1):29–39.
- Cervantes F, Pereira A. Does ruxolitinib prolong the survival of patients with myelofibrosis? Blood. 2017;129(7):832–837.
- Verstovsek S, Mesa RA, Gotlib J, for the COMFORT-I investigators, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10(1):55.
- Al-Ali HK, Griesshammer M, le Coutre P, et al. Safety and efficacy of ruxolitinib in an open-label, multicenter, single-arm phase 3b expanded-access study in patients with myelofibrosis: a snapshot of 1144 patients in the JUMP trial. Haematologica. 2016;101(9):1065–1073.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951.
- Barosi G, Mesa RA, Thiele J, International Working Group for Myelofibrosis Research and Treatment (IWG-MRT), et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22(2):437–438.
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901.
- Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122(8):1395–1398.
- Harrison CN, Mesa RA, Kiladjian J-J, et al. Health-related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib versus best available therapy. Br J Haematol. 2013;162(2):229–239.
- Carter GC, Liepa AM, Zimmermann AH, et al. Validation of the Functional Assessment of Cancer Therapy–Lymphoma (FACT-LYM) in patients with relapsed/refractory mantle cell lymphoma. Blood. 2008;112(11):2376.
- Cella D, Eton DT, Lai JS, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–561.
- Passamonti F, Cervantes F, Vannucchi AM, et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood. 2010;116(15):2857–2858.
- Verstovsek S, Mesa RA, Gotlib J, et al. The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo-controlled, Phase III study in patients with myelofibrosis. Br J Haematol. 2013;161(4):508–516.
- Palandri F, Palumbo GA, Bonifacio M, et al. Baseline factors associated with response to ruxolitinib: an independent study on 408 patients with myelofibrosis. Oncotarget. 2017;8(45):79073–79086.
