Abstract
This systematic review and meta-analysis aimed to determine the effectiveness of brentuximab vedotin (BV) in relapsed/refractory classical Hodgkin lymphoma (R/R cHL) in the clinical practice setting using most recent results. A total of 32 observational studies reporting on treatment patterns, overall response rate (ORR), complete response (CR) rate, progression-free survival (PFS), overall survival (OS), and adverse events were found. After four cycles, a random-effect model yielded pooled ORR and CR rates of 62.6% (95% confidence interval (CI): 56.0–68.9; I2 = 9.7%) and 32.9% (95% CI, 20.8–46.3, I2 = 64.8%), respectively. Regarding survival, 1-year, 2-year, and 5-year PFS ranged from 52.1% to 63.2%, 45.2% to 56.2%, and 31.9% to 33.0%, respectively. OS rates were 68.2–82.7%, 58.0–81.9%, and 58.0–62.0%, respectively. Most common adverse events were hematological toxicities (neutropenia: 13.3–23%, anemia: 8.8–39.0%, and thrombocytopenia: 4–4.6%), and grade ≥3 peripheral neuropathy (3.3–7.3%). This study supports the effectiveness and safety of BV in R/R cHL patients in the real-world setting.
Introduction
Hodgkin lymphoma (HL) is a cancer of the lymphatic system characterized by the presence of Reed-Sternberg cells [Citation1]. Although modern chemotherapy and radiotherapy regimens have made HL one of the most curable malignancies, 10–15% of early stage and 20–30% of advanced stage patients experience relapse after the initial treatment [Citation2]. Only 50% of patients with relapsed or refractory classical Hodgkin lymphoma (R/R cHL) achieve a prolonged complete remission with high-dose chemotherapy and autologous stem cell transplantation (ASCT) [Citation3], representing an important unmet medical need.
Brentuximab vedotin (BV, ADCETRIS®), an anti-CD30 antibody drug conjugate, was shown to be effective and well-tolerated for the treatment of R/R cHL. In a pivotal phase II trial, the overall response rate (ORR) was 75% and complete response (CR) was observed in 34% of patients [Citation4]. Previous meta-analyses compared these results to the activity of other drug therapies or experimental agents used for R/R cHL in the post ASCT setting and found that BV was superior to other therapies in terms of CR and overall survival (OS) [Citation5,Citation6]. In these analyses, BV data originated from the pivotal phase II trial only. Similar findings to the pivotal phase II trial were since obtained in observational studies; however, due to limited sample size (51–196 patients treated with BV), effectiveness estimates lacked precision [Citation7,Citation8]. To enhance the strength of evidence on the effectiveness of BV in the clinical practice setting for the treatment of R/R cHL in the adult population (age ≥18 years), a systematic review of observational studies was undertaken, followed by a meta-analysis of effectiveness outcomes.
Methods
The systematic review was conducted according to a protocol developed a priori and registered in PROSPERO (CRD42020173134).
Systematic review
Three sources of information were used: (i) literature search; (ii) pragmatic searches of web sources; and (iii) screening of reference list of retained studies (i.e. snowballing). Search period covered the past 10 years (1 January 2010 to 6 February 2020). The literature search was conducted in Ovid MEDLINE, Embase, and Web of Science using free-text keywords and thesaurus terms (i.e. MeSH and Emtree terms, respectively, for MEDLINE and Embase). Pragmatic searches involved a review of websites of relevant learned or clinical societies and related conference proceedings. Sources identified were screened independently by two reviewers (with conflicts resolved by a third independent assessor) using the following pre-defined inclusion criteria: (i) observational (non-interventional) studies including registries and case series; (ii) studies that included adult patients (age ≥18 years) with R/R cHL; (iii) sample size ≥20 patients; (iv) studies that reported effectiveness outcomes of BV used as a single agent; (v) original studies, conference proceedings, abstracts, reviews, or meta-analyses; (vi) publications written in English, French, or Spanish. Expert opinions, editorials, non-clinical studies, phase I–III clinical trials, and case reports were excluded. Data of interest were patterns of BV use, ORR, CR rate, progression-free survival (PFS), OS, and adverse events. Data extraction was conducted independently by two assessors (with conflicts resolved by a third) and the data extraction form was piloted prior to the start of data extraction. The review was conducted according to the methods proposed by the Cochrane group [Citation9] and the Institute of Medicine (IOM) of the National Academy of Medicine (NAM) [Citation10]. The methodological quality of retained full-text publications was assessed using the Joanna Briggs Institute (JBI) critical appraisal tools for original studies [Citation11]. To be considered of good methodological quality, studies were required to meet at least seven of the criteria from the checklist for cohort studies (11 items) or for case series (10 items). Owing to insufficient information, the methodological quality of studies published as abstracts only could not be determined.
