Abstract
The role of CNS involvement detected by flow cytometry (FCM) in patients with acute lymphoblastic leukemia has been discussed previously; however, its impact on survival has not been described enough. We analyzed a retrospective cohort of newly diagnosed ALL adult patients who had a cerebrospinal fluid (CSF) analysis by FCM and conventional cytology. We evaluated 81 patients; 19 (23.4%) were only positive by FCM, five (6.3%) were double-positive (DP) and 57 (70.4%) were double-negative (DN). The detection of CNS involvement was increased from 6% to 24%, employing FCM; In our final analysis, patients with FCM + had a lower survival of 7.01 months [95% CI (5.90–8.24)], compared with 11.71 months [IC95% (9.49–13.94)] in the DN group (p = 0.03).
Main text introduction
Acute lymphoblastic leukemia (ALL) is associated with central nervous system (CNS) infiltration in 3 − 7% of patients at diagnosis, and before CNS prophylaxis, 50% of pediatric patients relapsed with CNS involvement. This leads to a greater risk of developing chemoresistance, early relapse, and a shorter overall survival with neurologic damage that may be progressive and fatal. The use of CNS prophylaxis has been associated with better outcomes in pediatric, AYA, and adult patients [Citation1–3].
Risk factors associated with CNS infiltration in adults include T cell immunophenotype, high WBC count at presentation, Ph-positive, and a high LDH value [Citation1,Citation4]. The specific mechanisms and molecules leading to CNS disease in ALL remain unknown.
The gold standard for the detection of CNS involvement at diagnosis is cerebrospinal fluid (CSF) cytology, as described in most leukemia guidelines [Citation3,Citation4]. The presence of more than 5 leukocytes/μL or greater, or the presence of lymphoblasts in CSF, has a reported sensitivity and specificity of 28% and 100%, respectively [Citation1–3]. This low sensitivity is associated with an increased risk of false-negative results, mainly resulting from low CSF cellularity and inter-observer variation, when distinguishing normal and pathologic cells. Currently, it has been proposed that a group of patients might have undetected CSF involvement due to the actual low sensitivity methods. Flow cytometry can detect 10−4 phenotypically abnormal cells, and plays a pivotal role in determining bone marrow MRD when assessing the induction response. Several recently published studies on the detection of CNS disease in ALL suggest that CSF flow cytometry (FCM) is 50% more sensitive than standard cytology, thus establishing FCM as a complementary option in the timely detection of CNS involvement [Citation5–7].
CNS involvement detected by flow cytometry, but not by cytology, appears to reflect ‘occult central nervous infiltration’, but its clinical significance in terms of outcome is still a matter of debate, and its routine use is not recommended in current guidelines [Citation2].
Treatment of patients with CNS involvement includes intrathecal chemotherapy and/or radiotherapy, so the early identification of patients with CNS infiltration by flow cytometry may certainly impact the diseasés prognosis.
Our study’s aim is to evaluate the prognostic impact of CNS infiltration by flow cytometry in adult patients with recently diagnosed ALL.
Materials and methods
Population selection
This is a retrospective cohort study conducted in a reference oncologic center in Mexico City, between January 2017 and December 2019. Inclusion criteria: newly diagnosed ALL, age above 16 years, and with a CSF analysis by cytology and flow cytometry at diagnosis. Exclusion criteria: newly diagnosed patients that were initially treated elsewhere, and relapse patients. The primary endpoint was the impact of CNS disease detected by FCM on overall survival.
Cerebrospinal fluid (CSF) analysis
Fresh CSF samples were obtained and processed at the time of diagnosis without preservative agents. The cytopathology department prepared the samples for cytology analysis and reported positive cytology as CNS infiltration. FCM analysis was conducted in a FACS Canto II Cytometer (BD Systems), and analyzed by Infinicyte Software version 2.0 (Cytognos S.L., Salamanca, Spain). For a sample to be acceptable for analysis, it required at least 10 pathologic events. Total pathologic events per sample and sample viability were analyzed a posteriori.
