Novel treatment regimens combining chemotherapy with targeted agents are being developed for diffuse large B-cell lymphoma (DLBCL). These regimens are expected to show efficacy in biomarker-defined target populations. Personalized healthcare (PHC) studies are now being used to assess predictive biomarkers for clinical trial inclusion criteria [Citation1–5]; however, there are concerns that treatment delays due to prospective biomarker testing may exclude patients with aggressive disease, introducing bias in the clinical trial population [Citation6–9]. Data from a combined, prospective, observational cohort from the University of Iowa/Mayo Clinic Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource and the Lymphoma Study Association (LYSA) LNH-2003 clinical trials program showed that initiation of therapy ≥15 days from diagnosis was associated with less aggressive disease and more favorable outcomes than when initiated ≤14 days from diagnosis [Citation7–9]. To provide further insights, the present analysis evaluated whether a shorter vs. longer screening window, simulating the time for prospective biomarker testing, was associated with progression-free survival (PFS), using data from previously untreated DLBCL patients in the global, phase III GOYA study (NCT01287741) [Citation10]. Our findings suggest that treatment delay due to biomarker testing does not affect outcome on this model, and should not preclude enrollment of patients into clinical trials. Furthermore, patients with aggressive disease may benefit most from trials of novel biomarker-guided therapies.
Patients in GOYA were randomized to receive 8 cycles of rituximab or obinutuzumab plus 6–8 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) [Citation10]. Ethics approval and informed consent were obtained. To assess the association between treatment delay and outcome in this study, we evaluated the correlation between time from diagnosis-to-randomization and investigator-assessed PFS. For this analysis, patients were stratified into 7-day diagnosis-to-randomization intervals (i.e. 1–7 days, 8–14 days, etc.). Diagnosis was defined as the date of confirmation of the lymphoma-containing biopsy. Randomization was the date of assignment to a treatment arm once eligibility was confirmed.
To isolate the contribution of prospective testing times from overall treatment delay, we separated time from diagnosis-to-randomization into 2 steps: (1) time from diagnosis-to-initiation of screening (DS; where initiation of screening was the date screening activities started, as reported by Interactive Response Technology), and (2) time from initiation of screening to randomization (SR). The latter is the period when biomarker testing would take place in a trial with prospective testing. For the DS analysis, patients were stratified into 7-day intervals. As GOYA was not designed to include prospective biomarker testing, we assessed whether short vs. long SR times (an increase consistent with the turnaround time of biomarker testing) had an impact on outcomes for high-risk DLBCL patients. To simulate prospective testing, SR intervals of <6 days, 6–9 days, and >9 days were selected based on median SR times in GOYA (6 days) and median SR times in a trial with prospective biomarker testing (IMpower150, NCT02366143) [Citation11]. PFS between different DS and SR subgroups was compared using log-rank tests.
Median diagnosis-to-randomization time was 24 days (range 1–71 days). Patients with the shortest diagnosis-to-randomization time had the shortest PFS, with the largest difference in PFS being observed at a diagnosis-to-randomization threshold of 14 days (; hazard ratio [HR] for ≤14 days vs. >14 days, 0.63; 95% confidence interval [CI], 0.49–0.80; p < .0001). The 250 patients with a ≤ 14-day diagnosis-to-randomization interval had a 3-year PFS rate of 57% compared with 72% for the 879 patients with intervals >14 days. These results are broadly consistent with those reported by Maurer et al. [Citation7–9] in which patients with ≤14 days to treatment had a 24-month event-free survival rate of 56% compared with 72% for those with >14 days to treatment (p < .0001). Similarly, a retrospective, single-center study found that a diagnosis-to-treatment interval ≤14 days was associated with worse PFS than intervals >14 days (HR, 1.7; 95% CI, 1.16–2.58; p = .0067) [Citation12]. Previous studies also showed that patients with diagnosis-to-randomization intervals of ≤14 days were more likely to have features of aggressive disease than patients with >14-day intervals [Citation7–9,Citation12]. Consistent with these findings, DLBCL patients enrolled in GOYA with a ≤ 14-day diagnosis-to-randomization interval had a higher incidence of the following variables compared with those with intervals >14 days, suggesting more aggressive disease: double-hit lymphoma (9% [9/95] vs. 3% [9/353]; p = .002), >2 extranodal sites (34% [62/181] vs. 24% [149/629]; p = .004), bulky disease (≥7.5 cm: 48% [119/249] vs. 36% [312/869]; p = .0007), high lactate dehydrogenase (LDH; 82% [203/249] vs. 60% [525/868]; p < .0001), Ann Arbor stage IV disease (58% [145/249] vs. 48% [417/872]; p = .004), and high International Prognostic Index (IPI; 24% [61/249] vs. 18% [155/872]; p = .018). This correlation between short time to treatment and aggressive disease may reflect a desire by physicians to treat patients with the most aggressive DLBCL more promptly. No significant PFS difference was observed between patients with diagnosis-to-randomization intervals of 15–28 days and those with intervals >28 days ().
