Abstract
Patients with mycosis fungoides (MF) and Sézary syndrome (SS) often require multiple lines of systemic therapy. In the phase 3 MAVORIC study (NCT01728805), mogamulizumab demonstrated superiority to vorinostat in median progression-free survival (PFS) and confirmed overall response rate (ORR) in patients with MF/SS. This post hoc analysis examined the effects of number and type of prior systemic therapies on mogamulizumab response. MAVORIC patients randomized to mogamulizumab (1.0 mg/kg intravenously weekly) or vorinostat (400 mg orally daily) were grouped by number of prior therapies and immunomodulatory activity of immediate prior systemic therapy while also considering time elapsed since treatment. ORR, PFS, and duration of response (DOR) did not vary with number of prior therapies. ORR and DOR remained consistent regardless of immediate prior therapy type. Additionally, immunomodulatory activity of the last prior therapy and time from prior treatment generally did not affect the ORR or PFS observed in response to mogamulizumab.
Introduction
Cutaneous T-cell lymphomas (CTCL) are a rare class of non-Hodgkin lymphomas associated with significant morbidity and decreased quality of life from pain, itching, and disfigurement [Citation1–3]. The overall incidence of CTCL in the United States in 2011–2012 was 0.4 per 100,000 persons, with an estimated 1690 new cases diagnosed in 2016 [Citation4]. Mycosis fungoides (MF), the most common CTCL subtype, is frequently characterized by an indolent clinical course; Sézary syndrome (SS) is a rarer, more aggressive leukemic CTCL subtype [Citation5]. Together, MF and SS account for approximately 65% of CTCL cases [Citation6].
Less advanced stages of MF, which are often indolent, are initially treated with skin-directed therapy, while systemic therapies are used as later-line therapy for patients who prove refractory to skin-directed therapies and as primary treatment for patients with more advanced skin disease and/or systemic disease [Citation7–9]. Advanced CTCL has a poor prognosis and often a median survival <63 months [Citation10]. Systemic therapies such as retinoids (e.g. bexarotene, acitretin), histone deacetylase (HDAC) inhibitors (vorinostat, romidepsin), methotrexate, extracorporeal photopheresis (ECP), brentuximab vedotin, and interferons are often chosen over more conventional chemotherapy because of their less severe immunosuppression and lower rates of cumulative toxicities limiting duration of therapy, with multi-agent chemotherapy often reserved for patients whose disease has proved refractory to multiple prior therapies [Citation8,Citation11]. Systemic therapies represent a wide range of mechanistic approaches. Therapies with immunostimulatory mechanisms include interferon, whose proposed mechanisms in MF and SS include enhancement of T- and natural killer (NK)-cell cytotoxicity and inhibition of regulatory T-cell activity, and ECP, in which leukocytes are collected from whole blood, irradiated, and then reinfused into the patient, resulting in apoptosis of NK and T cells [Citation12,Citation13]. Lenalidomide, which has been shown to increase the activation and proliferation of both CD4T cells and NK cells, is also generally considered immunostimulatory [Citation14–17]. The rexinoid bexarotene is considered an immune-neutral therapy and has been shown to decrease the viability of CTCL tumor cells, likely by arresting proliferation due to activation of the p53/p73 pathway [Citation18]. The classes of conventional chemotherapies and methotrexate are considered immunoinhibitory. Although methotrexate is well-known as a folate antimetabolite that inhibits S-phase proliferation, it also has an anti-inflammatory mechanism, inhibiting prostaglandin E2 release and neutrophil chemotaxis [Citation19–23]. The specifics of its mechanism as an effective CTCL treatment have not yet been fully explained. HDAC inhibitors such as vorinostat and romidepsin appear to exert both pro- and anti-immune effects. For example, suppression of adaptive immunity may occur via their antiproliferative effects on tumor cells through promotion of cell cycle arrest and apoptosis and downregulation of innate immune receptor expression, which alters activation and function of dendritic and NK cells [Citation24,Citation25]. HDAC inhibitors have also been shown to enhance tumor cell recognition and promote the cytotoxic activity of NK cells by upregulating expression of stimulatory surface ligands and adhesion molecules, as well as tumor-associated antigens and MHC class I and II molecules [Citation26,Citation27].
