Ibrutinib, a first-in-class covalent inhibitor of Bruton’s tyrosine kinase (BTK) approved in the United States and European Union for adult patients with CLL [Citation1], has helped shift the treatment paradigm for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and other B-cell malignancies toward the use of novel targeted therapies. Multiple randomized phase 3 studies have demonstrated a significant progression-free survival (PFS) and/or overall survival (OS) benefit for ibrutinib, including superior first-line efficacy versus established chemotherapy/chemoimmunotherapies [Citation2–6]. Notably in the first-line setting, the median PFS has not been reached in these phase 3 studies. In the RESONATE-2 study, ibrutinib demonstrated sustained efficacy compared with chlorambucil in previously untreated CLL patients aged ≥65 years, and provided a 5-year PFS rate of 70% [Citation7]. For patients treated in the relapsed/refractory (R/R) setting, the phase 3 RESONATE study showed a median PFS of 44.1 months for ibrutinib versus 8.1 months for ofatumumab [Citation8].
We previously evaluated the impact of line of therapy on the efficacy and safety of single-agent ibrutinib [Citation9]; here we report updated results with long-term ibrutinib treatment. This integrated analysis included data from two phase 3 studies of single-agent ibrutinib: (1) in the first-line setting, 5-year follow-up data from RESONATE-2 (NCT01722487), and (2) in the relapsed/refractory setting, 6-year follow-up (final analysis) data from RESONATE (NCT01578707). The study designs for both trials have been previously published [Citation2,Citation3]. The studies were conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice and the principles of the Declaration of Helsinki. The protocols were approved by institutional review boards or independent ethics committees of all participating institutions. All patients provided written, informed consent. To ensure a homogenous population and dataset for this analysis, patients with chromosome 17p deletion were excluded from the RESONATE cohort in this analysis given original RESONATE-2 eligibility criteria that excluded patients with this abnormality. Subgroups were based on the number of prior lines of therapy. Patients treated with 1 and 2 prior lines were combined because of the small number of patients in each group.
This integrated analysis included 271 patients, 136 of whom were treated in the first-line setting. Of 135 patients with relapsed/refractory disease, 68 patients had 1-2 prior lines, and 67 patients had ≥3 prior lines of therapy. Characteristics of the patients at enrollment are found in Supplemental Table SI. Median follow-up times were 59.8 months for first-line, 66.2 months for 1-2 prior lines, and 65.1 months for ≥3 prior lines (Supplemental Table SII).
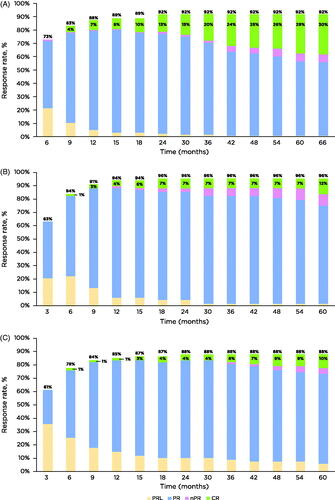
Overall response rates (ORRs; defined as partial response [PR] with lymphocytosis + PR + nodular PR + complete response [CR] with incomplete bone marrow recovery + CR) were high in all treatment settings, and were 92%, 96%, and 88% for those treated in the first-line setting, with 1-2 prior lines, and ≥3 prior lines, respectively. As has been shown previously [Citation9], the depth of remission increased over time, with no obvious plateau even at 5 years of treatment. Patients with fewer prior lines of therapy had an increased depth of remission over time, and at 5 years, 29% of patients treated with first-line ibrutinib, 12% of those with 1-2 prior lines, and 10% of patients with ≥3 prior lines of therapy achieved a CR/CRi (). When comparing across lines of therapy, patients treated in the first-line setting were at least 3 times more likely to achieve a CR versus patients treated in later lines—odds ratio for first-line versus 1-2 prior lines, 3.24 (95% confidence interval [CI], 1.42–7.38); for first-line versus ≥3 prior lines, 3.70 (95% CI, 1.56–8.78). There was no significant difference in the likelihood of achieving a CR among patients with 1-2 versus ≥3 prior lines of therapy—odds ratio, 1.14 (95% CI, 0.39–3.35).