Figure 1. Percentage of patients and progression-free survival by delay. (A) Delay from diagnosis-to-randomization. (B) Delay from diagnosis-to-initiation of screening. (C) Delay from initiation of screening to randomization.
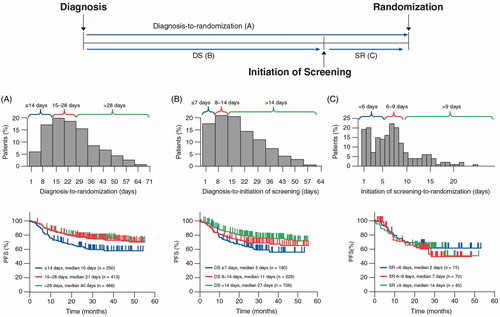
In step 1 of the 2-step analysis model, DS times of ≤7 days, 8–14 days, and >14 days were observed for 190, 228, and 706 patients, respectively. Patients with a shorter DS interval tended to have shorter PFS (). In particular, patients with DS ≤7 days had a 3-year PFS of 55% vs. 66% for patients with DS 8–14 days (HR, 0.76; 95% CI, 0.54–1.11), and 72% for those with DS >14 days (HR, 0.60 vs. 8–14 days; 95% CI, 0.45–0.80; overall log-rank, p = .0012). Consistent with the diagnosis-to-randomization analysis, patients with DS ≤7 days were more likely to have adverse disease characteristics (e.g. double-hit lymphoma, bulky disease, high LDH, and fever) than those with longer DS (). This novel observation that outcomes remain poor in these high-risk patients with DLBCL, despite a short DS interval, highlights a need to develop alternative therapeutic approaches, including novel targeted agents, in this patient cohort.
Table 1. Patient and disease characteristics by time from diagnosis-to-initiation of screening (safety population).
In step 2 of the 2-step analysis model, median SR time in patients with more aggressive disease (patients with DS ≤7 days) was 7 days (range 1–26 days). In these patients, an increase in SR time from <6 days to 6–9 and >9 days, which simulates a delay consistent with prospective biomarker testing, had no effect on PFS (HR for <6 vs. 6–9 days, 1.25; 95% CI, 0.63–2.48; HR for <6 vs. >9 days, 1.4; 95% CI, 0.78–2.53; overall log-rank p = 0.765; ). Similarly, for the highest risk patients (DS ≤7 days and high IPI score) and those with DS 8–14 days, a delay in screening time from <6 days to >9 days had no significant impact on PFS (p = .383 and p = .858, respectively). Thus, the longer screening times, which may be needed for prospective biomarker testing, do not appear to adversely affect outcomes. Furthermore, physicians could use steroids to help manage patients during SR.
In this model, biomarker testing (by anti-PD-L1 immunohistochemistry) during the screening period (derived from median SR time in Impower150) was feasible; however, more technically difficult biomarker assays, such as next generation sequencing (NGS) based testing including mutation or gene signature classifiers, may have a longer turnaround time and are not modeled here. As such, the possibility to test a biomarker in the time modeled in this study may depend on the type of assay.
