Abstract
Patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL) whose treatment failed with a Bruton’s tyrosine kinase inhibitor have poor outcomes. We investigated tafasitamab plus idelalisib (cohort A) or venetoclax (cohort B) in this patient population in a phase II study (NCT02639910). In total, 24 patients were enrolled (cohort A: n = 11, median time on study, 7.4 months; cohort B: n = 13, median time on study, 15.6 months). The most common treatment-emergent adverse event (TEAE) in cohort A was anemia (63.6%) and in cohort B was infusion-related reaction (53.8%). The most common severe TEAE was neutropenia (cohort A: 45.5%; cohort B: 46.2%). The best overall response rate was 90.9% (cohort A) and 76.9% (cohort B). Undetectable minimal residual disease in peripheral blood was achieved in 2/8 patients (cohort A) and 6/7 patients (cohort B). Overall, these results suggest that anti-CD19 antibody-based combinations may be important in the treatment of patients with CLL.
Introduction
Bruton’s tyrosine kinase (BTK) inhibitors including ibrutinib and acalabrutinib, as well as selective inhibitors of BCL2 such as venetoclax, with or without anti-CD20 antibody treatment, are approved in treatment-naïve and relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) [Citation1–11]. Idelalisib, a reversible inhibitor of phosphatidylinositol 3-kinase-δ, in combination with rituximab is approved in the relapsed setting [Citation12,Citation13].
Despite the efficacy and safety of BTK inhibitors, some R/R CLL and treatment-naïve CLL patients discontinue ibrutinib treatment (27%) [Citation2,Citation6] and acalabrutinib monotherapy (13–18%) [Citation5,Citation14] due to progressive disease (PD) or toxicities. Addition of an anti-CD20 antibody, such as rituximab or obinutuzumab, to a BTK inhibitor has not been shown to significantly improve efficacy over ibrutinib monotherapy in treatment-naïve patients [Citation5,Citation7]. Similarly, a recent retrospective analysis demonstrated a comparable efficacy between venetoclax monotherapy and venetoclax in combination with an anti-CD20 antibody in heavily pretreated patients [Citation8]. Such outcomes suggest that these patients experience a poor prognosis and unmet clinical need, generating interest in alternative therapeutic strategies in BTK-pretreated patients.
CD19 is stably expressed on B cells, in contrast to CD20 [Citation15], and expression is largely preserved within the CD20-negative tumor subset and post anti-CD20 targeted therapy, making it an attractive target in CLL [Citation16]. Combining an anti-CD19 antibody with idelalisib or venetoclax may therefore represent an alternative therapeutic strategy in BTK-pretreated patients.
TafasitamabFootnote1 (MOR208) is an Fc-enhanced, humanized, monoclonal antibody to CD19 with enhanced tumor cytotoxicity in CLL compared with non-engineered anti-CD19 analogs [Citation15,Citation17]. Tafasitamab monotherapy has shown clinical activity and a tolerable safety profile in a phase I study of patients with R/R CLL [Citation18]. However, combinations of drugs with distinct mechanisms of action may target tumor cells more effectively than single therapies.
This phase II study evaluated the safety and preliminary clinical activity of tafasitamab combined with idelalisib (cohort A) or venetoclax (cohort B) in patients with R/R CLL who had been pretreated with a BTK inhibitor.
Materials and methods
Study design
This multicenter, two-cohort, non-randomized, open-label phase II study (NCT02639910) was carried out across 12 sites in Austria, Germany, Italy, Poland, United Kingdom, and the US. The study was initiated in November 2016; the date for final analysis was October 2019. Patients were assigned to one of two independent cohorts (cohort A, tafasitamab–idelalisib; cohort B, tafasitamab–venetoclax) at the discretion of the investigator. The primary endpoint was safety, defined as the incidence and severity of adverse events (AEs). Secondary endpoints were overall response rate (ORR), immunogenicity, and the pharmacokinetic (PK) profile of tafasitamab. Exploratory endpoints included the proportion of patients with minimal residual disease (MRD) negativity as assessed in peripheral blood and bone marrow.
The study was approved by local independent ethics committees/institutional review boards, and conducted according to the International Council for Harmonisation Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent. The Independent Data Monitoring Committee recommended continuation of the study based on the safety results of a run-in phase comprising the first 10 patients who completed at least one 28-day cycle of treatment in cohort A or at least 5 weeks of combination treatment in cohort B.