Figure 1. Cumulative best response rate over time by first-line (A), 1-2 prior lines (B), and ≥3 prior lines of therapy (C). Response assessed by investigators by study time points. CR: complete response; CRi: complete remission with incomplete bone marrow recovery; PR: partial response; PRL: PR with lymphocytosis; nPR nodular PR.
Consistent with ORR and CR rates, PFS improved for patients treated with ibrutinib in earlier lines of treatment. Patients treated with first-line ibrutinib had a 34% reduction in risk of disease progression or death compared with treatment after 1-2 prior lines (hazard ratio [HR]: 0.66 [95% CI, 0.40–1.09]) and a 68% reduction in risk of disease progression or death compared with treatment after ≥3 prior lines (HR: 0.32 [95% CI, 0.21–0.49]). Similarly, ibrutinib after 1-2 prior lines reduced the risk of disease progression or death by 52% compared to treatment after ≥3 prior lines (HR: 0.48 [95% CI, 0.30–0.77]). Median PFS was not reached for patients treated with ibrutinib in the first-line setting or after 1-2 prior lines, but was 40.1 months for those with ≥3 prior lines of therapy. At 5 years, 70% of patients treated with first-line ibrutinib remained progression free, compared with 60% of those with 1-2 prior lines of therapy, and 33% of those with ≥3 prior lines ().
Figure 2. Progression-free survival (A-B) and overall survival (C-D) by prior lines of therapy for all patients and high-risk disease features. Tick marks on the curves indicate patients with censored data. CI: confidence interval; NE: not estimable; NR: not reached; OS: overall survival; PFS: progression-free survival.
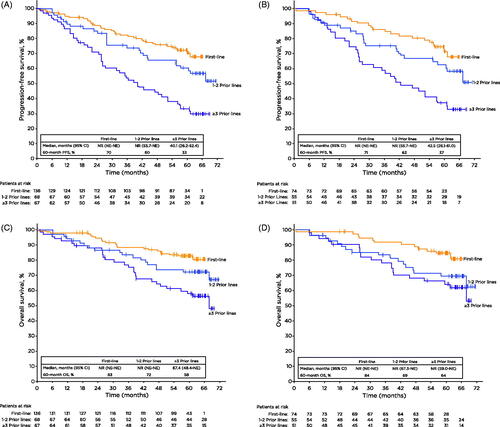
A greater effect of ibrutinib treatment in earlier lines of therapy was also seen for patients with one or more high-risk prognostic features (unmutated IGHV, chromosome 11q deletion [del(11q)], and/or TP53 mutation). Consistent with the overall population, first-line treatment in the high-risk population resulted in a 36% reduction in risk of disease progression or death compared with treatment after 1-2 prior lines (HR: 0.64 [95% CI, 0.35–1.18]) and a 67% reduction in the risk of disease progression or death versus ≥3 or more lines of therapy (HR: 0.33 [95% CI: 0.19 − 0.57]). When comparing 1-2 prior lines versus ≥3 lines of therapy, ibrutinib reduced the risk of disease progression or death by 49% (HR: 0.51 [95% CI: 0.30 − 0.87], ) in patients with high-risk disease. When examined individually, similar trends by lines of therapy were observed for IGHV mutation status and presence or absence of del(11q) (Supplemental Figure S1).
Overall survival (OS) was also influenced by lines of therapy; median OS was not reached for patients treated in the first-line or for those treated with 1-2 prior lines and was 67.4 months for ≥3 prior lines. At 5 years, OS rates were higher for patients treated with ibrutinib in earlier lines (first-line: 83%; 1-2 prior lines: 72%; ≥3 prior lines: 58%) (). OS for patients with composite high-risk prognostic features was also improved when patients were treated with ibrutinib in earlier lines of therapy ().
