Abstract
Platelet/endothelial cell adhesion molecule 1 (PECAM-1, CD31) is an immunoglobulin superfamily member expressed on the surface of platelets, leukocytes and endothelial cells. The role of CD31 in biology of lymphomas has not yet been systemically studied. Expression of cell surface CD31 was analyzed by flow cytometry on primary MCL cells isolated from peripheral blood, bone marrow or malignant effusions obtained from 29 newly diagnosed MCL patients. CD31 was significantly more expressed in patients with documented extranodal involvement. Knock-down of CD31 expression in JEKO1 and MINO MCL cell lines hampered their subcutaneous engraftment in immunodeficient mice and prolonged overall survival of intravenously-xenografted animals. In contrast, transgenic overexpression of CD31 accelerated growth of subcutaneous JEKO1 and MINO tumors, shortened overall survival of intravenously-xenografted mice, and resulted in significantly increased frequency of extramedullary murine tissue infiltration Our observations suggest that CD31 facilitate survival and regulate extranodal spread of MCL cells.
Introduction
Mantle cell lymphoma (MCL) is a B-cell neoplasm characterized by translocation t(11;14) leading to aberrant expression of cyclin D1, that accounts for approximately 2–10% of all non-Hodgkin lymphomas [Citation1,Citation2]. Although median overall survival increased by almost twofold within the past two decades, MCL is still considered an incurable, chronically-relapsing malignancy [Citation3,Citation4]. Therefore, studies of MCL pathogenesis unraveling potential new druggable targets are needed.
Besides tumor cell intrinsic molecular pathways, extrinsic signals from microenvironment are currently thought to contribute to MCL pathogenesis and disease spread. In vitro studies have shown that interactions of MCL cells with bone marrow stromal cells (BMSC) are mediated by chemokine receptors CXCR4 and CXCR5 and adhesion molecule VLA-4, and that these interactions increase survival and confer drug resistance of MCL cells [Citation5,Citation6].
Platelet/endothelial cell adhesion molecule 1 (PECAM-1, CD31) is an immunoglobulin superfamily member expressed on the surface of platelets, leukocytes and endothelial cells [Citation7]. CD31 is widely used as an endothelial marker in determining tumor microvascular density. It has been reported that high microvessel density has adverse prognostic impact in diffuse large B-cell lymphoma (DLBCL) [Citation8], and follicular lymphoma [Citation9]. CD31 expression, however, has been also detected on the surface of malignant lymphocytes [Citation10,Citation11]. The significance of this expression remains unknown. Several studies also reported that CD31/PECAM-1 functions as an inhibitor of mitochondrial apoptosis [Citation12,Citation13] and mediates anchorage-independent growth and anoikis resistance in cancer cells [Citation14]. We have demonstrated that engraftment and growth of MCL cells in immunodeficient mice is associated with upregulation of CD31 in vivo [Citation15].
In the current study we further analyzed the role of CD31 in engraftment, growth and spread of MCL.
Material AND methods
Cell culture
JEKO1 cell line was purchased from German Collection of Microorganisms and Cell Cultures (DSMZ), MINO was purchased from American Tissue Culture Collection (ATCC). Cell lines were cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 15% fetal bovine serum (FBS) and 1% penicillin/streptomycin. For downregulation experiments JEKO1 cells were infected with CD31/PECAM-1 Human GIPZ Lentiviral shRNA Particles (Thermo Fisher Scientific) at MOI (multiplicity of infection) 10 and the transduced cells were selected 2 days post-infection with the medium containing puromycin (4 µg/ml). MCL clones with stable upregulation of CD31 molecule were derived as follows: pUNO1-hPECAM1 (InvivoGen) was transfected into MCL cell lines. MCL clones were derived by blasticidin selection with subsequent single cell dilution. Level of CD31 expression was verified by flow cytometry.