Eligibility criteria
Eligible patients were adults with R/R CLL indicated for treatment according to the criteria of the 2008 International Workshop on Chronic Lymphocytic Leukemia (IWCLL) [Citation19]. In addition to experiencing R/R disease on, or intolerance to, a BTK inhibitor, patients had received a BTK inhibitor as monotherapy or combination treatment for at least 1 month as their most recent anti-cancer therapy. Patients with Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2 were included.
Exclusion criteria included transformed CLL/SLL or Richter’s syndrome, active and uncontrolled autoimmune cytopenia, having received a BTK inhibitor within 5 days prior to first dosing of tafasitamab, or not having discontinued CD20-targeted treatment within 14 days prior to dosing. Full eligibility criteria are listed in the Supplementary Appendix.
Study treatments
The planned treatment period comprised up to 24 cycles (28 days each). Patients who benefited from treatment could continue tafasitamab treatment beyond cycle 24 at the investigator’s discretion.
In cycles 1–3, tafasitamab 12 mg/kg was administered as an intravenous infusion on days 1, 8, 15, and 22 with an additional loading dose on cycle 1, day 4 (C1D4). In cycles 4–6, tafasitamab 12 mg/kg was administered every second week with infusions on day 1 and day 15, then monthly from cycle 7 onwards. The first infusion occurred at a rate of 70 mL/hour for the first 30 min, then increased to 125 mL/hour. The total infusion time was not to exceed 2.5 hours. Subsequent infusions were administered at a rate of 125 mL/hour over a 2-hour period. Premedication with antipyretics, histamine H1 receptor blockers, and glucocorticoids (methylprednisolone 80–120 mg per dose IV or equivalent) was administered for the first three infusions, and optionally thereafter for those not experiencing infusion-related reactions (IRRs). If IRRs were experienced, premedication was continued for subsequent infusions.
Patients assigned to cohort A self-administered idelalisib 150 mg orally, twice daily beginning on day 1 of each cycle. Patients assigned to cohort B received venetoclax orally to an individual ramp-up schedule according to the venetoclax label starting at day 8 of cycle 1 per protocol (Supplementary Figure 1). Dose de-escalations and treatment withdrawal were permitted if indicated by clinical or laboratory findings. If AEs suspected to be related to idelalisib or venetoclax occurred, the patient could continue with tafasitamab monotherapy.
Patients continued study treatment until documented disease progression, intolerable toxicity, withdrawal of consent, death, or physician decision.
Assessments
All patients had computed tomography (CT) or magnetic resonance imaging to determine tumor response at screening, at day 1 of cycle 4, cycle 7, and every 6 months thereafter, and at end of treatment. Response rates and progression were determined using IWCLL criteria [Citation19], with the modification that treatment-related lymphocytosis (in cohort A) was not considered PD in the absence of other signs of symptoms of disease progression. Patients who exhibited a partial response (PR) or complete response (CR) as per standard criteria were assessed by MRD testing (defined as <10−4 cells/leukocyte (<1 CLL cell/10,000 leukocytes)), employing allele-specific oligonucleotide polymerase chain reaction from peripheral blood at C1D1, C4D1, C7D1, C13D1, and so on, and from bone marrow if peripheral blood negativity was reached [Citation19]. Laboratory assessments are described in the Supplementary Appendix.
A safety follow-up assessment was scheduled for 30 days post-study to monitor for post-treatment-emergent adverse events (TEAEs), including serious AEs (SAEs). Laboratory and AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Statistical analysis
Data were summarized using descriptive statistics. No formal statistical hypothesis testing was planned. The primary endpoint was analyzed in the safety analysis set, which included patients who received at least one dose of any study drug and had at least one post-baseline safety evaluation. The secondary endpoint, ORR, was analyzed in patients who received more than one dose of tafasitamab and/or one dose of idelalisib or venetoclax. PK analyses were performed in patients who had received at least one dose of tafasitamab and had one quantifiable tafasitamab serum concentration. Immunogenicity evaluations were performed in patients who had at least one anti-tafasitamab antibody assessment. Patients without any post-baseline response assessment data were considered non-responders in the analyses for ORR. Statistical analyses were performed using SAS Software version 9.4 or above (SAS Institute, Cary, NC).