Variable definitions
FCM (FCM+) patients were defined as having at least 10 phenotypically pathologic events assessed with flow cytometry but with negative cytology; Double positive (DP) cases referred to patients with FCM (+) and positive cytology (Cyt+) – defined as CNS-2 or CNS-3–. Patients with only a positive FCM but negative cytology were excluded from this group. Patients were considered double-negative (DN) when neither FCM nor cytology demonstrated the presence of disease. Cases with traumatic taps were included in the analysis if the sample could provide valid cytology and FCM results.
Outcome definitions
A Complete Response (CR) was established once the patient had less than 5% blasts in the bone marrow smear, and no evidence of extramedullary disease; hematological recovery was defined as >1 × 103 neutrophils/mm3, >100 × 109 platelets/mm3, and with no transfusion requirements. Induction-related mortality (IRM) was defined as any death occurring after day 1 of induction therapy and before the next cycle. We considered that a patient´s case was refractory if CR was not achieved after 2 cycles of induction chemotherapy. Relapse was defined as the presence of >5% blasts in the bone marrow or extramedullary disease at any point after CR. We defined MRD-1 as < 0.1% pathologic events by FCM after induction, and MRD-2 as < 0.01% pathologic events by FCM at week 16; overall Survival (OS) refers to the time, in months, from diagnosis until patient death or final follow-up. Disease-free survival (DFS) refers to the time, in months, from complete remission (CR) to relapse, death, or last follow-up.
Results
Between January 2017 and December 2019, 92 patients were screened but only 81 could be evaluated due to missing data. The Median age was 28 years (16–70), 82.7% were considered AYA, and the distribution between males and females was roughly equal across the cohort (51% vs. 49%); 91% (74) had a B-cell phenotype, 44% (34) expressed CD20, and 24% (20) had leukocytosis (>30,000 cells × 103/mm3 in B ALL and over 100,000 cells × 103/mm3 for T ALL).
In terms of CNS disease at diagnosis, 30% of patients had associated infiltrative disease, 24% (19) were exclusively detected by FCM, and 6% (5) were detected by cytology and by flow (double-positive). Fifty-eight percent (58%) (36) of the analyzed karyotypes suggested an unfavorable prognosis. The male gender was associated with positive flow cytometry (77% males, vs. 23% females, p = 0.01). A high leucocyte count, a high LDH level, certain immunophenotype markers, cytogenetic risk, comorbidities, and T cell leukemia were not associated with CNS involvement by FCM. Baseline characteristics are shown in .
Table 1. Baseline characteristics.
Median analyzed events:14,733 (5,025–50,000) with a median sample viability of 38.5% (22–61.75). Forty-five percent (45%) of patients with CNS involvement detected with either technique had neurological symptoms, headache being the most common.
BFM was the most frequently administered chemotherapy regimen in 46 (58.2%) patients, followed by HyperCVAD in 28 (35.4%), treatment was given according to local protocols and the patient’s economic capacity. Patients with documented CNS involvement by FCM or cytology were treated with triple intrathecal chemotherapy two times a week until blast clearance in CSF by both techniques.
Outcomes
In the entire cohort, complete remission (CR) after induction was achieved in 53 (84.1%) patients, and 40 (64.5%) had the undetectable minimal residual disease (MRD) by FCM in the bone marrow sample. Ten (10) (15.4%) patients were refractory to treatment .
Table 2. Outcomes.
There were no differences in terms of CR (75% vs. 88.6%, p = 0.31), refractory disease (25% vs. 10%, p = 0.27) or disease relapse (53.3% vs. 44%, p = 0.81) between FCM + and DN groups. MRD-1 and MRD-2, as well as death, were more favorable in the DN group than in FCM+; however, the sample was not sufficiently large to reach a statistical difference.