Meta-analysis
For each effectiveness outcome of interest (ORR, CR, PFS, and OS), a qualitative assessment of the clinical and methodological heterogeneity of retained studies was conducted. Statistical heterogeneity was quantified through the I2 statistics, which is the percentage of variation across studies that is due to heterogeneity rather than chance. According to the Cochrane recommendations, when the I2 exceeds 50%, heterogeneity is considered substantial, and estimates should not be pooled [Citation12]. When heterogeneity exceeded the threshold, alternative subgroups analyses based on study design, populations, or BV treatment schedule were explored. Primary meta-analyses included studies of good methodological quality only. Two sets of sensitivity analyses were conducted: the first included studies of good and moderate quality, and the second included previous estimates as well as those published as abstracts only. When appropriate, estimates were pooled using a random effects model. The presence of publication bias was determined using a funnel plot. All statistical analyses were performed using MetaXL, version 5.3 [Citation13].
Results
Search results
The selection of sources through the different phases of the search is presented in the PRISMA flowchart (). The literature search yielded 2460 sources, of which 114 were retained for in-depth review. There were 103 sources that were further excluded at this stage for reasons listed in . Pragmatic searches and snowballing yielded 21 additional sources. Data were thus abstracted from 32 sources (13 original studies, 17 abstracts, one letter to the editors, and one website). The majority of studies originated from Europe (n = 24; 75.0%). Countries covered included France (n = 3), Germany (n = 2), Greece (n = 2), Italy (n = 4), Poland (n = 2), the United Kingdom (UK) (n = 5), and other countries (n = 6; Bulgaria, Czech Republic, Portugal, Slovakia, and Sweden). The remainder were from North America (n = 4; 12.5%, all from the United States) and Asia (n = 3; 9.4%, one from Kazakhstan, one from Japan and one from Taiwan). Also, a multiregional study conducted in six national centers was identified (countries not specified). Patient populations were relatively homogenous across studies. Specifically, the median age ranged between 27 years and 45.7 years, and most studies included a majority of patients who underwent ASCT prior to initiating BV. In the few studies that reported the Eastern Cooperative Oncology Group (ECOG) performance status, most patients had a score ≤2. A summary of study characteristics is presented in . Details regarding patient populations are summarized in .
Table 1. Characteristics of studies included in the systematic review on the effectiveness and safety of BV in R/R cHL patients (N = 32).
Table 2. Patient characteristics in studies included in the systematic review on the effectiveness and safety of BV in R/R cHL patients (N = 32).
Patterns of BV use in R/R cHL patients
According to 26 sources that reported patterns of BV usage in R/R cHL patients (n = 18 [Citation22] to 241 patients [Citation29]), BV was used as a single agent after a median of 3 prior regimens (range: 2–4). Dosing regimen was 1.8 mg/kg every three weeks and the median number of BV cycles administered ranged from 4 [Citation18,Citation32,Citation35] to 8 [Citation7,Citation14,Citation16,Citation20].
Treatment response
To evaluate treatment response, most studies relied on positron emission tomography (PET) or computed tomography (CT) scans, with most centers using either technique depending on availability. Also, a majority of studies based this assessment on the 2007 revised response criteria for malignant lymphoma from the International Working Group [Citation44] while a minority of studies considered the Lugano Classification (2014) [Citation20,Citation39]. Across studies, response was typically assessed after several cycles of BV treatment, ranging from two cycles to the end of treatment.
Based on results reported in 24 studies, the ORR of BV in R/R cHL patients ranged from 46.6% [Citation26] to 84.0% [Citation19]. In these studies, the study populations were representative of patients who are eligible to BV, according to the approved indication. Specifically, the proportion of patients who received previous ASCT ranged from 25.5% [Citation24] to 100% [Citation8,Citation17,Citation36] and 37.5% [Citation27] to 81% [Citation42] patients had refractory HL, defined as stable or progressive disease during first-line or salvage chemotherapy. However, almost half studies (n = 10; 41.7%) did not provide any details regarding the distribution of patients according to relapse or refractory status. Lower estimates of ORR (20.6% [Citation31] to 40.0% [Citation35]) were reported in case series from France [Citation29], Italy [Citation35], Portugal [Citation31], and the UK [Citation32]. In the case series from France (n = 241) and Portugal (n = 34), the estimates of ORR after 2–4 cycles of BV were within the previously reported range (58% [Citation29] and 67% [Citation31], respectively). However, at the end of treatment with BV, the ORR was 32% in France (median number of BV cycles = 6, range: 1–16) [Citation29] and 20.6% in Portugal (median number of BV cycles = 7.5) [Citation31]. In the case series from Italy (n = 30) [Citation35] and the UK (n = 26) [Citation32], BV was evaluated as a bridge to ASCT for R/R cHL patients who achieved a suboptimal response to salvage treatment, with reported ORR estimates of 40.0% and 38.5%, respectively, after four cycles.
