Abstract
B cell-derived lymphoproliferative disorders are associated with secondary immunodeficiency (SID); some patients require immunoglobulin replacement therapy (IgRT) to mitigate infections. Using IQVIA’s PharMetrics® Plus database, patients with SID who received IgPro10/IgPro20 in the 12 months post-diagnosis (IgRT users) were matched to patients with SID not receiving IgRT (non-IgRT users). The risk of severe infection was compared using within-patient change from baseline to follow-up as well as between cohorts. Overall, 277 IgRT users were matched to 1019 non-IgRT users. Before IgRT, more IgRT users experienced any bacterial infection (88.4% vs. 72.9%; p<.0001) or ≥1 severe bacterial infection (SBI) (42.2% vs. 31.8%; p=.0011) vs. non-IgRT users. During follow-up, risk of SBI among IgRT users (21.7%) reached parity with non-IgRT users (21.2%). IgRT was associated with a reduction in SBIs to levels comparable with the lower ‘baseline infection risk’ of non-IgRT users. These criteria help define SID patients who may benefit from IgRT.
Introduction
Secondary immunodeficiency (SID) refers to a compromised immune system by non-hereditary factors, including certain hematological malignancies, medications (notably, B-cell depleting or T-cell modulating therapies), solid organ or hematopoietic stem cell transplantation, radiation, protein-losing conditions, infections such as HIV, malnutrition, and age-related immunosenescence [Citation1]. One form of SID is hypogammaglobulinemia (HGG), which most commonly manifests as recurrent and/or severe respiratory tract infections, and may also be characterized by poor response to vaccines [Citation2].
B cell-derived lymphoproliferative disorders, such as chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and non-Hodgkin lymphoma (NHL), are frequently associated with SID-HGG, owing to both the impact of malignant lymphocytes and the fact that lymphocyte-targeting treatment of the malignancies can impact the capacity to generate antibodies [Citation3–6]. CLL, MM, and NHL affect 1.19 (95% confidence interval (CI): 1.12–1.25), 2 (95% CI: 1.84–2.26), and 6.39 (95% CI: 6.27–6.50) people in every 100,000 globally, respectively [Citation7]. HGG affects up to 85% of CLL patients [Citation3,Citation8], 52–90% of MM patients [Citation9], and 15–38% of NHL patients [Citation5]. The resulting infections can be life-threatening; in CLL, MM, and NHL, the attributable mortality rate from infections is approximately 50%, 22%, and 33%, respectively [Citation8,Citation10–12].
Given the prevalence of SID-HGG (herein referred to as ‘SID’) in these B-cell lymphoproliferative disorders, and the significant potential for associated morbidity and mortality, preventative measures would be expected to improve quality of life and overall outcomes for these patients. Immunoglobulin replacement therapy (IgRT) has been previously shown to mitigate the infection-associated complications of HGG, in both patients with inborn errors of immunity (where it is, in fact, standard of care), as well as in SID [Citation10,Citation13,Citation14]. Despite this evidence of benefit, a scoping analysis of various national guidelines demonstrated that they rarely recommend IgRT as first-line therapy in patients with newly diagnosed SID; rather, they include various criteria to guide clinicians on which patients are eligible for IgRT including: history of HGG, recurring or persistent infections, severe infections and/or progressive disease. Some guidelines and consensus statements include a failure of antibiotic therapy or conjugate pneumococcal immunization as pre-requisites for receipt of IgRT treatment [Citation10,Citation13,Citation15–20]. In the US specifically, the American Academy of Allergy, Asthma and Immunology (AAAAI) guidelines recommend recurrent severe bacterial infections (SBIs) as part of the pre-requisite for IgRT in CLL and MM patients [Citation19].
The real-world consequences of guideline recommendations have not been well characterized, other than in selected European countries [Citation21]. Notably, such data from the US are lacking. However, information on the use and clinical impact of IgRT policies, and on patient-specific factors would be invaluable in optimizing clinical advice, guidelines, healthcare resource utilization, and medical care.
This retrospective US claims database analysis was undertaken aimed to (a) describe characteristics of patients treated with IgRT (IgPro10 (Privigen®) or IgPro20 (Hizentra®); CSL Behring, King of Prussia, PA) and compare them with those of patients who did not receive IgRT and (b) evaluate the impact of IgPro10/IgPro20 in terms of reducing the odds of SBIs in patients with hematological malignancies.