In summary, we confirm that short time from diagnosis-to-randomization is associated with worse PFS and correlates with features of high-risk biology and aggressive disease, implying that underlying biology affects patient prognosis. Despite seemingly expedited work-up, these high-risk patients are associated with poor outcomes, highlighting the need for innovative therapies and trial designs. By dividing the time from diagnosis-to-randomization into two phases, we observed that an increase in screening time, consistent, in our model, with additional time required for prospective testing, did not adversely impact outcome (PFS). Our findings suggest that treatment delay due to biomarker testing does not affect outcome in patients with aggressive DLBCL and therefore should not be a limitation in their inclusion from clinical trials of targeted therapy.
Acknowledgments
The authors would like to thank the patients and their families, as well as the investigators from the GOYA study. The authors also gratefully acknowledge the contribution of Mehrdad Mobasher to this analysis. Medical writing support was provided by Tara N. Miller of Envision Pharma Group and Lynda McEvoy of Gardiner-Caldwell Communications, which was funded by F. Hoffmann-La Roche.
Disclosure statement
E.S.-G., F.V.P., J.R., J.V., M.B., and E.P. are employees of Genentech, Inc, and are equity holders of Roche Holding AG. J.L., A.S., C.H., M.O., M.K., and A.K. are employees and equity holders of Roche Products Ltd. L.H.S. has received consulting fees from Roche/Genentech, Amgen, Gilead Sciences, Lundbeck, Seattle Genetics, Janssen Pharmaceuticals, AbbVie, TG Therapeutics, and Celgene, and honoraria from Seattle Genetics, AbbVie, and TG Therapeutics. U.V. consults for or advises F. Hoffmann-La Roche Ltd, Celgene, Juno Therapeutics, and Janssen Pharmaceuticals, participates in speakers' bureaus for F. Hoffmann-La Roche Ltd, Janssen Pharmaceuticals, Celgene, Gilead Sciences, Servier, and Takeda, and has received research support from F. Hoffmann-La Roche Ltd and Celgene, and travel support from F. Hoffmann-La Roche Ltd, Alexion Pharmaceuticals, and Janssen Pharmaceuticals.
Data availability
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Additional information
Funding
References
- Nowakowski GS, Chiappella A, Witzig TE, et al. ROBUST: lenalidomide-R-CHOP versus placebo-R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma. Future Oncol. 2016;12(13):1553–1563.
- Younes A, Zinzani PL, Sehn LH, et al. A randomized, double-blind, placebo-controlled phase 3 study of ibrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in subjects with newly diagnosed nongerminal center B-cell subtype of diffuse large B-cell lymphoma (DLBCL). J Clin Oncol. 2014;32(15_suppl):TPS8615.
- Davies AJ, Barrans S, Maishman T, et al. Differential efficacy of bortezomib in subtypes of diffuse large B-cell lymphoma (DLBCL): a prospective randomised study stratified by transcriptome profiling: REMODL-B. Hematol Oncol. 2017;35(Suppl S2):130–131.
- Morschhauser F, Feugier P, Flinn IW, et al. Venetoclax plus rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) improves outcomes in BCL2-positive first-line diffuse large B-cell lymphoma (DLBCL): first safety, efficacy and biomarker analyses from the phase II CAVALLI study. Blood. 2018;132(Supplement 1):782.
- Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-Cell lymphoma. J Clin Oncol. 2019;37(15):1285–1295.
- Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-Cell lymphoma: clinical outcomes of the Phase III intergroup trial Alliance/CALGB 50303. J Clin Oncol. 2019;37(21):1790–1799.
- Maurer MJ, Ghesquières H, Link BK, et al. Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol. 2018;36(16):1603–1610.
- Maurer MJ, Ghesquières H, Link BK, et al. Diagnosis-to-treatment interval (DTI) remains associated with adverse clinical characteristics and outcome in newly diagnosed patients with diffuse large B-cell lymphoma treated on clinical trials. Blood. 2017;130(Suppl 1):4115.
- Maurer MJ, Link BK, Habermann TM, et al. Time from diagnosis to initiation of treatment of DLBCL and implication for potential selection bias in clinical trials. Blood. 2016;128(22):3034.
- Vitolo U, Trněný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3529–3537.
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301.
- Camus V, Dubois S, Jardin F, et al. Prognostic impact of diagnosis to treatment interval (DTI) in diffuse large B-cell lymphoma patients: a real-life monocentric study. Leuk Lymphoma. 2019;60(3):839–841.