Systemic therapy in MF/SS too often provides only modest benefit, with patients often experiencing disease relapse or progression during treatment [Citation28–31]. In the ALCANZA study, for example, 56% of brentuximab vedotin-treated patients achieved an objective response lasting at least 4 months, compared to 13% of patients treated with physician’s choice, and the median progression-free survival (PFS) was 17.2 months vs 3.5 months [Citation32]. However, 59% of patients treated with brentuximab vedotin received at least one subsequent therapy, and peripheral neuropathy is a known adverse effect with brentuximab vedotin that may preclude its long-term use [Citation32]. Given the limited number of treatments available and the chronicity of the disease, patients may cycle through multiple therapies to maintain disease control, highlighting the need for new effective therapies with non-cross-resistant mechanisms of action for patients [Citation28,Citation31].
Mogamulizumab, a first-in-class, defucosylated immunoglobulin G1 (IgG1) monoclonal antibody directed against C-C chemokine receptor 4 (CCR4), was first approved as monotherapy in Japan for treatment of relapsed or refractory CCR4-positive adult T-cell leukemia-lymphoma (ATL) in 2012 and for treatment of relapsed or refractory CCR4-positive CTCL in 2014 [Citation33–37]. In 2018, mogamulizumab gained approval in the United States, Europe, and Japan for the treatment of relapsed or refractory MF or SS after at least one prior systemic therapy [Citation33,Citation34,Citation37–40]. CCR4, which is involved in trafficking of lymphocytes to skin, is consistently overexpressed on the surface of tumor cells in CTCL [Citation34]. Mogamulizumab selectively binds to CCR4, which promotes crosslinking between membrane-bound Fc gamma receptors (FcγRs) on nonspecific effector cells, including NK cells, to the CCR4+ target cell, enhancing cytotoxic activity and causing lysis of the antibody-targeted cell [Citation41,Citation42].
MAVORIC (NCT01728805) was an open-label, randomized, controlled, international phase 3 study that demonstrated that in patients with relapsed/refractory MF/SS, mogamulizumab was superior to vorinostat in median PFS (7.7 vs 3.1 months, p < .0001) and confirmed overall global response rate (ORR [complete response (CR) plus partial response (PR)]; 28% vs 5%, p < .0001) [Citation35]. These efficacy results were obtained in a heavily pretreated population, who had received a median of 3 prior systemic therapies (range 0–18). To optimally apply these data on mogamulizumab in clinical practice, it is reasonable to investigate whether more heavily pretreated patients respond in a similar fashion to those with a history of fewer prior systemic therapies. In addition, given the mechanism of action of mogamulizumab, we sought to understand whether the type of prior therapy could influence response to mogamulizumab. For example, in vitro studies in which T-cell lymphoma cell lines were treated with HDAC inhibitors, including vorinostat and romidepsin, demonstrated a decrease in CCR4 surface and mRNA expression levels along with a decrease in mogamulizumab-induced cytotoxicity [Citation43]. CCR4 expression was also significantly reduced in skin samples from patients with primary CTCL who had received treatment with vorinostat [Citation43]. Further, a decline in cytolytic function of NK cells was observed in patients with CTCL after three cycles of romidepsin treatment, with a similar finding observed in NK cells from healthy donors after in vitro treatment with romidepsin [Citation24]. Given that mogamulizumab’s mechanism of action is dependent on intact NK or effector cell activity in patients as well as retained CCR4 expression, we sought to determine whether these in vitro findings translate to the clinic and whether the immune effects of prior systemic therapies or time from the last systemic treatment impact mogamulizumab’s efficacy [Citation41,Citation42].