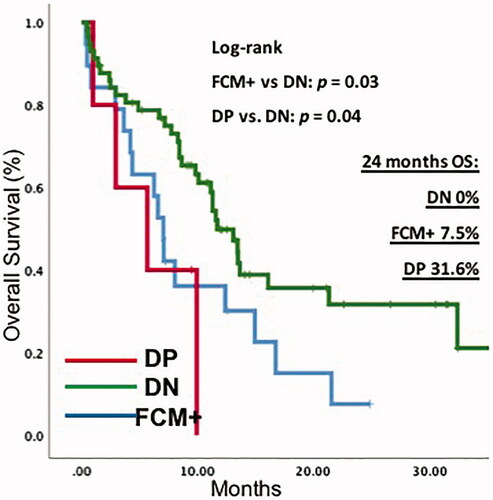
The median OS for the entire cohort was 11.3 months. According to the diagnostic method of CNS involvement, in the FCM + patients, the median OS was 7.07 months (95% CI 5.90–8.24) and 11.7 months (95% CI9.49–13.94) in DN patients; p = 0.03. There was no significant difference in OS between FCM + and the DP group (7.07 vs. 5.69 months; 95% CI 5.90–8.24 and 95% CI 0.00–11.55; p = 0.52).
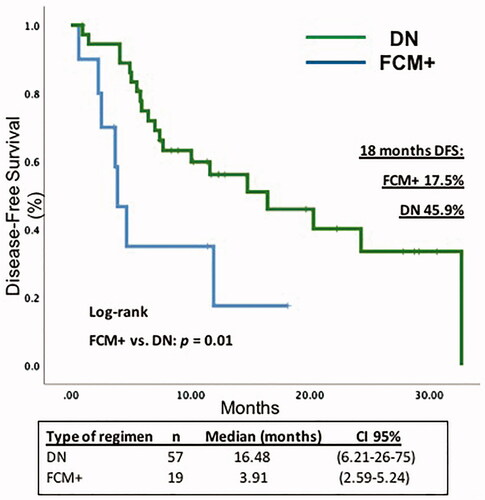
When comparing positive flow cytometry patients (FCM+), versus DN patients, the median DFS in FCM + patients was 3.91 months [95% CI (2.59–5.24)] vs. 16.48 months [95% CI 6.21–26.75] in the DN group. In the Cox regression model, the HR for survival in patients with FCM + was 1.85 (CI 1.004–3.421; p = 0.04) ( and ).
Discussion
Central nervous system involvement in acute lymphoblastic leukemia is detected by cytology in 5–10% of newly diagnosed patients. A positive test reflects a dire prognosis in terms of survival, relapse, and resistance to current chemotherapy regimens. According to most international guidelines, CSF cytology remains the ‘gold standard’ to establish the presence of CNS infiltration. However, cytology relies solely on the observer’s experience and ability to distinguish pathological cells from normal lymphocytes. In the past, the usefulness of flow cytometry had been debated in terms of whether its sensitivity was superior in the detection of early infiltration and whether its use could potentially impact patient prognosis and management [Citation1–4,Citation8–15]. In this observational study conducted with data obtained in a reference center in Mexico, we analyzed the impact of central nervous system infiltration detected by flow cytometry, on overall survival and event-free survival.
Currently, flow cytometry plays a critical role as an early marker of disease aggressiveness and high-risk relapse by minimal residual disease due to its sensitivity in the rapid detection of very small traces of disease in fresh samples [Citation5,Citation6]. Few studies have also aimed to describe its role in the detection of leukemic blasts in cerebrospinal fluid. Many studies have proposed its usefulness given the low sensitivity of cytology which is unable to detect infiltration with low cell counts since it relies solely on the observer’s experience and training in distinguishing pathological cells from normal lymphocytes [Citation6,Citation16–20].
Some markers as LDH, initial leukocyte count, immunophenotype markers, cytogenetics, and the presence of extramedullary involvement have been proposed before as high-risk associations related to CNS involvement [Citation1,Citation2,Citation21–23]. However, in our study, only the male gender was statistically associated with occult infiltration (FMC+ 77.8%; p = 0.01).
The detection of CNS involvement in our study increased from 6% to 30% when using FCM in comparison with cytology; similar findings have been reported previously and were directly associated with CNS relapse [Citation13,Citation20]. This has been described not only in patients with acute lymphoblastic leukemia but also in patients with other hematological malignancies. In a few reports that analyzed patients with non-Hodgkin lymphoma, leptomeningeal involvement was detected by cytometry in 22% of cases as opposed to 8% that were detected by cytology [Citation8,Citation24–29], which strongly correlates with our results. Mitri et al. [Citation21] found that flow cytometry reached a sensitivity and specificity of 100% in the detection of leukemic blasts in the leptomeningeal space. This suggests the possibility of standardizing flow cytometry as the initial method to detect CNS infiltration when approaching recently diagnosed acute lymphoblastic leukemia patients.
