Abstract
Idecabtagene vicleucel (ide-cel, bb2121), a chimeric antigen receptor (CAR) T cell therapy, has been investigated in patients with relapsed and refractory multiple myeloma (RRMM) who have received an immunomodulatory drug, proteasome inhibitor, and anti-CD38 antibody in the single-arm phase 2 KarMMa clinical trial. Two therapies with distinct mechanisms of action – selinexor plus dexamethasone (Sd) and belantamab mafodotin (BM) – are currently approved in the United States for heavily pretreated patients, including those who are triple-class refractory. To compare ide-cel versus Sd and ide-cel versus BM, matching-adjusted indirect comparisons were performed. Ide-cel extended progression-free survival (PFS) and overall survival (OS) versus both Sd and BM (hazard ratio (HR); 95% confidence interval (CI)). PFS: ide-cel versus Sd, 0.46; 0.28–0.75; ide-cel versus BM, 0.45; 0.27–0.77. OS: ide-cel versus Sd, 0.23; 0.13–0.42; ide-cel versus BM, 0.35; 0.14–0.87. These results suggest ide-cel offers clinically meaningful improvements over currently approved regimens for patients with heavily pretreated RRMM.
Introduction
The clinical outcomes of multiple myeloma (MM) have been transformed over the last decade with the introduction of three main classes of therapy, including immunomodulatory drugs (such as the IMiD® agents lenalidomide and pomalidomide), proteasome inhibitors (PIs) and, most recently, anti-CD38 monoclonal antibodies (mAbs). Drugs from all three classes are approved and recommended for use in patients with newly diagnosed MM or relapsed/refractory MM (RRMM), preferably in 2-, 3-, or 4-agent regimens [Citation1,Citation2]. Despite the longer survival time achieved with these novel treatments, the majority of patients will ultimately relapse and require new lines of therapy.
The choice of regimen following relapse is influenced by response and tolerance to prior therapies, as well as by disease and patient characteristics. For refractory patients (defined as failure to respond to primary or salvage therapy, or progression within 60 days of last therapy per International Myeloma Working Group (IMWG) criteria [Citation3]), the recommendation is to switch to a new regimen, preferably one with a novel mechanism of action [Citation1,Citation2]. However, triple-class refractory patients have poor outcomes, with a median overall survival (OS) of 9 months [Citation4]. Moreover, there is no consensus regarding the standard-of-care treatment for triple-class exposed patients [Citation1]. Following treatment with an anti-CD38 mAb, most patients (90%) will receive further therapy, although the regimens vary substantially and include daratumumab-containing regimens, elotuzumab with an immunomodulatory drug, carfilzomib-containing regimens, and various chemotherapies [Citation5]. Overall response rate (ORR) is just over 30% in these patients, and the median progression-free survival (PFS) and OS are 3.4 and 9.3 months, respectively [Citation5]. As 3- and 4-agent regimens are increasingly used in first- and second-line settings, new treatment classes are required to improve outcomes for patients in later lines of therapy.
This unmet need among triple-class exposed and refractory patients has driven the development of new therapies with novel mechanisms of action. A number of these novel agents target the B-cell maturation antigen (BCMA), a protein that is overexpressed on the surface of MM cells. Three common treatment modalities used to target BCMA are bispecific antibody constructs, antibody–drug conjugates, and chimeric antigen receptor (CAR)-modified T cell therapy [Citation6]. In CAR T cell therapy, antigen-specific T cells are generated by the introduction of genes encoding CARs [Citation7]. These CARs recognize intact cell-surface proteins and glycolipids, allowing T cells to function in a human leukocyte antigen-independent manner [Citation7]. One such CAR T cell therapy is idecabtagene vicleucel (ide-cel, bb2121), a CAR T cell therapy under investigation for the treatment of RRMM in the phase 2 KarMMa clinical trial (NCT03361748) [Citation8]. Ide-cel, therefore, has a distinct mechanism of action compared with approved agents for earlier RRMM, providing a novel therapy for triple-class exposed patients. The clinical activity of ide-cel in heavily pretreated patients has been demonstrated in a phase 2 study, where 73% of patients achieved an ORR, and median PFS was 8.8 months [Citation8].