Patients and methods
Data sources
A retrospective US database cohort analysis was conducted using IQVIA’s PharMetrics® Plus Database of patients extending from January 2010 to September 2018. Within this database window, adult patients with a SID diagnosis (codes: ICD-9-CM 279.0x (excluding 279.04), 279.3; ICD-10-CM D80.x (excluding D80.0), D84.9) that occurred between January 2011 and September 2017 were identified (first SID diagnosis was established as the ‘diagnosis date’) and stratified by the required underlying malignancy (CLL and/or MM and/or NHL). Patients were excluded if they had a SID diagnosis in the 12 months prior to the diagnosis date, to ensure the identified diagnosis was an incident one or if they had incomplete data or data quality issues. Ethics approval was not required for this type of study.
Study cohorts
Two groups of patients were established. ‘IgRT users’ were patients with a SID diagnosis who received ≥1 claim for IgPro10 or IgPro20 (and no other IgRT) in a 12-month period following the diagnosis date. To ensure comparison of ‘IgRT users’ to a similar severity group, a cohort of ‘non-IgRT users’ were designed to be age-matched (exact age at index date) and cancer subtype-matched patients who did not receive any IgRT (regardless of brand) in the 12 months following the diagnosis date. Each IgRT user was matched with up to five non-IgRT users. The treatment index date was defined as the date of the first IgRT claim for IgRT users. A pseudo-treatment index date was assigned to matched non-IgRT users by taking the hematological cancer diagnosis date and adding the days until the treatment index date for the matched IgRT user, and applying the same process to matched non-IgRT users. Patients were required to have had at least 12 months’ continuous health plan enrollment (CE) pretreatment index or pre-pseudo treatment index date (baseline period) and 12 months’ CE post-treatment index or post-pseudo treatment index date (follow-up period) to be included in the analysis.
Study outcomes
The following outcomes were compared:
Occurrence of SBIs (defined as those requiring intravenous (IV) antibiotics in the outpatient setting or those hospitalized with a bacterial infection diagnosis code (mutually exclusive)) between the IgRT users and non-IgRT users over the follow-up period.
Difference in (a) any bacterial infection and (b) SBIs (mean number) between baseline and follow-up period, for IgRT users vs. non-IgRT users.
Mean number of (a) any bacterial infections and (b) SBIs per patient, between the IgRT users and non-IgRT users over the follow-up period.
Diagnosis codes indicating bacterial/SBIs were identified using the criteria below:
Specific to a bacterial organism.
Specific to an infection that can only be caused by bacteria.
Specific to an infection primarily caused by bacteria (e.g. ear infection).
Specific to an infection that could have been caused by bacterial or non-bacterial pathogens, and bacteria were not considered to be the primary cause but was included for key anatomic sites: sinopulmonary (pneumonia, bronchopneumonia, sinus disease, etc.), skin and subcutaneous tissue, urinary tract infection, septicemia; unless the code specified a non-bacterial cause (like viral pneumonia).
A full list of the diagnostic codes is available upon request.
Statistical analyses: baseline characteristics
For the matched groups, baseline characteristics were compared using bivariate conditional logistic regressions. Conditional logistic regression is the application of a logistic model to each stratum individually, with the coefficients of the predictors conditionally modeled based on being a member of a certain stratum [Citation22–24]. Conditional logistic regression was considered appropriate for these data to ensure that the comparison accounted for the previously matched cases and controls (i.e. 1: many matched patients). Bivariate regression refers to the comparison of cases and controls without adjustment for other covariates, while multivariate regression does control for other covariates.
Statistical analyses: outcomes
To evaluate outcomes across IgRT users and non-IgRT users, the following analyses were conducted. Bivariate conditional logistic regression was conducted to compare occurrence of severe infections (outcome 1), in IgRT users vs. non-IgRT users. Further, multivariate conditional logistic regression models were estimated for the overall cohort and by cancer subtype to adjust outcome 1 for significantly higher occurrence of baseline risk factors (number of prior infections, use of antibiotics, cancer treatments, and hospitalization, among others) in IgRT users compared with non-IgRT users. Outcomes 2 and 3 were compared for the baseline and follow-up periods, as observed, for IgRT users and non-users, overall and by cancer subtype. These outcomes were also estimated with the main IgRT effect adjusted for baseline covariates using a generalized linear regression models (GLMs) approach with Poisson’s distribution and log-link transformation [Citation25,Citation26], and predicted values generated for the number of infections expected in follow-up (outcome 3).
Results
Patient characteristics during baseline period
The final sample comprised 277 IgRT users and 1019 non-IgRT users that were matched by age and cancer subtype. Most patients had CLL or NHL. Although IgRT users and non-IgRT users were not matched by sex, sex distribution was similar in both groups ().
Table 1. Baseline patient characteristics.
Overall, before receiving IgRT, IgRT users in this US study had significantly higher mean healthcare costs in the 12-month baseline period than non-IgRT users ($219,149 vs. $153,800; p<.0001); this trend was also observed for each cancer subtype (CLL: $169,575 vs. $98,668; MM: $331,425 vs. $206,642; NHL: $238,170 vs. $178,019). IgRT users also had a higher mean number of baseline physician office visits (34.5 vs. 27.6; p<.0001) compared with non-IgRT users, and a higher proportion had neutropenia, sinusitis, and/or bronchitis in the baseline period ().