Central nervous system infiltration detected by flow cytometry in children is a poor prognostic factor regardless of the moment of detection, at diagnosis or consolidation. Del Príncipe et al. [Citation2], analyzed an Italian population from 13 centers and reported that not only did flow cytometry detect the presence of CNS involvement more frequently than cytology (43 vs. 18 patients), but it was also an important marker unfavorably impacting prognosis; another study with adults on Hyper-CVAD chemotherapy, demonstrated that positive flow cytometry was a marker of relapse (hazard ratio 4.8, 95% confidence interval [CI] 1.2–20, p = 0.031). The estimated three-year cumulative incidence of CNS relapse in CSF + was 15% vs. 3.3% for CSF–; however, OS was not discussed [Citation2,Citation7,Citation13,Citation30,Citation31]. This is the first study in a Latin–American reference center that explores previous findings in adults with similar results as those herein presented. According to our results, it would be most relevant to conduct prospective trials with intensified therapies, including the possibility of performing allogeneic stem cell transplants in these high-risk patients, since directed treatment (intrathecal therapy or radiotherapy) does not appear to modify the adverse outcome [Citation2].
This study is clinically important in terms of ‘occult’ central nervous system infiltration detected in acute lymphoblastic leukemia, which directly impacts overall survival and progression-free survival, decreasing it from eleven to six months and from 16.4 to 4.6 months respectively.
The treatment of patients that relapse or have newly diagnosed ALL with CNS involvement is a challenge, regardless of the geographic location, socio-economic factors, or the proximity to an experienced reference center. Different strategies have been developed including radiotherapy, specific neurotrophic chemotherapy (cytarabine or methotrexate), intrathecal chemotherapy, or any combination of the above, and no standard of care has been clearly established [Citation10,Citation11]. Even newer strategies such as monoclonal antibodies or CART-cells have yielded poor results in these groups of patients [Citation32–39]. In this retrospective study, we did not analyze whether any strategy led to superior results; however, the number of patients with ‘occult’ involvement suggests that perhaps a more CNS focused strategy might have an effect on these patients if detected at diagnosis, and who with other methods might be easily misclassified as low-risk [Citation24].
Few mechanisms have attempted to explain why some patients relapse only in the CNS, and others never develop CNS infiltration. Although studied in childhood leukemia, this cellular behavior can be translated to adults. Some other cells, such as natural killer cells, adhesion molecules, chemotactic and growth factors might play a role in this selectivity, and may perhaps become a treatment target. However, these molecular markers have not been evaluated in a clinical setting, and their measurement is far from feasible [Citation8,Citation16,Citation40–43]
According to our results, the development of prospective trials with intensified therapies, including the possibility of performing allogeneic stem cell transplants in these high-risk cases, appears to be relevant.
The main limitations of our study, other than its retrospective nature, are the fact that it was conducted in a single-center, the general outcomes of the whole population are inferior to those obtained in other centers across the world, and there is no standardized method to classify traumatic punctures in equivocal samples.
In conclusion, flow cytometry should be considered in future prospective trials based on several publications that prove its impact on patient outcome; however, we must be careful with how the leukemic bulk is quantified, and cytometer markers must be standardized.
Authors’ contribution
Rosana Córdova-Serrano: Investigation, Writing – Original Draft. Almanza-Huante Emmanuel: Investigation, Writing – Original Draft. Hernández-Alcántara Areli: Flow cytometry analysis. Fernández-Sánchez Emmanuel: Data curation, Writing – Review and Editing, Supervision. Espinosa-Bautista Karla: Data curation, Writing – Review and Editing, Supervision.
Acknowledgments
We gratefully acknowledge the contributions of every resident, laboratory technician, and Associate Professor in the Department of Hematology at the Instituto Nacional de Cancerología.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Reman O, Pigneux A, Huguet F, et al. Central nervous system involvement in adult acute lymphoblastic leukemia (ALL) at diagnosis and/or at first relapse: results from the GET-LALA group. Blood. 2007;110(11):4326–4326.