Two therapies have recently been approved by the US Food and Drug Administration (FDA) for use in heavily pretreated patients, including those with triple-class exposed/refractory RRMM. Selinexor, a selective inhibitor of nuclear export (SINE), was approved in combination with dexamethasone (Sd) based on the results of a single-arm phase 2 study [Citation9], and belantamab mafodotin (BM), an anti-BCMA immunoconjugate, was approved based on a phase 2 study comparing two dosing regimens [Citation10]. In the absence of head-to-head studies comparing therapies for triple-class exposed patients [Citation11], we performed matching-adjusted indirect treatment comparisons (MAICs) to compare ide-cel versus Sd and BM, respectively.
Methods
Evidence for ide-cel
Individual patient-level data (IPD) regarding the efficacy of ide-cel were available from the phase 2 KarMMa clinical trial (NCT03361748). This is an ongoing, multicenter, single-arm clinical trial to evaluate the efficacy and safety of ide-cel in patients with RRMM who had received ≥3 prior regimens, were triple-class exposed (i.e. to an immunomodulatory drug, PI, and an anti-CD38 mAb) and refractory to their last regimen [Citation8]. Of the 140 patients enrolled, 128 received an ide-cel infusion at a target dose of 150 × 106 (n = 4), 300 × 106 (n = 70), or 450 × 106 (n = 54) CAR + T cells. Based on the dose–response, the 450 × 106 dose reflects the target dose, although the dose used in this study ranged from 150 to 450 × 106 cells. As of the 14 January 2020 data cutoff, the median duration of follow-up after ide-cel infusion was 13.3 months (range: 0.2–21.2), with 82 (64.1%) patients having been followed for ≥12 months from infusion [Citation8].
Evidence for Sd and BM
Study-level publications regarding the efficacy of Sd and BM were identified through a systematic literature review (SLR) of real-world studies and clinical trials evaluating treatments for triple-class exposed RRMM patients [Citation11]. To be inclusive, any studies that evaluated patients exposed to an anti-CD38 mAb were included, even if this exposure was not a requirement of the eligibility criteria. Four single-arm, multicenter trials evaluating Sd and BM were identified: STORM part 1 (phase 2) [Citation12] and STORM part 2 (phase 2) [Citation9] evaluated Sd, and DREAMM-1 (phase 1) [Citation13] and DREAMM-2 (phase 2) [Citation10] assessed BM. Of these studies, only the phase 2 studies, STORM part 2 and DREAMM-2, were included in the indirect comparisons versus Sd and BM, respectively, as patients in these studies represented the patient population in the KarMMa clinical trial, especially in terms of exposure and refractoriness to anti-CD38 mAbs. For each study, data on patient characteristics and outcomes were extracted. For PFS and OS, the IPD were reconstructed by digitizing the Kaplan–Meier (KM) curves using the Guyot algorithm [Citation14].
STORM part 1 evaluated patients exposed to ≥2 immunomodulatory drugs and two PIs, where only 39% of patients had received an anti-CD38 mAb [Citation12]. In contrast, STORM part 2 included patients with exposure to bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, glucocorticoids, and an alkylating agent; and required that patients have been refractory to ≥1 agent in each of the following classes: an immunomodulatory drug, a PI, and an anti-CD38 mAb. Patients (n = 123) received selinexor (80 mg) plus dexamethasone (20 mg) twice weekly until disease progression, death, or discontinuation. At the last date of follow-up, five patients (4%) continued to receive treatment, whereas 34 (28%) had discontinued treatment and remained in follow-up for long-term survival [Citation9].
DREAMM-1 evaluated patients who had previously received an alkylator, a PI, an immunomodulatory drug, and a prior stem cell transplantation (if eligible), and were refractory to the last line of treatment [Citation13]. However, only a third of patients (31.5%) had received an anti-CD38 mAb. In comparison, DREAMM-2 included patients with disease progression on or after receiving three or more previous lines of antimyeloma treatment and were refractory to an immunomodulatory drug or a PI, and were refractory or intolerant (or both) to an anti-CD38 mAb [Citation10]. Patients were randomized to receive intravenous BM 2.5 mg/kg (n = 97) or 3.4 mg/kg (n = 99) every 21 days until disease progression or unacceptable toxicity. The 2.5 mg/kg group reflects the dose that has been approved based on a median follow-up of 6.3 months [Citation10].