A higher proportion of IgRT users had a serum immunoglobulin G (IgG) test (86.3% vs. 60.6%; p<.0001), antibiotic use (95.7% vs. 87.0%; p<.0001; IV antibiotic use: 38.6% vs. 24.5%; p<.0001), and biologics use (i.e. monoclonal antibodies) for cancer treatment (35.7% vs. 22.8%; p<.0001) in the baseline period compared with non-IgRT users. Across cancer subtypes, use of biologics was higher for the NHL subtype (42.9% of IgRT users and 28.8% of non-IgRT users; p=.0004) and the CLL subtype (39.6% of IgRT users and 22.3% of non-IgRT users; p=.0003) subtypes, compared with the MM subtype (10.0% of IgRT users and 5.8% of non-IgRT users; p=.4714), where the use of targeted therapies overall (including proteasome inhibitors, e.g. bortezomib) was more frequent (52.5% of IgRT users and 50.0% of non-IgRT users; p=.7807) ().
Table 2. Hematologic malignancy treatments by cancer subtype and by IgRT group during the baseline period.
Occurrence of bacterial infections during the baseline period
In the baseline period (i.e. before IgRT use), a higher proportion of IgRT users than non-IgRT users experienced ≥1 bacterial infection (88.4% vs. 72.9%; p<.0001) () and had a higher mean number of bacterial infections (10.3 vs. 5.9; p<.0001) (). A higher proportion of IgRT users also experienced ≥1 SBI compared with non-IgRT users (42.2% vs. 31.8%; p=.0011) () and had a higher mean number of SBIs per patient in the 12-month baseline period (0.5 vs. 0.2; p=.0014) ().
Figure 1. Percentage of patients with bacterial infections during the baseline period, in IgRT or non-IgRT users. *All p<.0001 for IgRT versus non-IgRT users; except for severe BI (p=.0011). BI: bacterial infections; IgRT: immunoglobulin replacement therapy.
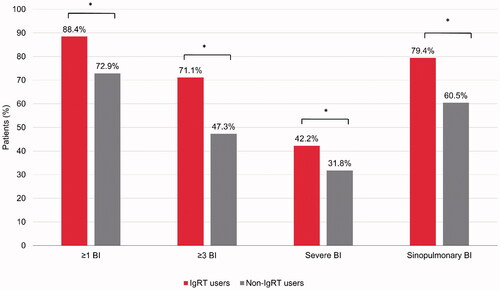
Figure 2. Mean number of bacterial infections per patient, in the 12-month baseline and follow-up periods (observed and predicted) for the overall cohort and cancer-specific subtypes. Follow-up period predicted values were estimated using a GLM approach with a Poisson distribution and log-link transformation. BI: bacterial infections; CLL: chronic lymphocytic leukemia; GLM: generalized linear regression model; IgRT: immunoglobulin replacement therapy; MM: multiple myeloma; NHL: non-Hodgkin lymphoma.
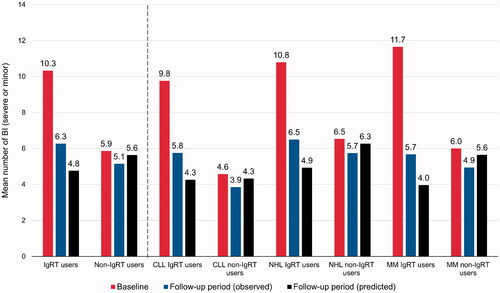
Figure 3. Percentage of patients with ≥1 severe bacterial infection during the baseline and follow-up periods, in IgRT or non-IgRT users. BI: bacterial infection; CLL: chronic lymphocytic leukemia; IgRT: immunoglobulin replacement therapy; MM: multiple myeloma; NHL: non-Hodgkin lymphoma.
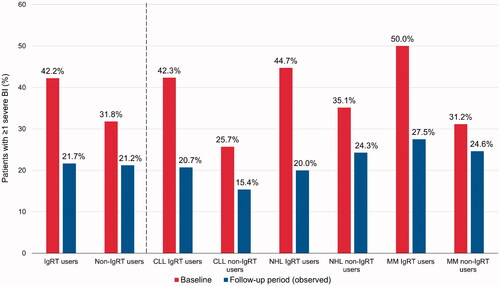
Figure 4. Mean number of severe bacterial infections per patient, in the 12-month baseline and follow-up periods (observed and predicted) for the overall cohort and cancer-specific subtypes. Follow-up period predicted values were estimated using a GLM approach with a Poisson distribution and log-link transformation. The impact of IgRT on severe BI was statistically significant during the follow-up period in the overall cohort and for the NHL and CLL subtypes, but not for the MM subtype, based on evidence from the corresponding GLM models. BI: bacterial infections; CLL: chronic lymphocytic leukemia; GLM: generalized linear regression model; IgRT: immunoglobulin replacement therapy; MM: multiple myeloma; NHL: non-Hodgkin lymphoma.