- Del Principe MI, Buzzatti E, Piciocchi A, et al. Clinical significance of occult central nervous system disease in adult acute lymphoblastic leukemia. A multicenter report from the campus all network. Haematol. 2019;106(1):39–45.
- Hoelzer D, Bassan R, Dombret H. Acute lymphoblastic leukemia in adult patients: ESMO clinical practice guidelines for diagnostis, treatment and follow up. Ann Oncol. 2016;27(suppl 5):v69–v82.
- Brown P, Shah B, Advani A, et al. 2020. Acute lymphoblastic leukemia. NCCN Guidelines version 2.2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- Tawfik B, Brown L, Fuda F, et al. Utility and proposed algorithm of CSF flow cytometry in hematologic malignancies. Ann Hematol. 2018;97(9):1707–1716.
- Jaime-Pérez J, Borrego-López M, Jiménez-Castillo R, et al. Comparison of conventional cytomorphology, flow cytometry immunophenotyping, and automated cell counting of CSF for detection of CNS involvement in acute lymphoblastic leukemia. Int J Lab Hematol. 2018;40(2):169–174.
- Subirá D, Castañón S, Román A, et al. Flow cytometry and the study of central nervous disease in patients with acute leukaemia. Br J Haematol. 2001;112(2):381–384.
- Lenk L, Alsadeq A, Schewe DM. Involvement of the central nervous system in acute lymphoblastic leukemia: opinions on molecular mechanisms and clinical implications based on recent data. Cancer Metastasis Rev. 2020;39(1):173–187.
- Levinsen M, Taskinen M, Abrahamsson J, et al. Clinical features and early treatment response of central nervous system involvement in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(8):1416–1421.
- Larson RA. Managing CNS disease in adults with acute lymphoblastic leukemia. Leuk Lymphoma. 2018;59(1):3–13.
- Cancela P, Murao M, Assumpção J, et al. Immunophenotyping of the cerebrospinal fluid as a prognostic factor at diagnosis of acute lymphoblastic leukemia in children and adolescents. Pediatr Hematol Oncol. 2017;34(2):53–65.
- Frishman-Levy L, Izraeli S. Advances in understanding the pathogenesis of CNS acute lymphoblastic leukaemia and potential for therapy. Br J Haematol. 2017;176(2):157–167.
- Garcia K, Stevenson P, Cherian S, et al. Multiparameter flow cytometry (MFC) of cerebrospinal fluid (CSF) from adults receiving hypercvad for acute lymphoblastic leukemia/lymphoma (ALL) identifies patients at higher risk of central nervous system (CNS) relapse: a single-center retrospective analysis. Blood. 2020;136(Supplement 1):28–30.
- Yang W, Xiaojuan Y, Zhang C, et al. The correlation studies between central nervous system involvement by flow cytometry and the prognosis of children acute lymphoblastic leukemia: a single chinese center's experience by Cclg- ALL. Blood. 2017;130(Supplement 1):1293.
- Baljevic M, Kantarjian H, Thomas D, et al. Incidence and outcome in adults with acute lymphoblastic leukemia with primary central nervous system involvement. Blood. 2013;122(21):2639–2639.
- Levinsen M, Marquart HV, Groth-Pedersen L, et al. Leukemic blasts are present at low levels in spinal fluid in one-third of childhood acute lymphoblastic leukemia cases. Pediatr Blood Cancer. 2016;63(11):1935–1942.
- Rassi F, Mitri Z, Heffner L, et al. Cerebrospinal fluid flow cytometry in acute lymphoblastic leukemia/lymphoma: is it beneficial? Blood. 2012;120(21):2576.
- Cerello Chapchap E, Feres C, Cameirão Bento L, et al. Central nervous system (CNS) infiltration assessment using cerebrospinal fluid flow cytometry in hematologic malignancies: a single center retrospective analysis of clinical outcomes. Blood. 2019;134(Supplement_1):5773.
- Nagafuji K, Miyamoto T, Eto T, et al. Minimal residual disease (MRD) status after induction therapy is a strong prognostic factor in the treatment of adult Ph (-) acute lymphoblastic leukemia (ALL): results of a prospective study (ALL MRD2008 study). Blood. 2018;132(Supplement 1):3970.