Methods
Study design
A post hoc analysis of data from the MAVORIC study, an open-label, randomized, controlled, international, phase 3 trial, was conducted [Citation35]. The detailed methodology of this trial has been published previously [Citation35]. Briefly, patients enrolled in MAVORIC had stage IB-IVB, histologically confirmed, relapsed or refractory MF or SS and had failed (refractory, progression, or toxicity) at least one previous systemic therapy [Citation35]. Patients were randomized 1:1 to receive mogamulizumab (1.0 mg/kg, administered intravenously once weekly for 5 weeks, then every 2 weeks) or vorinostat (400 mg daily, administered orally) until disease progression, unacceptable toxicity, drug intolerance, or other criteria for treatment discontinuation were met [Citation35]. Patients on vorinostat for at least two cycles who showed disease progression or grade ≥3 adverse events (excluding inadequately treated nausea, vomiting, diarrhea, and alopecia) despite dose reduction and appropriate management could cross over to treatment with mogamulizumab [Citation35]. The analysis described here was performed on the intent-to-treat (ITT) population, defined as all patients randomized to receive a therapy. The study was approved by institutional review boards or independent ethics commissions at each site in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Assessments/outcome measures
ORR was based on a global composite response score in each of four disease compartments (skin, blood, lymph nodes, and viscera) [Citation44], confirmed at two consecutive visits [Citation35], and was assessed for patient cohorts that had received 1, 2, 3, 4, 5, or ≥6 prior systemic therapies, cohorts divided by type of prior therapy, and cohorts divided by immune activity of the immediate prior therapy and time since most recent prior therapy. PFS was defined as the time from randomization until documented disease progression or death due to any cause [Citation35] and was assessed for cohorts defined by number of prior therapies, by type of prior therapy, and by immune activity of immediate prior therapy and time since most recent prior therapy. Duration of response (DOR) was defined as the time from first achievement of an overall response to progression or death and was assessed for patient cohorts defined by the most recent prior therapy. Based on the experience and judgment of these authors, systemic therapies given to patients immediately prior to mogamulizumab in MAVORIC were retrospectively assigned to immune activity categories (immunostimulatory, immune-neutral, or immunoinhibitory, with HDAC inhibitors categorized alone, as shown in ), and the time between the stop date of the immediate prior therapy and the date of the first mogamulizumab treatment was determined. Immediate prior therapy was determined by the last treatment date for systemic therapy. Analyses to assess the effect of prior treatment in subgroups of patients with or without blood involvement at baseline were also conducted. Patients with missing or partially missing dates of immediate prior systemic therapy and those who did not receive mogamulizumab after randomization were excluded from the analysis group.
Table 1. Classification of immediate prior systemic therapies in the MAVORIC trial for subgroup analyses.
Statistical analysis
PFS and ORR were analyzed using Cox proportional hazards and logistic regression models, respectively, with disease type, disease stage, region (United States, Japan, and Rest of World), time from immediate prior therapy to first treatment with mogamulizumab, immunomodulatory activity, and interaction term between time from immediate prior therapy and immunomodulatory activity as covariates.
Results
Number of prior therapies
The overall MAVORIC population was heavily pretreated, with 29% of all enrolled patients having received ≥5 prior systemic regimens. Among patients randomized to mogamulizumab, 30.1% of patients had received ≥5 prior systemic therapies, with similar findings in patients with less advanced (Stage IB/II) and more advanced (Stage III/IV) disease (). The median number of prior treatments was 3.0 (range 1–18).
Table 2. Baseline disease stage and median PFS, confirmed ORR, and median DOR after mogamulizumab treatment by number of prior systemic therapies in patients randomized to mogamulizumab (ITT population).