In vivo experiments
The studies were approved by the institutional Animal Care and Use Committee. Female immunodeficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (Jackson Laboratory) were maintained in individually ventilated cages. JEKO1 and MINO control cells and cells with downregulated/upregulated expression of CD31/PECAM-1 were harvested, suspended in phosphate buffered saline (PBS), and injected (5 × 106/mouse) subcutaneously and intravenously into the left abdominal flank of 8- to 12-week-old mice. When tumors in SC-injected mice reached 2 cm in any largest diameter, the animals were euthanized and tumors excised and weighed. IV-injected animals were euthanized immediately after they developed signs of advanced lymphoma (hardship, terminal paralysis), and were carefully inspected in search for extranodal/extramedullary (E/E) involvement.
Proliferation assays
WST8-based Quick Cell Proliferation Assay Kit (BioVision) was used to determine the effect of CD31 down- and up-regulation on cell proliferation. Briefly, 20 000 cells were incubated for the indicated time periods (0–96 h) and absorbance of the samples analyzed after 2-hour incubation.
Flow cytometry (FCM)
Peripheral blood and bone marrow aspirates were acquired from patients with MCL after obtaining informed consent according to the Declaration of Helsinki. Specimens were collected into the test tubes containing EDTA K anticoagulant. The samples were washed in PBS and stained with antibodies for 15 min at room temperature in the dark. The cells were then lysed with FACS Lysing Solution (BD Biosciences) for 10 min and twice washed with PBS. Following fluorochrome-conjugated MoAbs were used: CD5 PerCP-Cy5.5 (clone L17F12, BD Biosciences), CD19 PE-Cy7 (clone J3-119, Beckman Coulter), CD31 FITC (clone WM59, BD Biosciences), CD45 Krome Orange (clone J33, Beckman Coulter). MCL cells were defined by cell-surface co-expression of CD19 and CD5 (CD19 + CD5+) (with one exception, where the MCL cells were CD5 negative, but there was confirmed translocation (11;14)). Samples were analyzed by a FACSCanto (Becton Dickinson, San Jose, CA, USA) or Navios (Beckman Coulter, Miami, FL). Flow cytometers were set according to the EuroFlow instrument setup Standard Operating Protocol [Citation16]. Instrument performance was monitored daily using eight-peak beads (Spherotech). Flow cytometry results were processed with Kaluza software, version 1.5 (Beckman Coulter). Isotype-matched negative controls were used in all the assays to distinguish positive from negative cells. Because of routine practice and to give a clear message to clinicians both the percentage of positive CD31 cells and median fluorescence intensity (MFI) of CD31 was used in this study. MFI of a positive or negative population of cells was calculated by the Kaluza software.
Statistical analysis
The data were analyzed using GraphPad Software. The values represent means ± standard deviations of at least three independent experiments. The differences between experimental groups were determined by unpaired t test.
Results
Expression of CD31 on the surface of CD19 + CD5+ MCL cells
We analyzed the expression of CD31 on the surface of CD19 + CD5+ MCL cells obtained from the bone marrow (BM) of 20 patients with newly diagnosed MCL. Patient characteristics are shown in . The expression of CD31 on the surface of CD19 + CD5+ MCL cells was detectable in all MCL BM samples, however, the percentage of CD31+ cells as well as the CD31 MFI were highly variable. The percentage of CD31+ cells (out of CD19 + CD5+ cells) ranged from 2.4% to 96.2% (median % = 57.25) and the CD31 MFI from 289 to 1497 (median MFI = 669.5, ). The percentage of CD31+ positive cells correlated with CD31 MFI (r = 0.8959, p < 0.0001, ). No significant correlation was found between the level of CD31 and MCL international prognostic index (MIPI, ). In 12 out of 20 patients, we analyzed also the expression of CD31 on the surface of CD19 + CD5+ MCL cells obtained from the peripheral blood. The percentage and MFI values were comparable between bone marrow and peripheral blood (data not shown).
Figure 1. Expression of CD31 on CD19+/CD5+ lymphocytes in BM obtained from patients with newly diagnosed MCL; (A) % of CD31 + CD19 + CD5+ cells, median % = 57.25; (B) MFI of CD31 + CD19 + CD5+ cells, median MFI = 669.5; (C) MFI correlated with the percentage of positive cells (r = 0.8959, p < 0.0001).