An overview of study design and baseline patient characteristics for KarMMa, STORM part 2, and DREAMM-2 is presented in and in Supplementary Table 1.
Table 1. Patient characteristics at baseline in KarMMa, STORM part 2, and DREAMM-2 trials.
Matching-adjusted indirect comparisons
In the absence of a randomized trial comparing ide-cel with other therapies, pairwise unanchored indirect comparisons were performed to estimate the relative treatment effects of ide-cel versus Sd, and ide-cel versus BM, based on the three independent phase 2 non-randomized single-arm studies [Citation8–10]. Between-study differences in patient characteristics that may have influenced the outcomes (and consequently the treatment effects) were adjusted using MAIC [Citation15–18] to reduce the bias in the treatment effect estimates inherent in a naïve indirect comparison. This type of indirect treatment comparison (ITC) uses IPD from the index trial, such as KarMMa, to up (or down) weight patients most similar (or dissimilar) to those in an external study based on the published patient characteristics.
A logistic propensity score model was used to estimate weights for the individual patients from KarMMa (ide-cel) so that the weighted mean baseline characteristics matched those observed in the STORM part 2 (Sd) and DREAMM-2 (BM) publications, respectively. These weights were applied to ide-cel to predict the observed outcomes in each respective population using either a weighted logistic (for ORR) or a weighted Cox (for PFS and OS) regression. Robust estimates of the variance associated with the comparative treatment effects were calculated using sandwich estimators. Treatment effects were expressed in terms of odds ratios (ORs) or hazard ratios (HRs) of ide-cel versus the comparator along with their 95% confidence intervals (CIs). For each outcome of interest, a model without individual weights provided a naïve estimate of the treatment effect of ide-cel versus each comparator. A weighted MAIC model then provided an estimate of the treatment effect for ide-cel versus Sd or BM, which would have been observed in a population similar to the STORM part 2 or DREAMM-2 populations, respectively. All analyses were performed using R version 3.5.1 (Vienna, Austria) (http://www.r-project.org/).
Within the KarMMa trial, three target doses were evaluated. The base case considered all ide-cel-treated patients (n = 128) and sensitivity analyses considered (1) the overall, enrolled population (n = 140) and (2) patients who received the target dose of 450 × 106 CAR + T cells (n = 54). For STORM part 2, the modified intention-to-treat (mITT) population was used for the analysis, which included all eligible patients who received ≥1 dose of Sd (as reported on in the primary publication [Citation9]). DREAMM-2 outcomes were based on the mITT populations, comprising all randomized patients regardless of treatment administration, and focused on the 2.5 mg/kg approved dose. For each population/target dose, weights from KarMMa were estimated to match the patient characteristics reported in STORM part 2 and DREAMM-2.
Selection of patient characteristics for propensity model
Prognostic factors for inclusion in the MAIC were driven by a literature review of previous ITC studies in RRMM [Citation19,Citation20] and recommendations from clinical experts. The most relevant prognostic factors that differed between the studies were included as covariates in the propensity model: (1) median number of prior treatments; (2) median years from initial diagnosis; (3) the proportion of patients refractory to bortezomib, carfilzomib, lenalidomide, or pomalidomide (considered for each agent individually in DREAMM-2 and combined for STORM part 2); (4) the proportion of patients in stage II or lower (according to the International Staging System (ISS) for DREAMM-2 and revised ISS as reported in STORM part 2); (5) the proportion of patients with high-risk cytogenetics; and (6) the proportion of patients with extramedullary disease (DREAMM-2 only). Any missing covariates from KarMMa were imputed based on the mean covariate value for the included patients from the relevant population/dose in this study.
Results
Matching
After matching, baseline characteristics were well balanced between the ide-cel populations/target doses from KarMMa, the STORM part 2 population, and the DREAMM-2 2.5 mg/kg patient group (Supplementary Table 2).
The effective sample size (ESS) adjusts the sample size based on the weighting of the observations to reflect the extent of overlap in patient baseline characteristics between the study populations included in the MAIC [Citation21]. For the MAIC versus Sd, the ESS was reduced by 56% (ESS, n = 56) for the ide-cel-treated population (base case) and 55% (ESS, n = 64) and 82% (ESS, n = 10) for the overall and target-dose populations, respectively. For the MAIC comparison versus BM 2.5 mg/kg, the ESS was reduced by 65% (ESS, n = 45) in the ide-cel treated population, 58% (ESS, n = 59) in the overall population, and 78% (ESS, n = 12) in the target-dose population, respectively.