According to 22 studies, 21.1% [Citation41] to 45.8% [Citation21] R/R cHL patients treated with BV achieved CR. Lower estimates (14.3% [Citation42] to 16.7% [Citation21,Citation22]) were found in four case series that included either small sample sizes (n = 21 [Citation27], n = 24 [Citation30], and n = 18 [Citation22]) or covered a specific population [Citation31]. Conversely, in a conference proceeding that reported on a study conducted at the Memorial Sloan Kettering Cancer Center (MKSCC) in the United States, 13 (61.9%) of the 21 R/R cHL patients who received BV post-ASCT relapse achieved CR [Citation38].
Out of the 26 studies that reported estimates of ORR or CR, there were 12 full-text publications. These were considered for the meta-analysis and the qualitative assessment of clinical and methodological heterogeneity revealed no major differences across those 12 studies. In all studies, patients received BV in monotherapy, and the majority of patients (range: 50% [Citation21] to 87.5% [Citation27]) had advanced disease (stage III or IV) and a previous ASCT (range: 33.3% [Citation22] to 100% [Citation8,Citation36]). As the time period for the assessment of treatment response varied across studies, three subgroups were defined: (i) assessment after four cycles; (ii) assessment after 4–6 cycles; and (iii) assessment after more than six cycles. Seven of these 12 studies were deemed of good methodological quality [Citation8,Citation14,Citation15,Citation20,Citation22,Citation28,Citation36] and were included in the primary meta-analysis while the remaining five studies were of moderate methodological quality and hence, were only considered for the sensitivity analyses [Citation16,Citation21,Citation26,Citation27,Citation41]. In addition, there were 14 studies which consisted of abstracts or conference proceedings that were included in a second set of sensitivity analyses [Citation17–19,Citation23–25,Citation29–32,Citation34,Citation38,Citation39,Citation42].
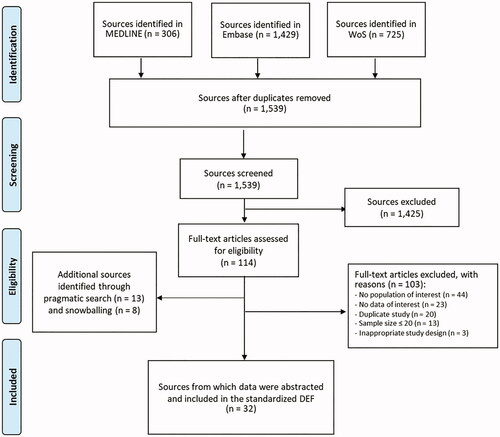
Pooled estimates of ORR according to the number of BV cycles are summarized in with details provided in Supplementary Table 1. The pooled estimate was 62.6% (95% confidence interval (CI), 56.0–68.9; I2 = 9.5%) after four cycles, 66.7% (95% CI, 58.5–74.5; I2 = 43.9%) after 4–6 cycles and 72.0% (95% CI, 49.3–90.4, I2 = 92.5%) after more than six cycles. Pooled estimates originating from good and moderate quality studies (sensitivity analysis 1) as well as those including data published as abstracts (sensitivity analysis 2) were similar to results of the main meta-analysis, thus confirming the robustness of findings (Supplementary Table 2). Also, as shown in the funnel plot (), the distribution of studies around the average estimate is relatively symmetrical, therefore, suggesting no or minor publication bias.
Figure 2. Meta-analysis of overall response rates. (A) Forest plot of estimates of overall response rates after 4 cycles of treatment with BV (1st subgroup); (B) forest plot of estimates of overall response rates after 4–6 cycles of treatment with BV (2nd subgroup); (C) forest plot of estimates of overall response rates after 6 or more cycles of treatment with BV (3rd subgroup); (D) funnel plot of full-text publications reporting overall response rate estimates (n = 12 publications).
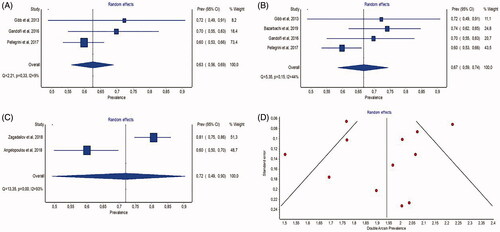
Pooled estimates of CR are summarized in and in . Results from the primary meta-analysis were 32.9% (95% CI, 20.8–46.3, I2 = 64.8%), 32.4% (95% CI, 24.3–41.1; I2 = 49.3%), and 33.4% (95% CI, 13.0–57.3, I2 = 92.9%), respectively, for each subgroup of cycles. Despite the substantial statistical heterogeneity (I2>50%) associated with two pooled estimates, both sensitivity analyses led to similar pooled estimates (). According to the funnel plot presented in , there was no or minor publication bias.