Results
Patient disposition
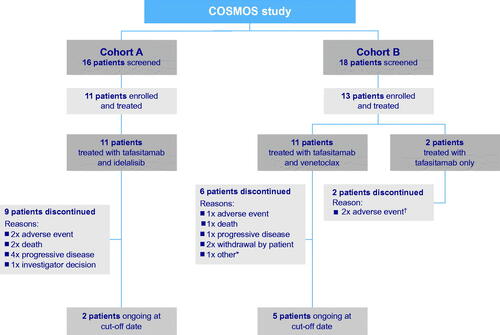
In total, 34 patients were screened and 24 were enrolled and treated: 11 in cohort A and 13 in cohort B (). Two patients in each cohort completed 24 months of combination therapy per protocol. Of the 11 patients enrolled to receive tafasitamab plus idelalisib in cohort A, nine discontinued the study, including two deaths: one related to disease progression, and one due to treatment-emergent cardiac failure. Of the 13 patients in cohort B, 11 received tafasitamab plus venetoclax and two received tafasitamab only. These two patients discontinued study treatment ahead of the planned start of venetoclax on day 8 of cycle 1. In cohort B, eight patients discontinued the study and one death occurred, which was due to treatment-emergent sepsis. The data cutoff was 8 October 2019.
Baseline characteristics
Cohort A
Patients had a median age of 69 years (range 51–79); six males and five females (Supplementary Table 1). All patients had an ECOG PS of 0 or 1. The median time since first CLL diagnosis was 95 months (range 44–296). The median number of prior treatments was five (range 2–9), and the median time on prior BTK inhibitor was 22 months (range 9–50). Prior treatment included anti-CD20 therapy (100%), fludarabine, cyclophosphamide, and rituximab (FCR) (73%), chemoimmunotherapy other than FCR (82%), venetoclax (9%), autologous stem cell transplantation (18%), and allogeneic stem cell transplantation (9%). The reasons for discontinuing BTK inhibitors prior to enrollment were PD (n= 9) and toxicity (n= 2). Biomarker assessments and mutational status were assessed in nine out of 11 patients.
Cohort B
The median age was 64 years (range 50–77); 10 males and three females (Supplementary Table 2). All patients had an ECOG PS of 0 or 1. The median time since first CLL diagnosis was 117 months (range 20–192). The median number of prior treatments was three (range 1–5). Patients were on prior BTK inhibitor for a median of 35 months (range 12–62), and the reasons for BTK inhibitor discontinuation were PD (n= 10), toxicity (n= 2), and unknown (n= 1). Prior treatment included anti-CD20 therapy (92%), FCR (77%), chemoimmunotherapy containing fludarabine (92%), and idelalisib (8%). Biomarker assessments and mutational status were assessed in all patients.
Safety
Cohort A
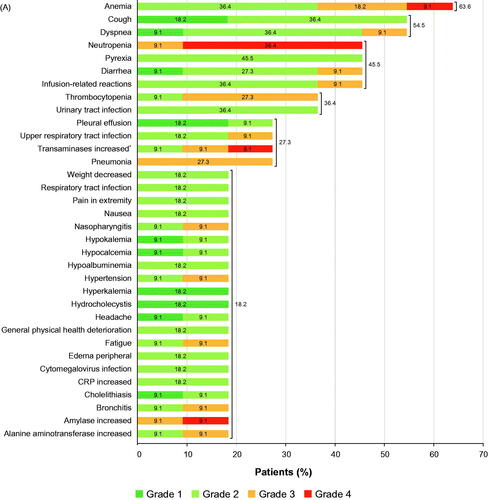
All patients were included in the safety analysis set; all patients experienced TEAEs (244 events). Most TEAEs were of toxicity grade 1 or 2 (181/244 events). The most common all-grade TEAEs were anemia (63.6%), dyspnea (54.5%), cough (54.5%), neutropenia (45.5%), pyrexia (45.5%), diarrhea (45.5%), and IRR (45.5%) (). The most common grade ≥3 TEAEs were hematologic and included neutropenia (45.5%), anemia (27.3%), and thrombocytopenia (27.3%).