Comparisons between baseline and follow-up period
In the follow-up period, SBIs were reported in a similar proportion of IgRT users (now on IgRT, dose determined by the clinical care provider) and non-IgRT users (21.7% vs. 21.2%; p=.8667) (). For the first occurring SBI, the most common anatomic site was sinopulmonary (51.7% and 48.6% among 60 IgRT users and 216 non-IgRT users with at least one SBI, respectively; p=.675).
In terms of the mean number of observed total bacterial infections per patient, the difference in change from baseline to observed values at follow-up was significantly greater for IgRT users than non-IgRT users: IgRT users 10.3–6.3, non-IgRT users 5.9–5.1, difference in change: –4.1 vs. −0.7 (p=.0001). Similar observations were made for each cancer subtype (CLL: IgRT users 9.8–5.8, non-IgRT users 4.6–3.9, difference in change: −4.0 vs. −0.7; p=.0140; NHL: IgRT users 10.8–6.5, non-IgRT users 6.5–5.7, difference in change: −4.3 vs. −0.8; p=.0014; MM: IgRT users 11.7–5.7, non-IgRT users 6.0–4.9, difference in change: −6.0 vs. −1.0; p=.0307) ().
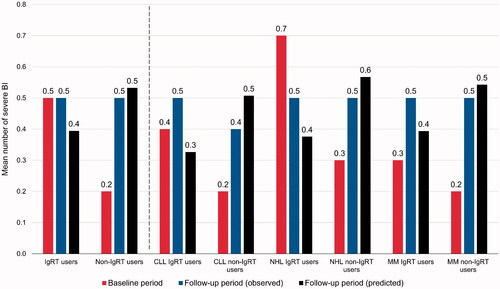
The difference in change from baseline to observed values at follow-up in the mean number of observed SBIs was not statistically significant between IgRT users and non-IgRT users overall (IgRT users 0.5–0.5, non-IgRT users 0.2–0.5, difference in change 0.0 vs. 0.3; p=.0704) or within the CLL subtype (IgRT users 0.4–0.5, non-IgRT users 0.2–0.4, difference in change 0.1 vs. 0.2; p=.6149]) and within the MM subtype (IgRT users 0.3–0.5, non-IgRT users 0.2–0.5, difference in change 0.2 vs. 0.3; p=.5789) (). In the NHL subtype, by contrast, the difference in change in the mean number of SBIs was significantly greater in IgRT users than non-IgRT users (IgRT users 0.7–0.5, non-IgRT users 0.3–0.5, difference in change −0.2 vs. 0.2 change; p=.0173) ().
Healthcare resource utilization for severe bacterial infections
Overall, during the follow-up period, SBIs were treated in the inpatient setting for 86.7% of IgRT users and 85.2% of non-IgRT users. The mean (standard deviation) length of stay for the first severe infection requiring hospitalization was 7.1 (6.0) and 10.8 (13.1) days (p=.0493) among IgRT users and non-IgRT users, respectively.
Multivariate conditional logistic regression model: risk of severe bacterial infections
In the follow-up period, the adjusted odds of SBIs were 31% lower in all IgRT users vs. non-IgRT users (odds ratio (OR)=0.69; p=.0401) (Supplementary Table 1). In the NHL subtype, the odds of having an SBI were 49% lower in IgRT users than IgRT non-users (OR = 0.51; p=.0036). In the CLL (OR = 0.93; p=.8243), and MM (OR = 0.77; p=.5438) subtypes, there was no significant difference between IgRT and non-IgRT users in the adjusted odds of SBIs in the follow-up period.
In the overall cohort, having ≥4 baseline-period bacterial infections (OR = 1.91; p<.0001), more baseline hospitalizations (OR = 1.13; p=.0006), or higher baseline costs (OR = 1.32; p=.0001) were additionally associated with a higher risk of SBIs in the follow-up period. In the CLL subtype, additional risk factors for SBIs in the follow-up period were baseline use of biologics (i.e. monoclonal antibodies) (OR = 0.47; p=.0174) and an emergency room visit during the baseline period (OR = 1.83; p=.0294). In the NHL subtype, baseline diabetes was also a significant risk factor (OR = 1.60; p=.0223). For the MM sub-type, having ≥4 baseline-period bacterial infections (OR = 3.22; p=.0018) was the only significant risk factor.