Figure 3. Meta-analysis of complete response rates. (A) Forest plot of estimates of complete response after 4 cycles of treatment with BV (1st subgroup); (B) forest plot of estimates of complete response after 4–6 cycles of treatment with BV (2nd subgroup); (C) forest plot of estimates of complete response after 6 or more cycles of treatment with BV (3rd subgroup); (D) funnel plot of full-text publications reporting complete response estimates (n = 12 publications).
Table 3. Pooled estimates of complete response.
Table 4. Sensitivity analysis of pooled estimates of complete response.
Survival in R/R cHL patients treated with BV
A total of 18 sources documented PFS in R/R cHL patients treated with BV. Twelve studies (66.7%) defined this parameter as the median duration from BV initiation until disease progression, relapse, or death from any cause, with estimates ranging from 5 [Citation34] to 16.6 [Citation26] months. Other measures of PFS identified included 1-year PFS (two studies, 52.1% [Citation16], and 63.2% [Citation26]), 2-year PFS (three studies, 45.2% [Citation26], 51% [Citation18], and 56.2% [Citation19]), 3-year PFS (one study, 36.7% [Citation20]), and 5-year PFS (two studies, 31.9% [Citation28] and 33.0% [Citation25]).
Nine full-text publications that reported estimates of the median PFS were considered for the meta-analysis. The qualitative assessment of clinical heterogeneity revealed no major differences across studies. However, in all these studies, only point estimates of PFS were reported. Therefore, a meta-analysis could not be conducted due to the lack of information on the distribution of data (i.e. interquartile range, 95% CI).
A total of 18 publications reported estimates of OS in R/R cHL patients treated with BV, with five reporting median OS: 17.8 [Citation16], 26.5 [Citation14], 33.2 [Citation42], 57.0 [Citation33], and 91.5 months [Citation43]. The lowest estimate was found in a case series of 136 R/R cHL patients ineligible to ASCT identified in Germany and the UK [Citation16] and, the highest estimate was reported in a conference proceeding describing patients treated with BV following a relapse after ASCT over a median follow-up period of 49.4 months [Citation43]. In four studies, median OS was not reached. In these studies, follow-up periods following BV initiation were 14 months (range: 1–36) [Citation34], 16 months [Citation29], 18.1 months (range: 1.5–26.3) [Citation41], and 55 months [Citation28]. Other studies reported estimates of 1-year OS (six studies, range: 68.2% [Citation16] to 82.7% [Citation15,Citation16,Citation26,Citation29,Citation31,Citation40]), 2-year OS (seven studies, range: 58.0% [Citation29] to 81.9% [Citation14,Citation18,Citation19,Citation26,Citation29,Citation34,Citation41]), 3-year OS (two studies, 41% [Citation26] and 74.6% [Citation20]), 5-year OS (three studies, range: 58% [Citation43] to 62.0% [Citation25]), and 10-year OS (one study, 33.0%) [Citation43].
Among full-text publications, the most commonly reported parameter was median OS (n = 3 studies). Hence, the qualitative assessment of clinical heterogeneity was conducted for these three studies only as for other estimates data were scarce with only one study each reporting 1-year OS [Citation15], 2-year OS [Citation14], and 3-year OS [Citation20]. The qualitative assessment of study methods and populations revealed no major heterogeneity. However, since the two studies deemed of good methodological quality [Citation14,Citation33] used different definition of OS, no meta-analysis could be conducted. The first defined OS as the time from BV initiation to death of any cause [Citation14] while the second defined it as the time from relapse/progression after ASCT until death of any cause or last follow-up [Citation23].
Safety of BV in R/R cHL patients
A total of 12 studies reported safety data on BV in R/R cHL patients. The most common adverse events, as obtained from medical charts or disease registries, consisted of hematological toxicities: Neutropenia (13.3% [Citation35] to 23% [Citation29] patients), anemia (8.8% [Citation16] to 39.0% [Citation29]), and thrombocytopenia (4% [Citation18] to 4.6% [Citation8]). Occurrence of peripheral neuropathy in BV-treated patients was reported in 11 sources. The estimated frequency of grade 1 or 2 peripheral neuropathy, as identified in a disease registry, was 36.2%, [Citation26], and that of grade 3 or higher found in medical charts (details not provided) ranged from 3.3% [Citation35] to 7.3% [Citation28]. As reported in an Italian series of 234 patients, resolution or improvement of peripheral neuropathy was observed in 90% of patients with a median time to resolution or improvement of 12 weeks [Citation28].