- Thastrup V, Marquart H, Levinsen M, et al. Central nervous system involvement detected by flow cytometry is a risk factor for relapse in childhood acute lymphoblastic leukemia. Blood. 2018;132(Supplement 1):657–657.
- Mitri Z, Siddiqui MT, El Rassi F, et al. Sensitivity and specificity of cerebrospinal fluid flow cytometry for the diagnosis of leukemic meningitis in acute lymphoblastic leukemia/lymphoma. Leuk Lymphoma. 2014;55(7):1498–1500.
- Tang J, Yu J, Cai J, et al. Prognostic factors for CNS control in children with acute lymphoblastic leukemia treated without cranial irradiation. Blood. 2021; 2020010438.
- Beldjord K, Chevret S, Asnafi V, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739–3749.
- Quijano S, López AM, Sancho J, et al. Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin’s lymphoma: improved sensitivity of flow cytometry. J Clin Oncol. 2009;27(9):1462–1469.
- Wilson W, Bromberg J, Stetler-Stevenson M, et al. Detection and outcome of occult leptomeningeal disease in diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2014;99(7):1228–1235.
- Muslimani A, Kizilbash S, Jaiyesimi I, et al. Evaluation of the diagnostic utility of cerebral spinal fluid (CSF) flow cytometry (FC) in detection of central nervous system (CNS) involvement by hematological malignancy. Blood. 2010;116(21):3837.
- Münch V, Trentin L, Herzig J, et al. Central nervous system involvement in acute lymphoblastic leukemia is mediated by vascular endothelial growth factor. Blood. 2017;130(5):643–654.
- Del Principe M, Gatti A, Johansson U, et al. ESCCA/ISCCA protocol for the analysis of cerebrospinal fluid by multiparametric flow-cytometry in hematological malignancies. Cytometry B Clin Cytom. 2021;100(3):269–281.
- Pui CH, Thiel E. Central nervous system disease in hematologic malignancies: historical perspective and practical applications. Semin Oncol. 2009;36(4 Suppl 2):S2–S16.
- Sancho JM, Ribera JM, Oriol A, et al. Central nervous system recurrence in adult patients with acute lymphoblastic leukemia: frequency and prognosis in 467 patients without cranial irradiation for prophylaxis. Cancer. 2006;106(12):2540–2546.
- Del Principe M, Buzzati E, Forghieri F, et al. Clinical significance of occult central nervous system disease in adult ACUTE lymphoblastic leukemia. a multicenter, report from the campus ALL/Gimema network. Blood. 2018;132(Supplement 1):658.
- Alfayez M, Kantarjian H, Short N, et al. Safety and efficacy of blinatumomab in patients with central nervous system (CNS) disease: a single institution experience. Blood. 2018;132(Supplement 1):2702–2702.
- DeVita VT, Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–8653.
- Pehlivan KC, Duncan BB, Lee DW. CAR-T cell therapy for acute lymphoblastic leukemia: transforming the treatment of relapsed and refractory disease. Curr Hematol Malig Rep. 2018;13(5):396–406.
- Zhou F, Wen Y, Jin R, et al. New attempts for central nervous infiltration of pediatric acute lymphoblastic leukemia. Cancer Metastasis Rev. 2019;38(4):657–671.
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847.
- Aldoss I, Song J, Stiller T, et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017;92(9):858–865.
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448.
- Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. New Eng J Med. 2019;378(5):449–459.
- Gaudichon J, Jakobczyk H, Debaize L, et al. Mechanisms of extramedullary relapse in acute lymphoblastic leukemia: reconciling biological concepts and clinical issues. Blood Rev. 2019;36:40–56.
- Muench V, Trentin L, Herzig J, et al. Migration of acute lymphoblastic leukemia cells into the central nervous system is regulated by VEGF. Blood. 2015;126(23):2634–2634.
- Ramirez M, Gomez A, Martinez C, et al. Chemokines in leukemic infiltration of the central nervous system in childhood acute lymphoblastic leukemia. Blood. 2009;114(22):1627–1627.
- Frishman-Levy L, Shemesh A, Bar-Sinai A, et al. Central nervous system acute lymphoblastic leukemia: role of natural killer cells. Blood. 2015;125(22):3420–3431.