Generalized linear regression models
As noted, multivariate GLM with Poisson distribution and log-link transformation were used to model predicted number of infections – any level of severity, and severe infections – for each of the IgRT and non-IgRT groups, and for the overall cohort, as well as for the cancer-specific subtype. Results from the GLMs are shown in Supplementary Table 2 (all bacterial infections) and Supplementary Table 3 (SBIs), and associated predicted values in and . As seen, adjusting for a number of baseline risk factors variously identified by the models as statistically significant (p<.05), the IgRT group was associated with a statistically significant reduction in the number of all infections in the overall sample, and in the NHL and MM subtypes. The IgRT group was also associated with a significant reduction in the number of severe infections in the overall group, and in the NHL and CLL subtypes. and confirmed that, after adjusting for baseline covariates, corresponding predicted number of all and severe infections for the follow-up period were in the range of observed values. Moreover, for all infections () predicted values were substantially lower than baseline observed values for the IgRT group, and at generally maintained values for the non-IgRT group.
Discussion
The current study constitutes the largest, comparative, real-world analysis of SID-related bacterial infections, and the impact of IgRT on these infections, in US patients with hematological malignancies. This study demonstrated that, in patients with SID complicating CLL, MM, and/or NHL, the use of IgRT (IgPro10/IgPro20) was associated with significantly greater reductions in the total number of bacterial infections after initiation of treatment, compared with the non-IgRT users in the corresponding period. The reduction in the total number of these infections in IgRT users ranged from 51% in MM patients to 40% in CLL patients and 41% in NHL patients; the level of reduction in the MM subtype in particular was consistent with findings from the literature [Citation27]. In terms of SBIs, IgPro10/IgPro20 use was associated with 31% adjusted risk reduction overall; among the hematological malignancies, the greatest benefit was observed in the NHL subtype, vs. non-IgRT users. In fact, the 49% adjusted risk reduction of SBIs with the IgPro10/IgPro20 group in the NHL subtype would indicate an overall number needed to treat (NNT) of two patients (i.e. one patient would benefit for every two treated). Given that about 90% of SBIs required hospitalization, with length of stays of 7–11 days and costs over $30,000 per hospitalization, it is expected that IgRT would translate also to some direct cost savings, in addition to reduction in patient morbidity and potential attributable mortality. When interpreting our results, it must be noted that non-IgRT users may have received other interventions (e.g. antibiotics) to manage infections. Furthermore, IgRT could be adjuvant to other interventions as well; therefore, these findings reflect IgRT risk reductions relative to the non-IgRT standard of care.
Our study highlights that IgRT treatment with IgPro10/IgPro20 was typically restricted to those with more severe SID. This finding suggests that IgRT use patterns in the US are generally consistent with AAAAI guidelines, as well as a recent consensus statement, which recommend recurrent and/or SBIs as part of the pre-requisite for IgRT in CLL and MM patients with SID [Citation19,Citation20]. Further, our data demonstrated that 90% of patients in this US study already had infections and/or prophylactic antibiotic use (i.e. prior to initiation of IgRT) and is consistent with findings from a recent global physician survey [Citation28], suggesting more restrictive IgRT use in the US compared with at least some countries in Europe, with the exception of the UK [Citation29].
There was clear evidence of greater infection severity in the baseline period among those patients who went on to receive IgRT. Notwithstanding greater infection severity in the IgRT treated group, a substantial proportion of those who did not receive IgRT still had a considerable infection history at baseline, including risk factors identified in our and other studies: 32% of these patients had a severe infection, 47% had three or more total bacterial infections, 61% had a sinopulmonary infection, 36% had neutropenia, and about one-third received immunosuppressive therapies including monoclonal antibodies, especially so in the NHL and CLL subtypes, all in the context of high antibiotic usage (87%). This is of particular concern as infections are the most common cause of death in these patients, accounting for between 22 and 50% of deaths in SID patients with hematological malignancies [Citation8,Citation10–12,Citation30]. Our specific evidence that patients with four or more infections in the year preceding IgRT initiation were at significantly higher risk of subsequent severe infections is consistent overall with the recent consensus definition of recurrent infections as one of the criteria for IgRT [Citation20]. Additionally, episodes of infection may delay chemotherapy of the underlying malignancy. Again, with nearly one-fifth of patients ending up with severe infections, earlier initiation of IgRT and expanded use of IgRT among those at high risk may be warranted, accounting for complementary risk factors such as neutropenia. Whether higher targeted serum IgG levels may provide additional benefit in mitigating risk of infection, as is seen in patients with HGG due to inborn errors of immunity [Citation31,Citation32], also remains to be determined, but was not within the scope of this analysis.