Discussion
This review identified 32 observational studies that reported on the effectiveness or safety of BV used as a single agent in R/R cHL patients, which enables synthesis of evidence without the inclusion of clinical trial results. The dosing regimen of BV used in the clinical practice setting is aligned with that used in the pivotal phase II trial and the recommendations included in the summary of product characteristics [Citation4]. In addition, reported estimates of treatment response measured using the ORR, CR, PFS, and OS are consistent with results reported in the pivotal trial, even though patients treated in the real-world clinical practice setting may include subgroups of patients with a worse prognosis. In the pivotal phase II trial, the ORR was 75%, which is similar to the pooled estimate of 72.0% after more than six cycles found in the present meta-analysis. Similar results were observed for the CR rate (34% in the clinical trial versus 33.4% in the meta-analysis) [Citation4]. In the pivotal phase II trial, the study population was restricted to patients with an ECOG performance status score of 0 or 1, while some observational studies included patients with ECOG PS 2 or higher [Citation16,Citation24]. Also, patients with previous allogeneic SCT were excluded from the trial, whereas several studies identified in the systematic review included patients with such history of transplantation [Citation14,Citation21,Citation25–27,Citation29,Citation37]. In four case series, lower estimates of ORR were reported (20.6% [Citation31] to 40.0% [Citation29,Citation31,Citation32,Citation35]). However, these results may be explained by characteristics of the study populations (i.e. heavily pretreated patients refractory to previous salvage treatments or patients ineligible to ASCT due to the persistence of the disease). Similarly for CR rate, lower estimates reported in four case series (14.3% [Citation42] to 16.7% [Citation22,Citation30]) can be explained by small sample sizes (n = 21 [Citation42], n = 24 [Citation30], and n = 18 [Citation22]) or specific patient populations (heavily pretreated patients who received a median of four prior regimens and were refractory to previous salvage therapies [Citation31]). Of note, the majority of studies retained in this review assessed treatment response based on the 2007 International Working Group criteria, while these criteria have been updated in 2014, known as the Lugano Classification. Despite safety data being relatively scarce in the literature, results were consistent with those reported in the pivotal phase II trial. The most common BV-related adverse events observed in the real-world clinical practice setting were neutropenia, anemia, thrombocytopenia, and peripheral neuropathy. For the latter, most patients had grade 1 or 2 toxicity and only a minority experienced grade 3 or 4 adverse events.
Similar results were observed in two previous reviews conducted by Zinzani et al. [Citation45,Citation46], in which the pooled ORR and CR rates were 67% and 26%, respectively. In these studies, only BV data originating from published reports of the BV Named Patient Program, up to the year 2015 were included whereas in the current review a larger scope was considered. Specifically, the search period spanned through the year 2019 and various study designs such as retrospective and prospective cohort studies as well as case series were considered. Also, in previous reviews, the timing of response evaluation was not considered in the pooled analyses of ORR and CR rates, while in the current review pooled estimates were stratified according to timing of evaluation of treatment response or the mean/median number of BV cycles received by patients, following a formal assessment of clinical heterogeneity. In addition, Dada et al. [Citation47] also conducted a systematic review and meta-analysis to evaluate the impact of BV on outcomes in pretreated patients with R/R cHL. Based on this meta-analysis, the pooled ORR and CR rates were 62.7% and 31.8%, respectively. In this review, both observational studies and clinical trials published between 2012 and 2015 were included. The current review therefore offers an update of findings on BV treatment response using only real-world evidence data obtained in observational studies.
This review presents several strengths. First, the methodological quality of studies was evaluated using validated assessment tools based on expert critical review. Second, the screening and data extraction processes were performed independently by two reviewers with discrepancies resolved by a third independent assessor. Third, this review includes data on the use of BV in the real-world setting over more than a decade (1996–2019), therefore, supporting its effectiveness in clinical practice. Finally, sensitivity analyses led to estimates that were similar to those obtained in the primary analyses which demonstrates the robustness of results.