Prior systemic therapy
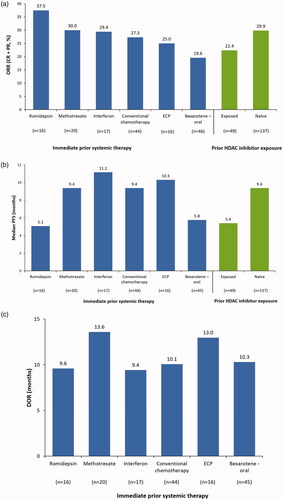
The most common systemic therapies used to treat patients with MF/SS immediately prior to their enrollment in MAVORIC and randomization to mogamulizumab were oral bexarotene (n = 46; 25%), chemotherapy (n = 44; 24%), methotrexate (n = 20; 11%), interferon (n = 17; 9%), ECP (n = 16; 9%), and romidepsin (n = 16; 9%), representing data from a total of 159 patients. Patients had similar confirmed ORRs regardless of the specific immediate prior therapy received (oral bexarotene, 20%; chemotherapy, 27%; methotrexate, 30%; interferon, 29%; ECP, 25%; romidepsin, 38%), which was also seen in patients with any prior exposure to an HDAC inhibitor (22%, n = 49) and in those who were HDAC inhibitor-naïve prior to enrollment in the study (30%, n = 137) (). Median PFS observed in patients treated with oral bexarotene immediately prior to mogamulizumab treatment was 5.8 months; with conventional chemotherapy, 9.4 months; with methotrexate, 9.4 months; with interferon, 11.2 months; with ECP, 10.3 months; and with romidepsin, 5.1 months (). In patients with prior exposure to an HDAC inhibitor (regardless of sequence), median PFS was 5.4 months, and in patients who were HDAC inhibitor-naïve, it was 9.4 months (). Patients treated with oral bexarotene immediately prior to mogamulizumab treatment experienced a DOR of 10.3 months; those treated with conventional chemotherapy, 10.1 months; patients treated with methotrexate, 13.6 months; patients receiving interferon, 9.4 months; those receiving ECP, 13.0 months; and patients treated with romidepsin, 9.6 months (). Number and type of prior treatments did not differentially affect ORR, PFS, or DOR in patients with or without baseline blood involvement (data not shown).
Figure 1. (A) Confirmed global ORR, (B) median PFS, and (C) DOR to mogamulizumab by immediate prior systemic therapy and prior HDAC inhibitor exposure (ITT population). CR: complete response; DOR: duration of response; ECP: extracorporeal photopheresis; HDAC: histone deacetylase; ORR: overall global response rate; PFS: progression-free survival; PR: partial response.
Type of and time since immediate prior systemic therapy
We also examined the impact of the immunomodulatory activity of the last prior systemic therapy used and the time from the last dose of the immediate prior therapy to the start of mogamulizumab treatment on response to mogamulizumab. Thirty-three patients had received immunostimulatory agents, 55 had received immune-neutral agents, 49 had received immunoinhibitory agents, and 13 patients were treated with HDAC inhibitors as their last systemic therapy prior to receiving mogamulizumab. Patient cohorts were generally similar with respect to demographics and disease characteristics across the four immediate prior systemic therapy types (). The median time from the immediate prior systemic therapy was 44 days among patients randomized to mogamulizumab (range 9–1094 days). Confirmed ORR, PFS, and DOR observed in cohorts receiving agents assigned to the categories of immunostimulatory, immune-neutral, or immunoinhibitory agents or HDAC inhibitors immediately prior to mogamulizumab treatment are shown in . According to the results of a logistic regression model, compared with the immune-neutral group, there was no significant relationship between immunostimulatory therapies (χ2=3.06, p = 0.08) or HDAC inhibitors (χ2=1.36, p = 0.24) and ORR (). There did appear to be a statistically significant relationship between immunoinhibitory therapies (χ2=4.28, p = 0.04) and ORR compared with the immune-neutral group (). Additionally, ORR showed no statistically significant relationship with the interaction term between time from immediate prior therapy and immunostimulatory therapies (χ2=1.57, p = 0.21), immunoinhibitory therapies (χ2=1.68, p = 0.19), or HDAC inhibitors (χ2=1.24, p = 0.27) (). The results of a Cox proportional hazard model showed that compared with the immune-neutral group, there was also no statistically significant interaction between immunostimulatory therapies (χ2=0.51, p = 0.48), immunoinhibitory therapies (χ2=0.0002, p = 0.99), or HDAC inhibitors (χ2=0.22, p = 0.64) and PFS. Similarly, PFS showed no statistically significant relationship with the interaction term between time from immediate prior therapy and immunostimulatory therapies (χ2=1.40, p = 0.24), immunoinhibitory therapies (χ2=0.001, p = 0.97), or HDAC inhibitors (χ2=2.97, p = 0.09) ().