Overall response rate
Ide-cel was more efficacious than both Sd and BM 2.5 mg/kg whether the analysis was based on the naïve or adjusted comparison using MAIC (). The unadjusted ORR for the ide-cel treated population was 73.4% compared with 26.6% for Sd and 31.6% for BM 2.5 mg/kg (). Thus, based on the naïve comparison, ide-cel was associated with a 7.8-fold improvement in ORR compared with Sd and a 6.2-fold improvement in ORR compared with BM 2.5 mg/kg. Ide-cel estimates changed little in weighted MAIC with a slight reduction in the OR versus Sd and BM 2.5 mg/kg, where both remained statistically significant (Sd: OR 7.74; 95% CI 3.83–15.62; BM 2.5 mg/kg: OR 5.12; 95% CI 2.35–11.13) (, ). Compared with the ide-cel-treated population, the overall population showed slightly less improvement for ide-cel versus Sd and BM 2.5 mg/kg, whereas results for the ide-cel 450 × 106 CAR + T cell target-dose population demonstrated greater improvement for ide-cel versus Sd and BM 2.5 mg/kg, respectively (). In the target-dose group, ide-cel was associated with a 14-fold improvement in ORR versus Sd (OR 13.97; 95% CI 3.09–63.23) () and 12.6-fold improvement versus BM 2.5 mg/kg (OR 12.62; 95% CI 3.69–43.10) based on the MAIC ().
Figure 1. ORR for the ide-cel treated population (adjusted) versus Sd and BM 2.5 mg/kg. BM: belantamab mafodotin; CI: confidence interval; ide-cel: idecabtagene vicleucel; MAIC: matching-adjusted indirect treatment comparison; OR: odds ratio; ORR: overall response rate; Sd: selinexor plus dexamethasone.
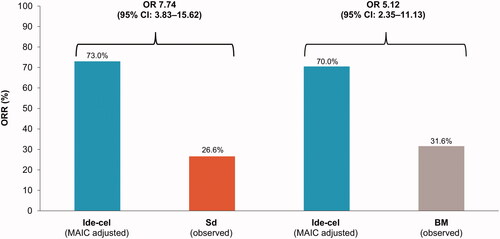
Figure 2. OR of ORR for (A) ide-cel versus Sd and (B) ide-cel versus BM 2.5 mg/kg for the base case and sensitivity analyses. BM: belantamab mafodotin; CI: confidence interval; ide-cel: idecabtagene vicleucel; MAIC: matching-adjusted indirect treatment comparison; OR: odds ratio; ORR: overall response rate; Sd: selinexor plus dexamethasone.
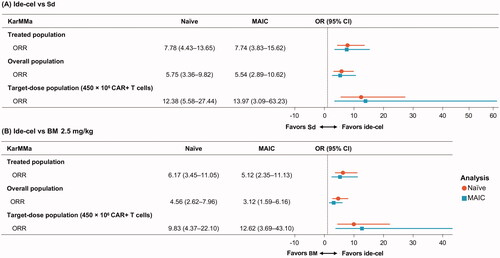
Table 2. Summary of the results for naïve and MAIC comparisons of ide-cel treated population versus Sd and versus BM, 2.5 mg/kg.
Progression-free survival and overall survival
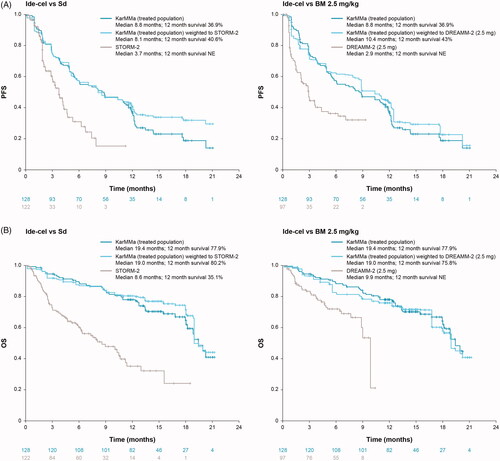
The KM plots for PFS and OS showed minor differences between the observed data and estimates adjusted based on the MAIC for the ide-cel-treated population (). In the observed ide-cel treated population, median PFS was 8.8 months and was estimated to be 8.1 months when matched to the Sd population (median 3.7 months), and 10.4 months when matched to the BM 2.5 mg/kg population (median 2.9 months) (). Although OS data are not mature enough to permit a definitive analysis, median survival was 19.4 months for ide-cel, whereas it was estimated to be 19.0 months when matched to both the Sd (median 8.6 months) or BM 2.5 mg/kg populations (median 9.9 months) ().