Despite the higher existing risk profile of patients who received IgRT, our study highlighted that their treatment with IgRT effectively restored their risk to a level on par with age- and cancer subtype-matched non-IgRT users. This may not only be due to the direct impact of the IgRT, but also that initiation of IgRT may have encouraged increased vigilance for infections from the clinical teams and more infection awareness/prevention from the patient. In the 12-month follow-up period, SBIs were reported in ∼20% of IgRT and non-IgRT users, with no significant difference between the groups. When statistically adjusted for the ‘sicker’ condition of the IgRT group, a 31% lower adjusted odds of severe infections was seen in the follow-up period vs. non-IgRT users, as noted earlier.
Our data are in line with that from both clinical studies and recent non-interventional studies of patients with hematological malignancies and SID, which show IgRT can reduce the frequency of severe and recurrent infections [Citation21,Citation27,Citation33–39]. It is more appropriate to review the reduction data than absolute numbers of infections due to variation in how infections are defined within the literature. Reiser et al. [Citation35] reported in a prospective, non-interventional study of patients with hematological malignancies (CLL, MM, and NHL) and SID that treatment with IgRT resulted in a 85.6% reduction in annualized major infections requiring IV antibiotics and reduction from 82% of patients with infections in the 12 months pre-IgRT, to 56% of patients in the year of IgRT. A 40% reduction in annualized major infections requiring IV antibiotics and 22% reduction in annualized total infections per patient was reported in another non-interventional study of CLL, MM, and NHL [Citation36]. Legendre et al. [Citation21] reported a reduction of 26.3% in annualized mean number of major infections with IgRT use. Specifically analyzing IgPro10, in a German non-interventional study of IgPro10 usage in patients with SID, Plath et al. [Citation37] reported the annualized infection rate was reduced by 70.4% post-IgPro10 use. In a prospective randomized cohort of MM patients treated with IgPro20, there was a 77% reduction in total infections per year and an 86% reduction rate in annualized SBIs per year for IgRT-treated patients vs. controls [Citation27]. The impact has varied between studies, which may reflect differences in dosing regimens used, as evidence suggests that IgPro10 benefit may be dependent on dose, with higher doses providing a greater reduction in infections compared with lower doses [Citation40,Citation41]. Altogether, these studies highlight the benefits of IgRT in mitigating infections in these conditions. The downstream effects of these benefits may be broad, potentially including improved quality of life for patients, decreased interruptions of their chemotherapy cycles, and potentially reduced healthcare costs related to infections, although further studies will be needed to confirm these benefits.
Although IgRT reduced the risk of infections broadly in this study, there were some subtle differences across cancer subtypes. Adjusting for baseline risk factors, IgRT was associated with reduced risk of severe infections in the overall cohort, and in the NHL and CLL subtypes, but not in the MM subtype. When we considered all infections including non-severe infections, IgRT was again associated with reduced risk overall and in the NHL subtype, but now additionally in the MM subtype, and not in the CLL subtype. These findings may reflect in part previously noted differences in use of underlying immuno-suppressive agents, including B-cell depleting monoclonal antibodies, which occurred to a much greater extent in the CLL and NHL subtype relative to the MM subtype. The MM subtype, by contrast, was characterized by a much greater use of ‘small molecule targeted therapies’ that included proteasome inhibitors (e.g. bortezomib) consistent with MM guidelines [Citation42], relative to NHL and CLL subtypes. The fact that IgRT was associated with significant risk reduction in all infections, but not severe infections, in MM patients may suggest that the use of (non-B-cell-depleting) proteasome inhibitors and other small molecule targeted therapies may change the infection phenotype from severe to non-severe infections in MM in particular. Nevertheless, it should be pointed out that with greater use of the plasma cell targeted CD-38 monoclonal antibodies (e.g. daratumumab) [Citation43], although not observed in our data due to their very recent approvals in MM, there may potentially be a resurgence of infection risk in MM over time and related need for IgRT. There were also subtle differences in use of specific monoclonal antibodies across NHL and CLL. The former was characterized by greater use of rituximab and the latter by somewhat greater, albeit low in absolute terms, use of ofatumumab, a newer generation CD-20 monoclonal antibody, which is specifically approved for refractory CLL and which may on account of its better targeting of CD20 and complement-dependent mode of action may be more likely to be used as lower dose monotherapy, with arguably lower aggregate infection risk [Citation44].
Further studies focused on quantifying exact levels of risk factors for infections in patients with hematological malignancies and SID, based on both disease phenotype and types of cancer treatments used, can support evidence-based development of risk factor algorithms, and assist clinicians in targeting IgRT for patients who may be the most vulnerable to infections.