Table 3. Baseline characteristics and outcomes by immediate prior therapy class in patients randomized to mogamulizumab (ITT population).
Table 4. Effect of immune activity of immediate prior systemic therapy on PFS and confirmed ORR with mogamulizumab.
Discussion
The MAVORIC phase 3 study results demonstrated that patients with MF/SS who were treated with mogamulizumab experienced a significantly longer median PFS (7.7 months [95% CI 5.7–10.3]), an improved ORR (28% [95% CI 21.6, 35.0]), and a longer median DOR (14.1 months [95% CI 9.4, 19.2]) compared with patients treated with vorinostat (median PFS 3.1 months [2.9, 4.1]; ORR 5% [2.2, 9.0]; median DOR 9.1 months [4.7, –]) [Citation35]. Post hoc analyses of the MAVORIC data suggest that the number of prior therapies, the specific immediate prior therapy, and the type of prior systemic therapy did not impact confirmed ORR, PFS, or DOR in mogamulizumab-treated patients with MF/SS in MAVORIC. When MAVORIC patients randomized to mogamulizumab were stratified by number of prior therapies, there was no observable effect on ORR, PFS, or DOR regardless of how many prior systemic therapies were administered. All patient groups experienced efficacy similar to the ITT population, a pattern that was seen regardless of the presence of blood involvement at baseline. Furthermore, the immediate prior systemic therapy did not significantly affect response rates or the duration of those responses, as response rates were generally similar whether the patient had received prior methotrexate, bexarotene, interferon, or the other most common immediate prior therapies [Citation35]. For subjects with MF/SS, mogamulizumab appears to be effective regardless of resistance to or time from other available systemic treatments [Citation35].
Published preclinical data have suggested that treatment with HDAC inhibitors could lead to a downregulation of HDAC-regulated CCR4 expression in lymphoma cells, which could attenuate the effects of mogamulizumab treatment [Citation24,Citation43]. The overall response in patients randomized to mogamulizumab whose immediate prior therapy was an HDAC inhibitor was 31%. In patients treated with romidepsin immediately prior to mogamulizumab, ORR was 38%, and in those who had had any prior exposure (regardless of sequence) to an HDAC inhibitor, ORR was 22%. These findings are consistent with the ORR of 31% previously reported in patients from MAVORIC who crossed over from vorinostat to mogamulizumab (n = 133) [Citation35]. Moreover, these overall response findings are similar to the ORR observed in the ITT population, 28%, and in the population of HDAC inhibitor-naïve patients, 30%. Taken together, these results suggest that any effect of HDAC inhibitors on CCR4 or NK cell activity was not clinically meaningful for subsequent mogamulizumab-treated patients.
When mogamulizumab treatment was examined in relation to the immediate prior systemic therapy’s effect on the immune system, no statistically significant impact on either the ORR or PFS was observed, with the exception of the impact of immunoinhibitory therapies on ORR (although the exploratory nature of these analyses suggests that additional data would be needed to confirm this effect). There was also no observed impact on ORR or PFS of the time elapsed between the stop date of the immediate prior therapy and the start of mogamulizumab therapy.