Several limitations of this review should be considered. First, identified studies were mostly those that led to publications in the scientific literature and that have been indexed in MEDLINE, Embase, or Web of Science using pre-defined MeSH terms and keywords. To mitigate this limitation, pragmatic searches, as well as snowballing were conducted. Another limitation consisted of the absence of pooled estimates for survival outcomes for which a meta-analysis was not feasible due to the heterogeneous definitions considered for OS and the lack of data on the distribution of median PFS estimates. In order to address this limitation, additional efforts including contacts with corresponding authors of publications reporting PFS estimates were conducted. However, no additional data regarding interquartile ranges or 95% CIs of median PFS estimates were available, and analysis could therefore not be conducted. Third, while the current review focused on the use of BV in R/R cHL alone, recent guidelines recommend its use in combination with other chemotherapy regimens such as doxorubicin, vinblastine, and dacarbazine in the first-line setting or as consolidation after ASCT [Citation48,Citation49]. Moreover, BV is also being used as a combination partner for salvage chemotherapy at first relapse in patients eligible for ASCT [Citation50–54]. This use of BV in earlier lines limits the generalizability of our current findings [Citation55], although retreatment with BV in patients who have previously obtained a response has been shown [Citation54,Citation56]. In addition, as the main focus of this review was to evaluate the effectiveness of BV in the real-life practice setting, safety data were relatively scarce and originated from studies which were not aimed to explore this outcome.
According to identified observational studies, covering a total of 2303 R/R cHL patients, reported estimates of treatment response measured through ORR, CR, PFS, and OS were consistent with those reported in the pivotal phase II trial as were safety results despite the fact that observational studies include broader and more heterogeneous populations. Specifically, in studies that were conducted in the clinical practice setting, patients with a worse prognosis or higher risk cHL than those included in the phase II trial could be included (i.e. patients with ECOG PS 2 or higher, patients who failed allogeneic SCT, patients ineligible for transplant). As a consequence, although lower estimates of ORR or CR may sometimes be reported in these specific populations, this study supports the effectiveness and safety of BV in R/R cHL patients in the real-world setting.
GLAL-2021-0129-File005.docx
Download MS Word (17.2 KB)Disclosure statement
von Tresckow reports personal fees from Amgen, personal fees from Pfizer, grants, personal fees and non-financial support from MSD, personal fees from Gilead, personal fees from Roche, grants, personal fees and non-financial support from Takeda, grants and non-financial support from Novartis. Bergamasco, Castillon, Arrendondo-Bisono, and Cristarella are employees of YOLARX Consultants that received funding from TAKEDA for the conduct of this study. Trinchese, Gavini, Bent-Ennakhil, and Zomas are employees of TAKEDA Pharmaceuticals. Moride is president of YOLARX Consultants that received funding from TAKEDA for the conduct of this study. Plattel reports advisory/consultancy activities for TAKEDA, BMS, and MSD.
Additional information
Funding
References
- National Cancer Institute. Cancer Stat Facts: Hodgkin lymphoma; 2019; [Internet]; [cited 2019 Apr 18]. National Cancer Institute. Available from: https://seer.cancer.gov/statfacts/html/hodg.html
- Shah GL, Moskowitz CH. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood. 2018;131(15):1689–1697.
- Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934–4937.
- Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–2189.
- Bonthapally V, Wu E, Macalalad A, et al. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma post-autologous transplant: meta-analysis versus historical data. Curr Med Res. 2015;31(5):993–1001.
- Bonthapally V, Yang H, Ayyagar R, et al. Brentuximab vedotin compared with other therapies in relapsed/refractory Hodgkin lymphoma post autologous stem cell transplant: median overall survival meta-analysis. Curr Med Res. 2015;31(7):1377–1389.
- Ladicka M, Sopko L, Chudej J. Real world data on the efficacy and safety of brentuximab vedotin in relapsed/refractory Hodgkin lymphoma in Slovakia. HemaSphere. 2018;2(Suppl. 2):911.
- Zagadailov EA, Corman S, Chirikov V, et al. Real-world effectiveness of brentuximab vedotin versus physicians' choice chemotherapy in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplantation in the United Kingdom and Germany. Leuk Lymphoma. 2018;59(6):1413–1419.
- Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane handbook for systematic reviews of interventions. version 6.1 [updated Sep 2020]. Cochrane; 2020. Available from: www.training.cochrane.org/handbook
- IOM. Standards for systematic reviews; 2011; [Internet]; [cited 2018 Mar 21]. Institute of Medicine. Available from: https://iom.nationalacademies.org/Reports/2011/Finding-What-Works-in-Health-Care-Standards-for-Systematic-Reviews/Standards.aspx
- JBI. Critical appraisal tools; 2018; [Internet]; [cited 2018 Aug 10]. The Joanna Briggs Institute. Available from: joannabriggs.org/research/critical-appraisal-tools.html
- Ryan R. Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Group reviews: planning the analysis at protocol stage; 2016; [Internet]; [cited 2019 Jun 28]. Cochrane Consumers and Communication Review Group. Available from: http://cccrg.cochrane.org
- EpiGear International; 2020; [Internet]; [cited 2020 Apr 18]. MetaXL version 5.3. Available from: https://www.epigear.com/index_files/metaxl.html
- Angelopoulou MK, Vassilakopoulos TP, Batsis I, et al. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma. The Hellenic experience. Hematol Oncol. 2018;36(1):174–181.