One key strength of this study is that it constitutes the largest comparative, real-world analysis of SID-related infections in patients with hematological malignancies, with and without IgRT. This study also provides real-world US evidence on the use of IgRT, specifically IgPro10 or IgPro20, highlighting that IgRT appears to be used clinically for more severe SID patients. A limitation of this study is that data are based on a claims database for insured patients within the US healthcare system and may not necessarily be representative of global data. The need for 12-months pre- and post-IgRT treatment data may also have excluded some patients with shorter survival. Furthermore, in this study based on an administrative claims database, we were unable to observe disease progression as a potential risk factor for infections.
Conclusions
This real-world study of patients with select hematological malignancies demonstrated that use of IgRT (IgPro10 or IgPro20) in the US is characterized by a high level of clinically important infection burden in terms of underlying severity of infections and therefore suspicion of SID, and though generally consistent with guidelines, was characterized by a generally reactive approach deferring initiation of IgRT until after occurrence of severe infections in the context of potential prior opportunities to risk-stratify patients with a more proactive approach. Treatment with IgPro10 or IgPro20 was associated with a reduction in the adjusted risk of SBIs to levels similar to those whose medical providers did not think met their clinical threshold to receive IgRT. It is hoped that these findings will guide IgRT utilization in at-risk patients based on some of the specific risk factors identified in this study, leading to potential subsequent reduction of severe or recurrent bacterial infection risk.
Supplemental Material
Download MS Word (37.1 KB)Acknowledgements
Editorial assistance was provided by Meridian HealthComms Ltd.
Disclosure statement
RM is an employee and shareholder of CSL Behring. VD is an employee of IQVIA which received funding from CSL Behring for this study. BDS received fees from CSL Behring for advisory board activity and travel support. SJ has been a speaker and involved in advisory boards, conferences, clinical trials, DSMB or projects with CSL Behring, Shire, Takeda, Thermofisher, Swedish Orphan Biovitrum, Biotest, Binding Site, BPL, Octapharma, Sanofi, LFB, Pharming, Biocryst, Zarodex, Weatherden, and UCB Pharma. MD has received consulting fees from IQVIA. DCV has received salary support (clinician-scientist, Junior 2) from Fonds de la recherche en santé du Québec and travel fees from CSL Behring and has been involved in clinical studies with Cidara Therapeutics and Janssen Pharmaceuticals; DCV has been a speaker and/or involved in advisory boards with CSL Behring, Merck Canada, Novartis Canada, Shire/Takeda, and UCB Pharma.
Data availability statement
The data that support the findings of this study are available from the corresponding author, RM, upon reasonable request.
Additional information
Funding
References
- Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2010;125(2 Suppl. 2):S195–S203.
- Jolles S, Chapel H, Litzman J. When to initiate immunoglobulin replacement therapy (IGRT) in antibody deficiency: a practical approach. Clin Exp Immunol. 2017;188(3):333–341.
- Hamblin A, Hamblin T. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49–62.
- Seppänen M. Immunoglobulin G treatment of secondary immunodeficiencies in the era of novel therapies. Clin Exp Immunol. 2014;178:10–13.
- Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13(2):106–111.
- Tete SM, Bijl M, Sahota SS, et al. Immune defects in the risk of infection and response to vaccination in monoclonal gammopathy of undetermined significance and multiple myeloma. Front Immunol. 2014;5:257.
- IHME. Global burden of disease results tool; 2019. Available from: http://ghdx.healthdata.org/gbd-results-too
- Morrison VA. Infectious complications of chronic lymphocytic leukaemia: pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol. 2010;23(1):145–153.
- Dhalla F, Misbah SA. Secondary antibody deficiencies. Curr Opin Allergy Clin Immunol. 2015;15(6):505–513.
- Oscier D, Dearden C, Eren E, et al. Guidelines on the diagnosis, investigation and management of chronic lymphocytic leukaemia. Br J Haematol. 2012;159:541.
- Blimark C, Holmberg E, Mellqvist U-H, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107–113.
- Ostrow S, Diggs CH, Sutherland J, et al. Causes of death in patients with non‐Hodgkin's lymphoma. Cancer. 1981;48(3):779–782.
- Anderson D, Ali K, Blanchette V, et al. Guidelines on the use of intravenous immune globulin for hematologic conditions. Transfus Med Rev. 2007;21(2 Suppl. 1):S9–S56.
- Čolović N, Bogdanović A, Čemerikić-Martinović V, et al. Prognostic significance of serum immunoglobulins in B-chronic lymphocytic leukemia. Arch Oncol. 2001;9:79–82.
- Snowden JA, Ahmedzai SH, Ashcroft J, et al. Guidelines for supportive care in multiple myeloma 2011. Br J Haematol. 2011;154(1):76–103.
- Department of Health. Clinical guidelines for immunoglobulin use. 2nd ed. London: Department of Health; 2011.