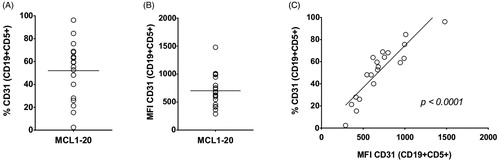
Table 1. Baseline characteristics of patients (MIPI = Mantle Cell Lymphoma International Prognostic Index, ND = not determined). BM infiltration and leukemisation was set by FCM.
Establishment of MCL clones with manipulated expression of CD31
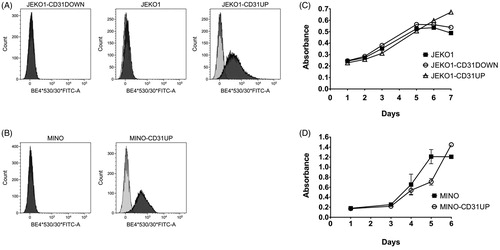
Recently, we have shown that CD31 is upregulated in vivo, upon engraftment of most of the tested MCL cell lines in immunodeficient mice [Citation15]. To further investigate the role of CD31 in the engraftment, growth and spread of MCL cells in vivo we used two MCL cell lines with low expression of CD31 (flow cytometry), JEKO1 and MINO, and derived their respective clones with transgenic (over)expression of CD31 (JEKO1-CD31UP, MINO-CD31UP) (). Because CD31 is upregulated in vivo upon engraftment in immunodeficient mice on JEKO1 cells, but not on MINO cells, we derived a JEKO1 clone with stable knock-down of CD31 expression (JEKO1-CD31DOWN) in order to shut down the in vivo-induced CD31 upregulation ().
CD31 impacts engraftment and growth of MCL cell lines in vivo
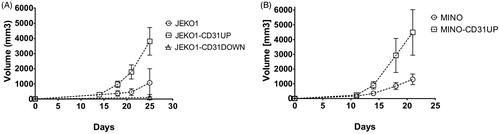
While in vitro proliferation rates were not significantly different between CD31-manipulated compared to the original cell lines (), we observed marked changes of the engraftment rates and SC growth in vivo. Xenotransplantation of JEKO1-CD31UP and MINO-CD31UP clonal cells resulted in accelerated tumor growth compared to controls (). Sustained overexpression of CD31 on the engrafted MCL cells was confirmed ex vivo by flow cytometry (Supplementary Figure 1). Disruption of CD31 upregulation in JEKO1-CD31DOWN clonal cells was associated with impaired engraftment and retarded growth of the corresponding MCL xenografts (). Engraftment was detected only in 2 out of 8 JEKO1-CD31DOWN-injected mice with the two resulting xenografts growing slower as compared to controls.
CD31 expression positively correlates with survival of systemically (IV)-xenografted mice
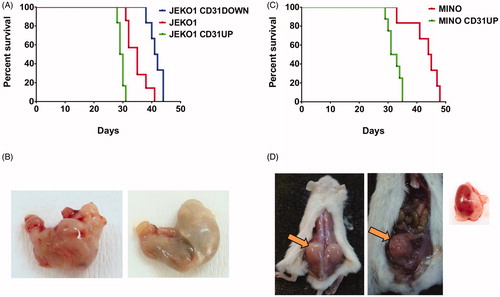
Survival of mice xenografted with MCL cells with transgenic overexpression of CD31 was significantly shorter compared to mice xenografted with the corresponding parental cell lines. Median survival of JEKO1-CD31UP compared to JEKO1-CD31DOWN-bearing mice reached 29.5 and 41.5 days, respectively (p = 0.0006, ). Median survival of MINO-CD31UP compared to MINO WT-bearing mice reached 32 and 44.5 days, respectively (p = 0.003, ). Moreover, mice xenografted with MCL cells with transgenic overexpression of CD31 demonstrated significantly increased frequency of infiltration of E/E tissues (e.g. muscles, kidneys, stomach, uterus and soft tissues) compared to mice xenografted with the corresponding controls. Five out of six mice (= 83.3%) IV-xenografted with JEKO1-CD31UP developed E/E lesions, but none of 6 IV-xenografted mice by JEKO1-CD31DOWN developed any lesions. Analogically, five out of eight (= 62.5%) MINO-CD31UP IV-xenografted mice developed E/E lesions. In contrast, only one out of eight mice (= 12.5%) IV-xenografted with parental MINO cells developed E/E lesions (). Infiltration with MCL cells was confirmed by immunohistochemistry.