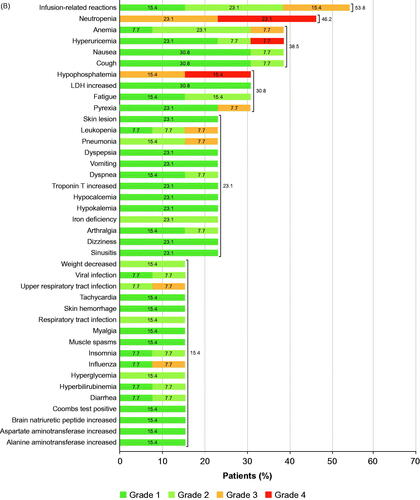
Figure 2. (A) TEAEs with ≥10% incidence in cohort A (tafasitamab plus idelalisib) (a patient with more than one TEAE per preferred term is counted once for that preferred term); *transaminases increased included alanine aminotransferase increased and/or aspartate aminotransferase increased. (B) TEAEs with ≥10% incidence in cohort B (tafasitamab plus venetoclax) (a patient with more than one TEAE for a preferred term is counted once for that preferred term). CRP: C-reactive protein; LDH: lactate dehydrogenase; TEAE: treatment-emergent adverse event. Disclaimer: Some values may not add up to the total given due to rounding.
Eight patients (72.7%) had treatment-emergent SAEs (19 events). Hematologic SAEs were anemia, thrombocytopenia, and pancytopenia, while the most common non-hematologic SAE was pneumonia (). Seven patients (63.6%) had SAEs (10 events) related to any study drug (tafasitamab and/or idelalisib).
Table 1. Summary of SAEs in cohort A (tafasitamab plus idelalisib) and cohort B (tafasitamab plus venetoclax).
Tafasitamab treatment was interrupted due to TEAEs in 10 patients (90.9%), mainly due to IRRs (five patients, 45.5%; grade 2 in four patients, and grade 3 in one patient). Tafasitamab was permanently discontinued in two patients (18.2%) due to TEAEs of aspartate aminotransferase increased and acute pancreatitis; no patient discontinued tafasitamab permanently due to IRRs. Idelalisib treatment was interrupted due to TEAEs in nine patients (81.8%). Five patients (45.5%) permanently discontinued idelalisib due to TEAEs of bronchitis, septic shock, cardiac failure, acute pancreatitis, aspartate aminotransferase increased, and transaminases increased (the latter two events in one patient). Two out of the five patients who discontinued idelalisib (due to bronchitis and septic shock) continued treatment with tafasitamab.
Cohort B
All patients were included in the safety analysis set; all patients experienced TEAEs (281 events). Most TEAEs were of toxicity grade 1 or 2 (228/281 events). The most common all-grade TEAEs were IRRs (53.8%), neutropenia (46.2%), anemia (38.5%), hyperuricemia (38.5%), nausea (38.5%), and cough (38.5%) (). The most common grade ≥3 TEAEs were neutropenia (six patients, 46.2%) and hypophosphatemia (four patients, 30.8%).
Nine patients (69.2%) had treatment-emergent SAEs (17 events). Hematologic SAEs were anemia and febrile neutropenia, while the most common non-hematologic SAE was pyrexia (23.1%) (). Grade 3 SAEs of IRRs were reported in two patients (15.4%), who recovered quickly after stopping tafasitamab and administering corrective treatment; both patients were withdrawn from the study. Importantly, only one patient (7.7%) had tumor lysis syndrome (grade 3). Six patients (46.2%) had SAEs (eight events) related to any study drug (tafasitamab and/or venetoclax).
Six patients (46.2%) had IRRs related to tafasitamab, four were grade ≤2. Tafasitamab treatment was interrupted due to TEAEs in eight patients (61.5%); five (38.5%) were due to IRRs. Venetoclax treatment was interrupted due to TEAEs in nine patients (69.2%).
Tafasitamab was permanently discontinued in four patients (30.8%) due to TEAEs of diarrhea and neutropenia (one patient each) and IRR (two patients). Three patients (23.1%) permanently discontinued venetoclax due to TEAEs of diarrhea, cough, and neutropenia (one patient each). The patient who discontinued venetoclax, due to cough, continued treatment with tafasitamab.