- National Blood Authority. Criteria for the clinical use of intravenous immunoglobulin in Australia. 2nd ed. Australia: Canberra; 2012.
- European Medicines Agency. Guideline on core SmPC for human normal immunoglobulin for intravenous administration (IVIg). London; 2018.
- Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1–S46.
- Jolles S, Michallet M, Agostini C, et al. Treating secondary antibody deficiency in patients with haematological malignancy: European Expert Consensus. Eur J Haematol. 2021;106(4):439–449.
- Legendre P, Chahwan D, Marjanovic Z, et al. Utilization of intravenous or subcutaneous immunoglobulins in secondary immune deficiency (ULTIMATE): a retrospective multicenter study. Clin Immunol. 2020; 215:108419.
- Breslow NE, Day NE. Statistical methods in cancer research. Volume I – the analysis of case-control studies. Lyon: International Agency for Research on Cancer; 1980;5–338.
- Vittinghoff E, Glidden DV, Shiboski SC, et al. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. New York: Springer; 2012.
- Langholz B, Goldstein L. Conditional logistic analysis of case-control studies with complex sampling. Biostatistics. 2001;2(1):63–84.
- Cameron AC. Regression analysis of count data. Cambridge: Cambridge University Press; 1998.
- Jones AM. Models for count data. London: Applied Health Economics; 2013.
- Vacca A, Melaccio A, Sportelli A, et al. Subcutaneous immunoglobulins in patients with multiple myeloma and secondary hypogammaglobulinemia: a randomized trial. Clin Immunol. 2018;191:110–115.
- Na IK, Buckland M, Agostini C, et al. Current clinical practice and challenges in the management of secondary immunodeficiency in hematological malignancies. Eur J Haematol. 2019;102(6):447–456.
- Patel SY, Carbone J, Jolles S. The expanding field of secondary antibody deficiency: causes, diagnosis, and management. Front Immunol. 2019;10:33.
- Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49(8):1211–1225.
- Orange JS, Grossman WJ, Navickis RJ, et al. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137(1):21–30.
- Orange JS, Belohradsky BH, Berger M, et al. Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy. Clin Exp Immunol. 2012;169(2):172–181.
- Chapel H, Lee M, Hargreaves R, et al. Randomised trial of intravenous immunoglobulin as prophylaxis against infection in plateau-phase multiple myeloma. Lancet. 1994;343(8905):1059–1063.
- Boughton B, Jackson N, Lim S, et al. Randomized trial of intravenous immunoglobulin prophylaxis for patients with chronic lymphocytic leukaemia and secondary hypogammaglobulinaemia. Clin Lab Haematol. 2008;17(1):75–80.
- Reiser M, Borte M, Huscher D, et al. Management of patients with malignancies and secondary immunodeficiencies treated with immunoglobulins in clinical practice: long-term data of the SIGNS study. Eur J Haematol. 2017;99(2):169–177.
- Benbrahim O, Viallard J-F, Choquet S, et al. The use of octagam and gammanorm in immunodeficiency associated with hematological malignancies: a prospective study from 21 French Hematology Departments. Hematology. 2019;24(1):173–182.
- Plath M, Slawik H, Reiser M, et al. Privigen® in secondary immunodeficiencies – interim analysis of a multicenter non-interventional study in Germany. Annual Meeting of the German, Austrian and Swiss Societies for Hematology and Medical Oncology; Basel; 2015.
- Spadaro G, Pecoraro A, De Renzo A, et al. Intravenous versus subcutaneous immunoglobulin replacement in secondary hypogammaglobulinemia. Clin Immunol. 2016;166–167:103–104.
- Viallard J, Agape P, Barlogis V, et al. Treatment with Hizentra in patients with primary and secondary immunodeficiencies: a real-life, non-interventional trial. BMC Immunol. 2016;17(1):34.
- Reiser M, Otremba B, Plath M, et al. Correlation between efficacy of the intravenous immunoglobulin preparation IgPro10 and IgG plasma concentrations achieved in secondary immunodeficiencies (SID) – results from a multicenter observation study. Poster ESID6-0592, Presented at ESID 2016. Barcelona, Spain; 2016.
- Ehlers H-U, Otremba BJ, Plath M, et al. Correlation between immunoglobulin dose and incidence of severe and serious infections in secondary immunodeficiencies. J Clin Oncol. 2017;35(15 Suppl.):e21686.
- Mikhael J, Ismaila N, Cheung MC, et al. Treatment of multiple myeloma: ASCO and CCO joint clinical practice guideline. J Clin Oncol. 2019;37(14):1228–1263.
- van de Donk NW, Moreau P, Plesner T, et al. Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood. 2016;127(6):681–695.
- Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1749–1755.