At the time of analysis, 58% of patients treated in the first-line setting (RESONATE-2) remained on ibrutinib treatment (Supplemental Table SII). At the time of study closure for relapsed/refractory patients (RESONATE), 38% of patients with 1-2 prior and 18% of patients with ≥3 prior lines remained on ibrutinib treatment. Consistent with observed PFS, discontinuation due to progressive disease was uncommon for patients treated in the first-line (6%) compared with patients in the relapsed/refractory groups (1-2 prior lines: 22%; ≥3 prior lines: 37%). Most commonly reported adverse events (AEs) with ibrutinib in the first-line and relapsed/refractory setting have been previously published [Citation7,Citation8]. AEs leading to ibrutinib discontinuations and dose reductions occurring in >1 patient across all subgroups are described in Supplemental Table SIII. Discontinuations and dose reductions due to neutropenia occurred more frequently in patients with more prior lines of therapy, possibly as a consequence of cumulative toxicity from prior myelosuppressive regimens. Discontinuations and dose reductions due to atrial fibrillation or infections were low. No patients discontinued ibrutinib or needed dose reductions due to hypertension of any grade; grade ≥3 hypertension occurred in 9% of the total population.
Together, these data support the earlier use of ibrutinib, especially in the first-line setting where superior efficacy to chemoimmunotherapy has been demonstrated in multiple phase 3 studies [Citation4–6]. While lead-time in disease course and treatments received prior to ibrutinib cannot be fully accounted for, long-term data from this combined analysis demonstrated that ibrutinib was effective across all lines of therapy, and remissions were deeper and longer for patients treated in earlier lines of therapy. The longer remissions in previously untreated patients correlate with lower rates of BTK mutations, PLCG2 mutations, and co-occurrence of BTK and PLCG2 mutations compared with R/R patients with similar follow-up [Citation10]. This finding of improved efficacy in earlier-lines of treatment is not unexpected and furthermore provides evidence against strategies of “saving” effective therapies like ibrutinib for subsequent treatments.
Discontinuations due to AEs were not meaningfully different across lines of therapy, and discontinuations due to progressive disease were infrequent in patients treated in the first-line setting. This is reassuring when taken together with other results, including real-world data, which have shown that survival outcomes are better for patients who discontinued treatment due to AEs compared to patients who discontinued due to progressive disease [Citation11–13]. Of patients who discontinued ibrutinib in RESONATE-2 and RESONATE, 78% (7/9) and 37% (10/27), respectively, reported a response to next-line treatment [Citation7,Citation8]. Next-line treatment regimens received included standard chemoimmunotherapy, chemotherapy, or novel agents [Citation7,Citation8]. With additional novel treatment options (e.g. venetoclax) and combinations becoming available, further studies of treatment sequencing will be necessary to better understand optimal approaches.
Overall, these data continue to support the use of ibrutinib across all lines of therapy and illustrate the importance of first-line ibrutinib, where the long-term benefits are greatest compared to later lines of therapy.
Disclosures statement
JW: consultancy/advisory role with AbbVie, Arqule, AstraZeneca, BeiGene, Janssen, and Pharmacyclics LLC, an AbbVie Company; research funding from AbbVie, Karyopharm, Loxo Oncology, Morphosys, and Verastem; AT: honoraria from and speakers’ bureau for AbbVie, AstraZeneca, BeiGene, and Janssen; TM: honoraria from AbbVie, AstraZeneca, Gilead, Janssen, and Novartis; consultancy/advisory role with Morphosys and Sunesis; travel expenses from AbbVie, Gilead, and Janssen; TS: consultancy/advisory role with AstraZeneca, BeiGene, Celgene, Juno Therapeutics, and Kite Pharma; research funding to institution from AstraZeneca, BeiGene, Celgene, Juno Therapeutics, Kite Pharma, Oncternal, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; speakers' bureau for AstraZeneca, Janssen, and