- Bazarbachi A, Boumendil A, Finel H, et al. Brentuximab vedotin for recurrent Hodgkin lymphoma after allogeneic hematopoietic stem cell transplantation: a report from the EBMT Lymphoma Working Party. Cancer. 2019;125(1):90–98.
- Brockelmann PJ, Zagadailov EA, Corman SL, et al. Brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma who are ineligible for autologous stem cell transplant: a Germany and United Kingdom retrospective study. Eur J Haematol. 2017;99(6):553–558.
- Chavda ND, Robinson S, Boumendil A, et al. Outcome of patients with Hodgkin lymphoma treated with brentuximab vedotin for relapse after autologous stem cell transplant: a retrospective analysis of the LWP-EBMT. Blood. 2019;134(Suppl. 1):4018.
- Czyz A, Lojko-Dankowska A, Joks M, et al. Brentuximab vedotin alone and in combination with bendamustine as salvage therapy for primary refractory or relapsed Hodgkin lymphoma: multicentre experience of the Polish Lymphoma Research Group (PLRG). Blood. 2018;132(Suppl. 1):5375.
- Czyz A, Lojko-Dankowska A, Giza A, et al. Improved treatment outcomes for patients with Hodgkin lymphoma relapsing after autologous hematopoietic stem cell transplantation in the brentuximab vedotin era – the real-life report from the Polish Lymphoma Research Group. Blood. 2019;134(Suppl. 1):5276.
- Gandolfi L, Pellegrini C, Casadei B, et al. Long-term responders after brentuximab vedotin: single-center experience on relapsed and refractory Hodgkin lymphoma and anaplastic large cell lymphoma patients. Oncologist. 2016;21(12):1436–1441.
- Garciaz S, Coso D, Peyrade F, et al. Brentuximab vedotin followed by allogeneic transplantation as salvage regimen in patients with relapsed and/or refractory Hodgkin's lymphoma. Hematol Oncol. 2014;32(4):187–191.
- Gibb A, Jones C, Bloor A, et al. Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica. 2013;98(4):611–614.
- Goranova-Marinova VS, Ignatova K, Ganeva P, et al. Prognostic factors influencing outcome after therapy with brentuximab vedotin in patients with relapsed or refractory Hodgkin's lymphoma. Ann Oncol. 2019;30(Suppl. 5):v437–v438.
- Ionova T, Afanasiev B, Andrievskih M, et al. Response to brentuximab vedotin and quality of life in patients with relapsed/refractory Hodgkin lymphoma (RR HL) in the real world setting. Blood. 2019;134(Suppl. 1):5296.
- Jansson C, Lagerlof I, Linderoth J, et al. Real life data of brentuximab vedotin use in relapsed/refractory HL in Sweden. HemaSphere. 2018;2(3 Suppl. 3):47–48.
- Král Z, Michalka J, Móciková H, et al. Treatment of relapsed/refractory Hodgkin lymphoma: real-world data from the Czech Republic and Slovakia. J Cancer. 2019;10(21):5041–5048.
- Monjanel H, Deville L, Ram-Wolff C, et al. Brentuximab vedotin in heavily treated Hodgkin and anaplastic large-cell lymphoma, a single centre study on 45 patients. Br J Haematol. 2014;166(2):306–308.
- Pellegrini C, Broccoli A, Pulsoni A, et al. Italian real life experience with brentuximab vedotin: results of a large observational study on 234 relapsed/refractory Hodgkin's lymphoma. Oncotarget. 2017;8(53):91703–91710.
- Perrot A, Monjanel H, Bouabdallah R, et al. Brentuximab vedotin as single agent in refractory or relapsed CD30-positive Hodgkin lymphoma: the French name patient program experience in 241 patients. Haematologica. 2014;99(Suppl. 1):498.
- Piddock K, Atabani S, Gibb A, et al. Real world experience with brentuximab vedotin in relapsed/refractory CD30 positive lymphoma: outcomes in 33 patients after prolonged follow-up at a single UK Centre. Br J Haematol. 2017;176(Suppl. 1):8.
- Pierdomenico F, Pinto AL, Coutinho J, et al. Limited efficacy of brentuximab vedotin in a heavily pre-treated Hodgkin lymphoma population. Haematologica. 2016;101(Suppl. 5):57.
- Sarma A, Potter V, Wrench D, et al. Brentuximab vedotin as a bridge to transplant in classical Hodgkin lymphoma: single tertiary centre real-world experience. Br J Haematol. 2016;173(Suppl. 1):108–109.
- Tsirigotis P, Vassilakopoulos T, Batsis I, et al. Positive impact of brentuximab vedotin on overall survival of patients with classical Hodgkin lymphoma who relapse or progress after autologous stem cell transplantation: a nationwide analysis. Hematol Oncol. 2018;36(4):645–650.