Figure 3. (A) PFS and (B) OS KM plots for ide-cel treated population (observed and adjusted) versus Sd and BM 2.5 mg/kg. BM: belantamab mafodotin; ide-cel: idecabtagene vicleucel; KM: Kaplan–Meier; NE: not estimated; OS: overall survival; PFS: progression-free survival; Sd: selinexor plus dexamethasone.
In terms of PFS, ide-cel was more efficacious than both Sd (HR 0.46; 95% CI 0.28–0.75) and BM 2.5 mg/kg (HR 0.45; 95% CI 0.27–0.77) based on the adjusted estimates. This finding was consistent across the populations and target dose group based on both the naïve and adjusted estimates (). With regard to other KarMMa patient groups, ide-cel was more beneficial regardless of the analytical approach (naïve or adjusted) or patient population analyzed (treated patients or target dose subgroup).
Figure 4. Hazard ratios of PFS and OS for (A) ide-cel versus Sd and (B) ide-cel versus BM 2.5 mg/kg for base case and sensitivity analyses. BM: belantamab mafodotin; CI: confidence interval; HR: hazard ratio; ide-cel: idecabtagene vicleucel; MAIC: matching-adjusted indirect comparison; OS: overall survival; PFS: progression-free survival; Sd: selinexor plus dexamethasone.
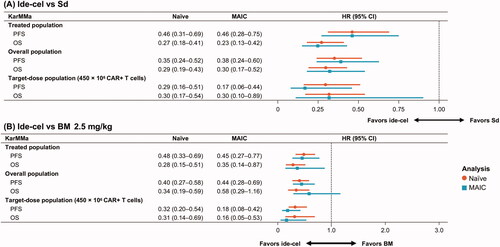
With respect to OS, ide-cel was also associated with statistically significant improvements versus Sd (HR 0.27; 95% CI 0.13–0.42) and BM 2.5 mg/kg (HR 0.35; 95% CI 0.14–0.87) based on the adjusted comparisons. OS was also improved for ide-cel versus Sd or BM based on the naïve estimate, as well as for the naïve and adjusted estimates for the overall and target-dose groups (). For the comparison versus BM 2.5 mg/kg, ide-cel was associated with slightly longer extension in the target-dose group compared with the overall population with both being statistically significant, whereas the survival benefits were slightly less in the overall population, where only the naïve estimate was statistically significant.
Discussion
The findings from this MAIC suggest that ide-cel offers statistically significant and clinically meaningful improvements in ORR, PFS, and OS compared with both Sd and BM 2.5 mg/kg. In the adjusted comparisons, ide-cel was associated with a 7.8-fold increase in ORR versus Sd and 5-fold increase in ORR versus BM 2.5 mg/kg, which represents significant improvements that are likely to be clinically meaningful for patients. For all outcomes, adjusted comparisons for both the overall population and the target-dose group remained significantly in favor of ide-cel, except for OS for ide-cel versus BM 2.5 mg/kg in the overall ide-cel population where the CIs were wide and included the null effect. Results for the naïve comparisons were largely similar to those for the adjusted comparisons, but were more uncertain.
Unanchored ITCs aim to adjust for baseline effect modifiers and prognostic variables that may influence efficacy outcomes in order to provide a robust comparison of treatments across different studies [Citation15,Citation16]. The variables included in the propensity score analyses in these MAICs were based on a review of published literature and clinical expert opinion. Thus, they were as relevant as possible for the clinical outcomes given the patient population. A total of nine covariates were used, which is more than have been used in many previous MAICs, as identified by Phillippo et al. [Citation21]. A sensitivity analysis including additional covariates, such as autologous stem cell transplantation, sex, Eastern Cooperative Oncology Group performance status, age, and renal insufficiency, did not affect the results substantially.