Figure 4. Survival curves of mice after IV xenotransplantation of different MCL clones with manipulated CD31 expression (A, C) and examples of E/E involvement: (B) stomach infiltration (left) by JEKO1-CD31UP cells compared to normal stomach from JEKO1 IV (right), (D) examples of E/E involvement appearing after MINO-CD31UP IV xenotransplant from left to right: infiltration of soft tissues, ovarium and kidney.
Expression of CD31 is associated with E/E involvement in MCL patients
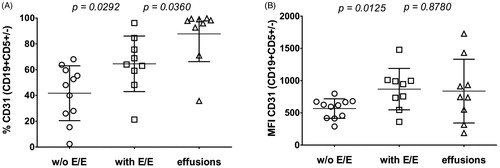
Based on our murine data we analyzed potential correlation of CD31 expression on MCL cells obtained from the bone marrow of patients with newly diagnosed MCL with E/E involvement. The level and percentage of CD31 expression on the surface of CD19 + CD5+ MCL cells were both significantly higher in patients with E/E involvement compared to patients with no E/E involvement as detected by CT or PET-CT (mean MFI 868.3 ± 107.0 and 566.7 ± 151.5, respectively (p = 0.0125), and percentage positivity 64.0% and 48.1% (p = 0.0292), respectively) (, ). In addition, we analyzed CD31 expression on MCL cells obtained from patients with malignant effusions (i.e. directly on MCL cells from an E/E compartment) and confirmed that percentage and level of CD31 expression were mostly comparable to MCL cells obtained from the bone marrow of patients with detectable E/E involvement (MFI 837.7 ± 164.8 compared to 868.3 ± 107.0, p = 0.88; percentage 97.9% compared to 64.0%, p = 0.036) ().
Discussion
Because we previously demonstrated that CD31 was upregulated upon engraftment of MCL cells in immunodeficient mice and because CD31 is involved in angiogenesis, we decided to study in more detail the role of CD31 in the engraftment, growth and spread of MCL.
We demonstrated that expression of CD31 on MCL cells is highly variable and positively correlates with the extent of E/E involvement. Using a panel of murine models of MCL we showed that CD31 positively regulates engraftment, growth and spread of MCL cells in vivo. CD31 overexpressing MCL clones were more biologically aggressive in vivo compared to the parental MCL cell lines including higher numbers of E/E involvement and shorter overall survival of IV-xenografted animals. Interestingly, the highest levels of CD31 expression were observed on MCL cells obtained from malignant effusions. Therefore, it strongly suggests that higher expression of CD31 facilitates spread, engraftment and/or survival of MCL cells in the E/E tissues. In the context of the observed results obtained exclusively from patients with a nodal, SOX11-positive MCL disease it would be interesting to analyze expression of CD31 also on non-nodal, SOX11-negative MCL. In the current study such an analysis was not implemented due to lack of SOX11-negative MCL samples.
Precise molecular mechanisms by which CD31 upregulation regulates the observed shift in the biological behavior of MCL cells remain elusive. However, because no measurable effects of CD31 upregulation was observed in vitro, we propose that in vivo microenvironmental factors are prerequisite for triggering the CD31-mediated phenotype changes. Such microenvironmental factors might include hypoxia-induced changes or cell-cell contacts, e.g. via CD38, a known ligand of CD31 and another key regulator of lymphoma cell biology.
The excess of CD31 on acute myeloid leukemia (AML) cells was associated with a higher peripheral white blood cell count due to higher transendothelial migration of AML cells from the bone marrow [Citation17]. Similarly, expression of CD31 enhanced migration of acute lymphoblastic leukemia (ALL) cells through brain microvascular endothelial cells [Citation10]. Moreover, it was recently reported that upregulation of VEGF in ALL is associated with increased frequency of CNS involvement [Citation18]. As CD31 is a critical regulator of angiogenesis, it could be hypothesized that the molecular pathways responsible for the increased E/E involvement (observed in our study) and for the enhanced CNS involvement (observed in the study by Münch et al.) might be at least partially shared.