- Viviani S, Guidetti A, Dalto S, et al. Brentuximab vedotin (BV) an effective treatment for transplant ineligible patients with relapsed/refractory (R/R) Hodgkin lymphoma (HL). Haematologica. 2015;100(Suppl. 1):455–456.
- Zinzani PL, Pellegrini C, Cantonetti M, et al. Brentuximab vedotin in transplant-naïve relapsed/refractory Hodgkin lymphoma: experience in 30 patients. Oncologist. 2015;20(12):1413–1416.
- Badar T, Epperla N, Szabo A, et al. Trends in postrelapse survival in classic Hodgkin lymphoma patients after experiencing therapy failure following auto-HCT. Blood Adv. 2020;4(1):47–54.
- Houk A, Gopal AK, Libby EN, et al. Prediction of progression-free survival with brentuximab vedotin therapy for relapsed Hodgkin lymphoma: a retrospective analysis. Blood. 2014;124(21):3086.
- Rajeeve S, Shah GL, Moskowitz CH, et al. Survival for relapsed/refractory Hodgkin lymphoma patients with recurrent or persistent disease following autologous hematopoietic stem cell transplantation treated in the modern ERA. Blood. 2018;132(Suppl. 1):2148.
- Svoboda J, Ballard HJ, Bair SM, et al. Current selection patterns, toxicities and outcomes of pre-transplant salvage treatment regimens in patients with relapsed/refractory Hodgkin lymphoma: results of a multicenter retrospective analysis. Blood. 2019;134(Suppl. 1):2855.
- ClinicalTrials: brentuximab vedotin (recombinant) for IV infusion – special drug use surveillance (all-case surveillance). Relapsed or refractory CD30+ Hodgkin's lymphoma or anaplastic large cell lymphoma; 2020; [Internet]; [cited 2020 Mar 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT02139592?term = NCT02139592&draw = 2&rank = 1
- Tien FM, Tsai CH, Liu JH, et al. Brentuximab vedotin as a salvage treatment for relapsed and refractory Hodgkin lymphoma patients in Taiwan. J Formos Med Assoc. 2019;118(10):1466–1470.
- Jazyltayeva A, Gabbasova S, Kemelbekov N, et al. Preliminary results of using brentuximab vedotin in relapse/refractory Hodgkin's lymphoma. HemaSphere. 2019;3(S1):908.
- Karuturi MS, Arai S, Chen RW, et al. Overall survival benefit for patients with relapsed Hodgkin lymphoma treated with brentuximab vedotin after autologous stem cell transplant. Blood. 2012;120(21):3701.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586.
- Zinzani PL, Sasse S, Radford J, et al. Experience of brentuximab vedotin in relapsed/refractory Hodgkin lymphoma and relapsed/refractory systemic anaplastic large-cell lymphoma in the Named Patient Program: review of the literature. Crit Rev Oncol Hematol. 2015;95(3):359–369.
- Zinzani PL, Sasse S, Radford J, et al. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma: an updated review of published data from the named patient program. Crit Rev Oncol Hematol. 2016;104:65–70.
- Dada R, Zekri J, Al Saadi R. Brentuximab vedotin in pretreated Hodgkin lymphoma patients: a systematic review and meta-analysis. Expert Opin Biol Ther. 2016;16(6):739–745.
- Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin's lymphoma. N Engl J Med. 2018;378(4):331–344.
- Moskowitz CH, Walewski J, Nademanee A, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132(25):2639–2642.
- Chen R, Palmer JM, Martin P, et al. Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2015;21(12):2136–2140.
- Moskowitz AJ, Schoder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284–292.
- LaCasce AS, Bociek G, Sawas A, et al. Brentuximab vedotin plus bendamustine: a highly active salvage treatment regimen for patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;126(23):3982.
- Garcia-Sanz R, Sureda A, Alonso-Alvarez S, et al. Evaluation of the Regimen Brentuximab Vedotin Plus ESHAP (BRESHAP) in refractory or relapsed Hodgkin lymphoma patients: preliminary results of a Phase I-II Trial from the Spanish Group of Lymphoma and Bone Marrow Transplantation (GELTAMO). Blood. 2015;126(23):582.
- Kersten MJ, Driessen J, Zijlstra JM, et al. Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: the phase II HOVON/LLPC Transplant BRaVE study. Haematologica. 2021;106(4):1129–1137.
- Vassilakopoulos TP. Relapsed or refractory classical Hodgkin lymphoma: which immunotherapy, and when? Lancet Oncol. 2021;22(4):417–419.
- Bartlett NL, Chen R, Fanale MA, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24.