The choice and number of covariates involves a tradeoff between including more covariates to achieve better matching and a greater reduction in sample size, with the latter meaning that the results are based on a small patient population that is less representative of the study as a whole. In these MAICs, the sample size reduction was 56% for the comparison versus Sd (i.e. from n = 128 to an ESS of n = 56) and 65% (from n = 128 to an ESS of n = 45) for the comparison versus BM 2.5 mg/kg in the ide-cel treated population. These are similar to the ESS and percentage reduction reported for other recent MAICs in oncology [Citation21]. However, the ESS for the ide-cel target-dose population in the two comparisons was 10 (Sd) and 12 (BM 2.5 mg/kg) patients, corresponding to reductions of 82% and 78%, respectively. These larger percent reductions in ESS reflect the fact that the target dose ide-cel population was less refractory to prior therapies than the treated or overall ide-cel populations, and thus overlapped less with the patient populations included in STORM part 2 and DREAMM-2. For example, while the proportion of patients refractory to bortezomib, carfilzomib, lenalidomide, and pomalidomide was 68% in STORM part 2 and 26% in the treated and overall ide-cel populations, it was only 15% in the ide-cel target-dose population. Similarly, while the proportion of bortezomib-refractory patients was 76% in DREAMM-2 and 61% in the treated and overall ide-cel populations, it was only 44% in the ide-cel target-dose population. These differences in combination with the small sample size limit interpretation of the results for the target-dose population.
These analyses have several limitations, many of which are common to all MAICs. In the absence of IPD for all studies, it is challenging to quantify the extent of residual bias in the treatment effect estimates and it is likely that some confounding variables remain unbalanced. These include the fact that some prognostic factors and/or effect modifiers may not have been adjusted for in the analysis because they were not reported. For example, of the prognostic factors identified in the published ITCs, the following characteristics could not be included as they were not reported in either DREAMM-2 or STORM part 2: β2 microglobulin level (identified in six previous ITCs), prior exposure to individual treatments (three previous ITCs), race (three previous ITCs), and immunoglobulin class heavy chain (two previous ITCs). Only published data were available for STORM part 2 and DREAMM-2. Thus, although an established algorithm was used to reconstruct IPD from these studies based on the KM curves for time-to-event outcomes, there were some minor discrepancies in the numbers at risk over time as a result of the timing of reconstructed censored observations.
Another drawback of the current study is the limited follow-up available in the three clinical trials, in particular with respect to DREAMM-2, which had only 6.3 months in median follow-up based on their initial publication. Longer term follow-up was recently published (January 2020) for DREAMM-2 [Citation22], which reported a median OS of 14.9 months (95% CI 9.9–not evaluable) for BM 2.5 mg/kg. Although this was published after the completion of the SLR and analyses, updated estimates suggest that the interpretation of the findings was consistent with the original analysis, both in terms of the direction of the effects and the statistical significance of the naïve and weighted estimates. Therefore, despite longer follow-up from DREAMM-2, ide-cel continued to show superiority to BM 2.5 mg/kg both in the overall population and the target-dose population. Given the immaturity of the results from all three clinical trials, it will be important to update the current analyses as more follow-up becomes available from the trials in order to better understand the long-term survival estimates.
In conclusion, the results from the MAICs reported here suggest that ide-cel may represent an important new treatment option for patients with RRMM who have been exposed to an immunomodulatory drug, a PI, and an anti-CD38 mAb. Efficacy estimates for ide-cel show a statistically significant and clinically meaningful benefit over those reported for Sd and BM 2.5 mg/kg, currently, the only therapies approved for heavily pretreated patients, which includes those with triple-class exposed disease. A recently reported quality-of-life analysis further supports the efficacy outcomes and demonstrated that ide-cel provides meaningful improvements in measures of both global function and symptoms related to disease [Citation23]. Longer follow-up is required to evaluate an OS benefit.
Author contributions
All authors contributed toward the study design, data analysis and interpretation, and preparation of the manuscript; approved the final submitted version; and met all ICMJE criteria.