Although the overall MAVORIC study population was large enough to allow post hoc subgroup analyses designed to investigate specific questions, the number of patients within certain treatment groups must be considered when assessing the findings from this post hoc analysis. The numbers of patients in each prior systemic therapy group were relatively small, precluding a multivariate analysis and limiting the strength of the conclusions. The MAVORIC study was not specifically powered to detect differences between subgroups of patients receiving different prior systemic therapies. As a result, analysis examined only the treatment immediately prior to mogamulizumab treatment, without fully considering other past treatments or the sequence of prior treatments, which could have also affected the outcome of mogamulizumab therapy. The time elapsed from the immediate prior systemic therapy was also not controlled, and as a result, potentially impactful biologic or immune effects of the immediate prior therapy may have been reduced or resolved by the time of the first mogamulizumab treatment.
Overall, this post hoc analysis demonstrated that clinical response to mogamulizumab treatment in the MAVORIC trial was generally consistent regardless of the number of prior therapies, the specific prior systemic therapy, or the type of prior systemic therapy. Importantly, prior treatment with HDAC inhibitors, which are thought to downregulate CCR4, did not negatively affect response rates to mogamulizumab. Further research is required to identify whether there is an optimal sequence for mogamulizumab therapy in the treatment of relapsed/refractory MF and SS patients; however, this analysis does not show any change in efficacy of mogamulizumab dependent on the previous sequence of treatments.
Previous presentations
Data from this paper were presented at the American Society of Hematology Annual Meeting, 1–4 December 2018, San Diego, CA; at the 47th Meeting of the Italian Society of Hematology, 7–10 October 2019, Roma, Italy; at the T-Cell Lymphoma Forum, 10–12 January 2019, La Jolla, CA; and at the Italian Society of Dermatology 24th World Congress of Dermatology, 10–15 June 2019, Milan, Italy.
Acknowledgments
Medical writing and editorial assistance were provided by Michelle Jones, PhD, and Anthony DiLauro, PhD, of MedVal Scientific Information Services (Princeton, NJ, USA) and were funded by Kyowa Kirin, Inc. (Bedminster, NJ, USA).
Disclosures statement
S.H. has received research funding from ADC, Affimed, Aileron, Celgene, Corvus, Daiichi Sankyo, Forty Seven, Infinity/Verastem, Kyowa Kirin, Millennium/Takeda, Portola, Seattle Genetics, Spectrum, and Trillium and has received advisor/consultancy fees from Acrotech, ADC, Affimed, Aileron, Astex, BeiGene, Bristol Myers Squibb, C4 Therapeutics, Celgene, Corvus, Forty Seven, Infinity/Verastem, Innate, Janssen, Kura, Kyowa Kirin, Merck, Millennium/Takeda, MiRagen, Mundipharma, ONO Pharmaceuticals, Portola, Seattle Genetics, Trillium, and Vividion.
P.L.Z. has received research funding from Bristol Myers Squibb, Celltrion, Johnson & Johnson, MSD, Portola, Roche, and Verastem; advisor/consultancy fees from Bayer, Bristol Myers Squibb, Celgene, Celltrion, Gilead, Janssen, Merck, MSD, Pfizer, Roche, Servier, TG Pharmaceuticals, and Verastem; and has served on speakers’ bureaus for AstraZeneca, Bristol Myers Squibb, Celgene, Celltrion, Gilead, Janssen, MSD, Servier, and Verastem.
M.B. has received advisor/consultancy fees from Helsinn/Recordati, Innate, Kyowa Kirin, Takeda, 4SC, and miRagen and has equity ownership in Innate.
Y.H.K. has received research funding from Eisai, Elorac, Forty Seven, Galderma, Horizon, Innate, Kyowa Kirin, Merck, miRagen, Neumedicines, Portola, Soligenix, Seattle Genetics, Takeda, TetraLogic, and Trillium and has received advisor/consultancy fees from Corvus, Eisai, Horizon, Innate, Kyowa Kirin, Medivir, Neumedicines, Seattle Genetics, and Takeda.