Pharmacyclics LLC, an AbbVie Company; PH: consultancy/advisory role with AbbVie, AstraZeneca, and Janssen; research funding from AbbVie, AstraZeneca, Gilead, Janssen, Novartis/GSK, Pharmacyclics LLC, an AbbVie Company, and Roche; speakers’ bureau for AbbVie AstraZeneca, Janssen, and Roche; travel expenses from AbbVie and Janssen; JCB: stock ownership in Vincerx; consultancy/advisory role with AstraZeneca, Janssen, Kura, Novartis, and Syndax; research funding from Pharmacyclics LLC, an AbbVie Company, and Zencor; patents, royalties, or other intellectual property with The Ohio State University; travel expenses from Gilead, Janssen, Novartis, Pharmacyclics LLC, an AbbVie Company, and TG Therapeutics; PG: honoraria from and consultancy/advisory role with AbbVie, Acerta/AstraZeneca, Adaptive, ArQule/MSD, BeiGene, Celgene/Juno Therapeutics/BMS, Gilead, Janssen, Lilly/Loxo, and Roche; research funding from AbbVie, AstraZeneca, Gilead, Janssen, Novartis, and Sunesis; SPM: honoraria from and speakers’ bureau for AbbVie, AstraZeneca, and Janssen; SD: employment with Pharmacyclics LLC, an AbbVie Company, and Horizon Therapeutics; stock ownership in AbbVie, Bristol Myers Squibb, Exelixis, Gilead, GlaxoSmithKline, Horizon Therapeutics, Myovant Sciences, and Revance Therapeutics; CIA: employment with Pharmacyclics LLC, an AbbVie Company, and Eisai Inc.; stock ownership in AbbVie; JPD: employment with Pharmacyclics LLC, an AbbVie Company; stock ownership in AbbVie; SMO: consultancy/advisory role with AbbVie, Alexion, Amgen, Aptose Biosciences Inc., Astellas, Autolus, Bristol Myers Squibb, Celgene, Eisai, Gilead, GlaxoSmithKline, Janssen Oncology, Johnson and Johnson, Juno Therapeutics, Merck, NOVA Research Company, Pfizer, Pharmacyclics LLC, an AbbVie Company, Sunesis, TG Therapeutics, Vaniam Group LLC, Verastem, and Vida Ventures; research funding from Acerta, Caribou, Gilead, Kite, Pfizer, Pharmacyclics LLC, an AbbVie Company, Regeneron, Sunesis, and TG Therapeutics; PMB: consultancy/advisory role with AbbVie, AstraZeneca, Celgene, Genentech, Gilead, Janssen, Merck, Morphosys, Pharmacyclics LLC, an AbbVie Company, Seattle Genetics, and TG Therapeutics.
GLAL-2021-0357-File004.docx
Download MS Word (273.8 KB)Acknowledgments
The authors thank the patients who participated in this study, and their supportive families, as well as the investigators, sub-investigators, and coordinators at each of the study sites. The study was supported by Pharmacyclics LLC, an AbbVie Company (Sunnyvale, CA, USA). Editorial support was provided by Emily Chastain, PhD, and was funded by Pharmacyclics LLC, an AbbVie Company.
Data availability statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Additional information
Funding
References
- IMBRUVICA® (ibrutinib) [prescribing information]. Sunnyvale (CA): Pharmacyclics LLC; 2020. [cited 2020 Dec 7]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/205552s031,210563s007lbl.pdf.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437.
- Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223.
- Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56.
- Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443.
- Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528.
- Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–798.
- Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–1363.
- O'Brien SM, Byrd JC, Hillmen P, et al. Outcomes with ibrutinib by line of therapy and post-ibrutinib discontinuation in patients with chronic lymphocytic leukemia: phase 3 analysis. Am J Hematol. 2019;94(5):554–562.
- Wiestner A, Ghia P, Byrd JC, et al. Rarity of B-cell receptor pathway mutations in progression-free patients with chronic lymphocytic leukemia (CLL) during first-line versus relapsed/refractory (R/R) treatment with ibrutinib. Blood. 2020;136(Supplement 1):32–33.
- Williams AM, Baran AM, Casulo C, et al. Ibrutinib dose adherence and therapeutic efficacy in non-Hodgkin lymphoma: a single-center experience. Clin Lymphoma Myeloma Leuk. 2019;19(1):41–47.
- Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–879.
- Jain P, Thompson PA, Keating M, et al. Long-term outcomes for patients with chronic lymphocytic leukemia who discontinue ibrutinib. Cancer. 2017;123(12):2268–2273.