Efficacy – ORR
Cohort A
A best ORR in cohort A was achieved in 10 out of 11 patients (90.9%) (). This included one CR (9.1%), and nine PR (81.8%). The observed median time on study was 7.4 months (range 2.1–28.2) (). As shown in , nine patients discontinued treatment. At data cutoff, two of 11 patients were still receiving tafasitamab treatment more than 2 years after treatment initiation.
Figure 3. Best ORR in cohort A (tafasitamab plus idelalisib) and cohort B (tafasitamab plus venetoclax). CR: complete response; NE: not evaluable or missing; ORR: overall response rate; PR: partial response; SD: stable disease.
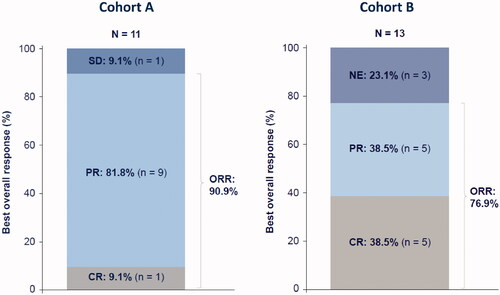
Figure 4. (A) Time on study in cohort A (tafasitamab plus idelalisib). Data cutoff: 8 October 2019. Median time on study was 7.4 months. Patient number indicated at the end of each bar (see Supplementary Table 1). *Heart failure; †acute pancreatitis; ‡PD with cardiorespiratory failure resulting in death; §patient reached clinical remission, thus became eligible for subsequent transplantation (bridging); ¶aspartate aminotransferase increased. Time on study (months) was defined as ((date of last documented visit or death – date of first visit)+1)×0.0328767. (B) Time on study in cohort B (tafasitamab plus venetoclax). Data cutoff: 8 October 2019. Median time on study was 15.6 months. Patient number indicated at the end of each bar (see Supplementary Table 2). *Sepsis; †diarrhea; ‡infusion-related reactions. Other: discontinuation after C24 at the discretion of the investigator and patient, per protocol. AE: adverse event; CR: complete response; ICF: informed consent form; MRD: minimum residual disease; NE: not evaluable or missing; PD: progressive disease; PR: partial response; SD: stable disease. Time on study (months) was defined as ((date of last documented visit or death – date of first visit)+1)×0.0328767.
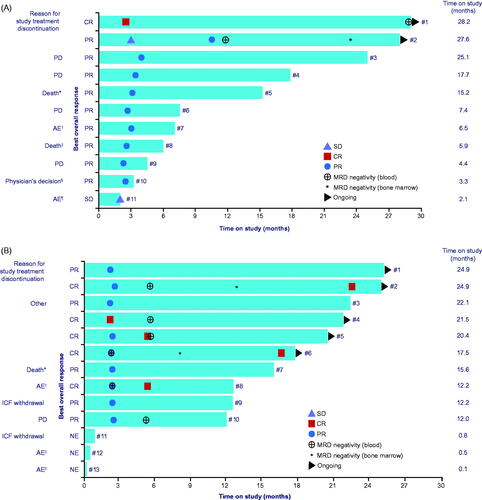
Cohort B
A best ORR in cohort B was achieved in 10 out of 13 patients (76.9%) (). This included CR and PR in five patients each (38.5% each). Three patients in cohort B (23.1%) did not undergo any post-baseline radiographic response assessment by CT. The observed median time on study was 15.6 months (range 0.1–24.9) (). As shown in , six patients discontinued treatment. At data cutoff, two of five patients were still receiving tafasitamab treatment more than 2 years after treatment initiation.
PK and immunogenicity
Initial weekly dosing at 12 mg/kg (including a loading dose at C1D4) led to mean Cmax and trough level of ∼509 mg/L and ∼294 mg/L, respectively. During biweekly dosing (cycles 4–6) and four-weekly dosing (from cycle 7), tafasitamab serum trough levels of ∼200 mg/L and ∼100 mg/L were observed, respectively. Tafasitamab serum levels were similar between the two treatment arms.
At baseline, two patients (one in each cohort) tested positive for anti-drug antibodies (ADAs) with low antibody titers. After start of treatment with tafasitamab, no patient tested positive for ADAs. No treatment-emergent or treatment-boosted ADAs were observed.