A.J.M. has received research funding from ADC, Bristol Myers Squibb, Incyte, Kyowa Kirin, Merck, miRagen, and Seattle Genetics and has received advisory/consultancy fees from ADC, Bristol Myers Squibb, Cell Medica, Erytech, Imbrium, Janpix, Kyowa Kirin, Merck, miRagen, Purdue, Seattle Genetics, and Takeda.
P.P. has received advisor/consultancy fees from Innate.
K.D., W.S., and F.H. are employees of Kyowa Kirin.
J.S. has received advisor/consultancy fees from 4SC, Actelion, Helsinn, Innate, Kyowa Kirin, Mallinckrodt, and Takeda and is an employee of the UK National Health System.
M.J. and A.D. have no other conflicts of interest apart from the funding MedVal received for medical writing and editorial assistance.
Additional information
Funding
References
- Demierre MF, Tien A, Miller D. Health-related quality-of-life assessment in patients with cutaneous T-cell lymphoma. Arch Dermatol. 2005;141(3):325–330.
- Demierre MF, Gan S, Jones J, et al. Significant impact of cutaneous T-cell lymphoma on patients’ quality of life: results of a 2005 National Cutaneous Lymphoma Foundation Survey. Cancer. 2006;107(10):2504–2511.
- Molloy K, Jonak C, Woei AJF, et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the Prospective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2020;182(3):770–779.
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–3785.
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713–1722.
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - Update 2017. Eur J Cancer. 2017;77:57–74.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: primary cutaneous lymphomas version 2. 2020. [cited 2020 Jun 16]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv30–iv40.
- Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous Lymphoma International Consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33(32):3766–3773.
- Hughes CF, Khot A, McCormack C, et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sézary syndrome: a comparative study of systemic therapy. Blood. 2015;125(1):71–81.
- Spaccarelli N, Rook AH. The use of interferons in the treatment of cutaneous T-cell lymphoma. Dermatol Clin. 2015;33(4):731–745.
- Cho A, Jantschitsch C, Knobler R. Extracorporeal photopheresis-an overview. Front Med (Lausanne). 2018;5:236.
- Acebes-Huerta A, Huergo-Zapico L, Gonzalez-Rodriguez AP, et al. Lenalidomide induces immunomodulation in chronic lymphocytic leukemia and enhances antitumor immune responses mediated by NK and CD4 T cells. Biomed Res Int. 2014;2014:265840.
- González-Rodríguez AP, Payer AR, Acebes-Huerta A, et al. Lenalidomide and chronic lymphocytic leukemia. Biomed Res Int. 2013;2013:932010.
- Giuliani M, Janji B, Berchem G. Activation of NK cells and disruption of PD-L1/PD-1 axis: two different ways for lenalidomide to block myeloma progression. Oncotarget. 2017;8(14):24031–24044.
- González-Rodriguez AP, Villa-Álvarez M, Sordo-Bahamonde C, et al. NK cells in the treatment of hematological malignancies. J Clin Med. 2019;8(10):1557.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160(3):519–526.
- Olek-Hrab K, Maj J, Chmielowska E, et al. Methotrexate in the treatment of mycosis fungoides - a multicenter observational study in 79 patients. Eur Rev Med Pharmacol Sci. 2018;22(11):3586–3594.
- Brown PM, Pratt AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol. 2016;12(12):731–742.
- Cutolo M, Sulli A, Pizzorni C, et al. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001;60(8):729–735.
- Vergne P, Liagre B, Bertin P, et al. Methotrexate and cyclooxygenase metabolism in cultured human rheumatoid synoviocytes. J Rheumatol. 1998;25(3):433–440.
- Walsdorfer U, Christophers E, Schröder JM. Methotrexate inhibits polymorphonuclear leucocyte chemotaxis in psoriasis. Br J Dermatol. 1983;108(4):451–456.