GLAL-2020-1550-File008.docx
Download MS Word (24 KB)Disclosure statement
Paula Rodriguez-Otero has served on advisory boards for AbbVie, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Kite, Oncopeptides, Sanofi, and Takeda, and has received honoraria from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Kite, and Sanofi. Faith E. Davies has received honoraria from, and has served on advisory boards for Adaptive, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Oncopeptides, Roche, Sanofi, and Takeda, and has received research funding from Bristol Myers Squibb and Janssen. Michel Delforge has received research funding from Amgen, Bristol Myers Squibb, and Janssen, and has served as a consultant for Amgen, Bristol Myers Squibb, Janssen, Sanofi, and Takeda. Katja Weisel has received honoraria from, and has served on advisory board for AbbVie, Adaptive Biotech, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Karyopharm, Oncopeptides, Roche, Sanofi, and Takeda, has received research funding (institution) from Amgen, Bristol Myers Squibb, Janssen, and Sanofi, and has received non-financial support from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Sanofi, and Takeda. Kristen Hege is an employee of Bristol Myers Squibb. Sujith Dhanasiri is an employee and has equity in Bristol Myers Squibb. Dieter Ayers, Shannon Cope, Ali Mojebi, and Jeroen P. Jansen are employees of PRECISIONheor, which received funding from Bristol Myers Squibb to conduct this study.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl. 4):iv52–iv61.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V.3.2021. © National Comprehensive Cancer Network, Inc.; 2020; [cited 2020 Nov 16]. Available from: NCCN.org
- Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–4695.
- Gandhi UH, Lakshman A, Gahvari Z, et al. Natural history of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody-based treatment. Blood. 2018;132(Suppl. 1):3233.
- Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266–2275.
- Shah N, Chari A, Scott E, et al. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34(4):985–1005.
- Friedman KM, Garrett TE, Evans JW, et al. Effective targeting of multiple B-cell maturation antigen-expressing hematological malignances by anti-B-cell maturation antigen chimeric antigen receptor T cells. Hum Gene Ther. 2018;29(5):585–601.
- Munshi NC, Anderson LD Jr., Shah N, et al. Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): initial KarMMa results. J Clin Oncol. 2020;38(Suppl. 15):8503.
- Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727–738.
- Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207–221.
- Davies F, Rodriguez-Otero P, Weisel K, et al. A systematic literature review to assess efficacy of treatments in triple-class exposed relapsed and refractory multiple myeloma patients. HemaSphere. 2020;4(Suppl. 1):EP1033.
- Vogl DT, Dingli D, Cornell RF, et al. Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(9):859–866.
- Trudel S, Lendvai N, Popat R, et al. Targeting B-cell maturation antigen with GSK2857916 antibody–drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19(12):1641–1653.
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9.
- Phillippo DM, Ades AE, Dias S, et al. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submission to NICE; 2016; [cited 2020 Nov 20]. Available from: http://www.nicedsu.org.uk
- Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–211.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947.
- Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–945.
- Cope S, Toor K, Popoff E, et al. Critical appraisal of published indirect comparisons and network meta-analyses of competing interventions for multiple myeloma. Value Health. 2020;23(4):441–450.
- Jagannath S, Lin Y, Goldschmidt H, et al. KarMMa-RW: a study of real-world treatment patterns in heavily pretreated patients with relapsed and refractory multiple myeloma (RRMM) and comparison of outcomes to KarMMa. J Clin Oncol. 2020;38(Suppl. 15):8525.
- Phillippo DM, Dias S, Elsada A, et al. Population adjustment methods for indirect comparisons: a review of national institute for health and care excellence technology appraisals. Int J Technol Assess Health Care. 2019;35(3):221–228.
- Lonial S, Lee HC, Badros A, et al. Pivotal DREAMM-2 study: single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) refractory to proteasome inhibitors (PIs), immunomodulatory agents, and refractory and/or intolerant to anti-CD38 monoclonal antibodies (mAbs). J Clin Oncol. 2020;38(Suppl. 15):8536.
- Delforge M, San Miguel J, Bertin KB, et al. Quality of life in patients with relapsed and refractory multiple myeloma treated with the BCMA-targeted CAR T cell therapy idecabtagene vicleucel (ide-cel; bb2121): results from the KarMMa trial. HemaSphere. 2020;4(Suppl. 1):EP1000.