Exploratory endpoints
Proportion of patients with MRD negativity
In cohort A, MRD status in peripheral blood was assessed in eight out of 11 patients (72.7%). Two patients achieved MRD negativity in peripheral blood (25% of tested, 18.2% of all patients): one in cycle 14 and one in cycle 31. In the three patients in whom MRD status was assessed in bone marrow, one patient (9.1% of all patients) achieved MRD negativity in cycle 27.
In cohort B, MRD status in peripheral blood was assessed in seven out of 13 patients (53.9%). Six patients achieved MRD negativity in peripheral blood (85.7% of tested, 46.2% of all patients): two at C4D1 (15.4% of all patients) and four at C7D1 (30.8% of all patients). In the four patients in whom MRD status was assessed in bone marrow, two patients (15.4% of all patients) achieved MRD negativity: one in cycle 10 and one in cycle 15.
Peripheral CD5+ B-CLL cell changes from baseline
As shown in Supplementary Figure 2, there was a rapid and effective depletion in peripheral CD5+ B-CLL cells over time, with a median reduction relative to the baseline of 99% and 100% for cohort A and B, respectively. In cohort A, the median number of peripheral CD5+ B-CLL cells rapidly decreased from 11,272 cells/µL at baseline to 50 cells/µL at C4D15. In cohort B, the median number of peripheral CD5+ B-CLL cells rapidly decreased from 3,588 cells/µL at baseline to 1 cell/µL at C2D15.
Discussion
Given that the expression of CD19 on B cells is present from an early stage through to mature B cells, targeting CD19 offers a broad opportunity in B-cell malignancies. This phase II study demonstrated that tafasitamab plus idelalisib or venetoclax may have efficacy in heavily pretreated patients with R/R CLL who had discontinued BTK inhibitor treatment, although both efficacy and safety data were derived from limited sample sizes. The safety profile of tafasitamab in combination with either small-molecule inhibitor was dependent on the combination partner. In both cohorts, the incidence of AEs including hematologic events and infections was comparable to that reported for idelalisib or venetoclax monotherapy, indicating that the addition of tafasitamab to either idelalisib or venetoclax does not significantly increase the safety burden for patients [Citation9,Citation10,Citation12,Citation13]. Notably, episodes of diarrhea typically associated with idelalisib were not observed in this study, possibly due to short exposure time. The most frequently reported grade ≥3 TEAEs were cytopenias, most commonly neutropenia (cohort A, 45.5%; cohort B, 46.2%), which was comparable with other studies [Citation4,Citation20,Citation21]. In the randomized combination trial of idelalisib with rituximab versus placebo with rituximab (NCT01539512), the incidence of grade 3/4 neutropenia was 34% and 22%, respectively [Citation20]. The overall incidence of neutropenia was 60.8% (grade 3/4: 57.7%) in the phase III MURANO (venetoclax + rituximab) study of patients with R/R CLL who had received 1–3 previous treatments including one chemotherapy-containing regimen [Citation21].
Despite the use of premedication in this study, almost all IRRs occurred during the patients’ first infusions, as described for other B cell-targeting monoclonal antibody therapies in this patient population [Citation22–25]. Most IRRs reported in this study were non-serious AEs of grade 1 or 2, and patients were able to complete their dose after a temporary pause of the infusion and symptomatic treatment. An intra-individual dose ramp-up of tafasitamab in patients with high tumor burden and high leukocyte counts may have the potential to reduce the incidence and severity of IRRs. Following the implementation of this strategy in an investigator-initiated trial (NCT02005289), no grade 3 IRRs were observed in patients with R/R CLL [Citation26,Citation27]. Tafasitamab displayed a typical PK profile as expected for a monoclonal antibody [Citation28].