- Kelly-Sell MJ, Kim YH, Straus S, et al. The histone deacetylase inhibitor, romidepsin, suppresses cellular immune functions of cutaneous T-cell lymphoma patients. Am J Hematol. 2012;87(4):354–360.
- Roger T, Lugrin J, Le Roy D, et al. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection. Blood. 2011;117(4):1205–1217.
- Dickinson M, Johnstone RW, Prince HM. Histone deacetylase inhibitors: potential targets responsible for their anti-cancer effect. Invest New Drugs. 2010;28(S1):3–S20.
- Kroesen M, Gielen P, Brok IC, et al. HDAC inhibitors and immunotherapy; a double edged sword? Oncotarget. 2014;5(16):6558–6572.
- Arulogun SO, Prince HM, Ng J, et al. Long-term outcomes of patients with advanced-stage cutaneous T-cell lymphoma and large cell transformation. Blood. 2008;112(8):3082–3087.
- Duvic M, Tetzlaff MT, Gangar P, et al. Results of a phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphoma and lymphomatoid papulosis. J Clin Oncol. 2015;33(32):3759–3765.
- Foss F, Duvic M, Lerner A, et al. Clinical efficacy of romidepsin in tumor stage and folliculotropic mycosis fungoides. Clin Lymphoma Myeloma Leuk. 2016;16(11):637–643.
- Talpur R, Singh L, Daulat S, et al. Long-term outcomes of 1,263 patients with mycosis fungoides and Sézary syndrome from 1982 to 2009. Clin Cancer Res. 2012;18(18):5051–5060.
- Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390(10094):555–566.
- Ishii T, Ishida T, Utsunomiya A, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 2010;16(5):1520–1531.
- Ferenczi K, Fuhlbrigge RC, Pinkus J, et al. Increased CCR4 expression in cutaneous T cell lymphoma. J Invest Dermatol. 2002;119(6):1405–1410.
- Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192–1204.
- Kyowa Hakko Kirin Co., Ltd. New drug application approval for Poteligeo® (mogamulizumab) injection in Japan, a therapeutic antibody for adult T-cell leukemia-lymphoma (ATL) [press release]. 2012. [cited 2017 Sep 25]. Available from: http://www.kyowa-kirin.com/news_releases/2012/e20120330_04.html.
- Kyowa Hakko Kirin Co., Ltd. Approval for additional indication for PTCL and CTCL of Mogamulizumab [press release]. 2014. [cited 2016 Feb 8]. Available from: http://www.kyowa-kirin.com/news_releases/2014/e20140317_01.html.
- Kyowa Kirin, Inc. Poteligeo® (mogamulizumab-kpkc) injection, for intravenous use [prescribing information]. 2018. [cited 2018 Sep 13]. Available from: https://www.poteligeohcp.com/assets/files/full-prescribing-information.pdf.
- Kyowa Hakko Kirin Co., Ltd. Kyowa Kirin announces Poteligeo® receives marketing authorisation in Europe for the treatment of mycosis fungoides and Sézary syndrome [press release]. 2018. [cited 2020 Mar 23]. Available from: https://www.kyowakirin.com/media_center/news_releases/2018/e20181126_01.html.
- Kyowa Hakko Kirin Co., Ltd. Kyowa Hakko Kirin receives the partial change approval of Poteligeo® in Japan [press release]. 2018. [cited 2020 Jun 12]. Available from: https://www.kyowakirin.com/media_center/news_releases/2018/e20180821_01.html.
- Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res. 2004;10(16):5494–5500.
- Duvic M, Evans M, Wang C. Mogamulizumab for the treatment of cutaneous T-cell lymphoma: recent advances and clinical potential. Ther Adv Hematol. 2016;7(3):171–174.
- Kitadate A, Ikeda S, Abe F, et al. Histone deacetylase inhibitors downregulate CCR4 expression and decrease mogamulizumab efficacy in CCR4-positive mature T-cell lymphomas. Haematologica. 2018;103(1):126–135.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598–2607.