In conclusion CD31 is an important regulator of engraftment, growth and spread of MCL cells in vivo. Level of expression of CD31 positively correlates with the number of E/E sites infiltrated by lymphoma cells in lymphoma-bearing mice as well as in MCL patients, which makes CD31 a potential druggable target.
ilal_a_1849678_sm4857.jpg
Download JPEG Image (173.9 KB)Disclosure statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Funding
References
- Dreyling M, European Mantle Cell Lymphoma N. Mantle cell lymphoma: biology, clinical presentation, and therapeutic approaches. Am Soc Clin Oncol Educ Book. 2014;(34):191–198.
- Smedby KE, Hjalgrim H. Epidemiology and etiology of mantle cell lymphoma and other non-Hodgkin lymphoma subtypes. Semin Cancer Biol. 2011;21(5):293–298.
- Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27(4):511–518.
- Martin P, Chadburn A, Christos P, et al. Intensive treatment strategies may not provide superior outcomes in mantle cell lymphoma: overall survival exceeding 7 years with standard therapies. Ann Oncol. 2008;19(7):1327–1330.
- Kurtova AV, Tamayo AT, Ford RJ, et al. Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): importance for interactions with the stromal microenvironment and specific targeting. Blood. 2009;113(19):4604–4613.
- Medina DJ, Goodell L, Glod J, et al. Mesenchymal stromal cells protect mantle cell lymphoma cells from spontaneous and drug-induced apoptosis through secretion of B-cell activating factor and activation of the canonical and non-canonical nuclear factor κB pathways. Haematologica. 2012;97(8):1255–1263.
- Jackson DE. The unfolding tale of PECAM-1. FEBS Lett. 2003; 540(1-3):7–14.
- Cardesa-Salzmann TM, Colomo L, Gutierrez G, et al. High microvessel density determines a poor outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus chemotherapy. Haematologica. 2011;96(7):996–1001.
- Taskinen M, Jantunen E, Kosma V-M, et al. Prognostic impact of CD31-positive microvessel density in follicular lymphoma patients treated with immunochemotherapy. Eur J Cancer. 2010;46(13):2506–2512.
- Akers SM, O'Leary HA, Minnear FL, et al. VE-cadherin and PECAM-1 enhance ALL migration across brain microvascular endothelial cell monolayers. Exp Hematol. 2010;38(9):733–743.
- Boyd RS, Jukes-Jones R, Walewska R, et al. Protein profiling of plasma membranes defines aberrant signaling pathways in mantle cell lymphoma. Mol Cell Proteomics. 2009;8(7):1501–1515.
- Gao C, Sun W, Christofidou-Solomidou M, et al. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102(1):169–179.
- Poggi A, Prevosto C, Catellani S, et al. Engagement of CD31 delivers an activating signal that contributes to the survival of chronic lymphocytic leukaemia cells. Br J Haematol. 2010;151(3):252–264.
- Zhang X, Xu L-h, Yu Q. Cell aggregation induces phosphorylation of PECAM-1 and Pyk2 and promotes tumor cell anchorage-independent growth. Mol Cancer. 2010;9(1):7.
- Molinsky J, Klanova M, Maswabi B, et al. In vivo growth of mantle cell lymphoma xenografts in immunodeficient mice is positively regulated by VEGF and associated with significant up-regulation of CD31/PECAM1. Folia Biol . 2013;59(1):26–31.
- Kalina T, Flores-Montero J, van der Velden VH, et al.; EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26(9):1986–2010.
- Gallay N, Anani L, Lopez A, et al. The Role of Platelet/Endothelial Cell Adhesion Molecule–1 (CD31) and CD38 Antigens in Marrow Microenvironmental Retention of Acute Myelogenous Leukemia Cells. Cancer Res. 2007;67(18):8624–8632.
- Munch V, Trentin L, Herzig J, et al. Central nervous system involvement in acute lymphoblastic leukemia is mediated by vascular endothelial growth factor. Blood. 2017;130(5):643–654.