Addition of the anti-CD20 monoclonal antibodies rituximab or obinutuzumab to a BTK inhibitor has not been shown to significantly improve outcomes in patients with treatment-naïve CLL. In a phase III study in older patients, the efficacy of rituximab plus ibrutinib was comparable to ibrutinib monotherapy in terms of progression-free survival [Citation7], and a post hoc analysis in the phase III ELEVATE-TN study showed only a modest benefit of adding obinutuzumab to acalabrutinib versus acalabrutinib monotherapy [Citation5]. Moreover, a recent retrospective analysis demonstrated comparable efficacy for venetoclax plus CD20-directed therapy versus venetoclax monotherapy in heavily pretreated patients [Citation8]. By comparison, the high response rates observed in this study suggest that combinations of tafasitamab with idelalisib or venetoclax are both clinically active in heavily pretreated patients with R/R CLL. Given that the patient populations in this study were generally considered to have a poor prognosis, it was notable that MRD negativity was achieved in peripheral blood and bone marrow in both cohorts. In cohort A, the best ORR was higher than those reported for rituximab plus idelalisib in R/R CLL [Citation20]. In cohort B, the CR and MRD negativity rates were remarkable despite the small sample size and were similar to data reported for the MURANO trial [Citation21]. However, these patient populations are not directly comparable to the present study, with only 2.6% of all patients in the MURANO study and none of the patients in the pivotal idelalisib study having previously been treated with a BTK inhibitor [Citation20,Citation21]. CR and MRD negativity rates in cohort B were also higher than reported with venetoclax monotherapy in patients with R/R CLL pretreated with a BTK inhibitor [Citation29].
Widespread use of anti-CD20 antibody treatment in previous lines of therapy means that many patients are likely to display refractoriness to anti-CD20 therapy. Interestingly, under ibrutinib treatment, the expression of the CD20 antigen is rapidly and persistently downregulated on the surface of CLL cells [Citation30]. The disease bulk at the time of anti-CD20 administration may also explain the limited effect in clinical trials in which an anti-CD20 monoclonal antibody was combined with ibrutinib for the first 6 months of therapy [Citation31]. In view of these observations, combination therapy approaches, particularly directed against alternative targets such as the more stably expressed CD19, are relevant.
Although a formal subgroup analysis was not performed due to the small sample size, a correlation between prognostic markers and response rates was not observed. There was no evidence of an inferior outcome for those patients who had poor prognosis features.
Overall, the results of this study suggest that anti-CD19 antibody-based combinations may be important in the R/R treatment setting since acalabrutinib with or without obinutuzumab or ibrutinib with or without rituximab have shown modest or no significant additional benefit over each BTK inhibitor alone [Citation7,Citation32].
Authors contributions
P.S., W.J., S.S, F.S., M.D-H., J.W., W.B., P.K., and J.A.W. designed the research. P.S., W.J., M.M, J.S., S.S., J.W., and J.A.W. performed research. P.S., W.J., J.M.M., M.M., P.N., J.S., C-M.W., and J.A.W. collected data. P.S, W.J., J.M.M., M.M., P.N., J.S., S.S., F.S., M.D-H., J.W. W.B., P.K., C-M.W., and J.A.W. analyzed and interpreted data. W.J. and J.W. performed statistical analysis. All authors contributed to writing the manuscript and provided final approval.
GLAL-2020-1625-File014.docx
Download MS Word (17.9 KB)GLAL-2020-1625-File013.docx
Download MS Word (17.8 KB)GLAL-2020-1625-File012.docx
Download MS Word (34.5 KB)GLAL-2020-1625-File011.docx
Download MS Word (65.7 KB)GLAL-2020-1625-File010.docx
Download MS Word (21 KB)Acknowledgements
We thank the patients and their families, clinical researchers, and their teams and hospitals that have participated in this study. We would also like to thank Guido Würth and Rainer Boxhammer of MorphoSys for their contributions to this manuscript. Medical writing assistance was provided by Ross Jarratt at Syneos Health and funded by MorphoSys AG.
Disclosure statement
P.S. has acted as a consultant for, received honoraria from, and provided paid expert testimony for AstraZeneca, AbbVie, Janssen, Gilead, Roche, MSD, BMS, CTI, Incyte, and Takeda-Millennium within the past 2 years and has also received research funding from Roche Diagnostics. W.J. has acted as a consultant for AstraZeneca, Debiopharm, Janssen, Gilead, and Roche within the past 2 years and has received research funding from Morphosys, Acerta, AstraZeneca, Janssen, BeiGene, Bayer, Celltrion, Debiopharm, Epizyme, Merck, MEI Pharma, Servier, Roche, and TG therapeutics. R.G. has acted as a consultant within the past 2 years and been a member of the board of directors or its advisory committees for each of Celgene, Novartis, Roche, BMS, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead, and Daiichi Sankyo. R.G. has also received research funding from Celgene, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, Gilead, and Roche in addition to receiving honoraria from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, AbbVie, Gilead, and Daiichi Sankyo. V.V. has acted as a consultant for AbbVie and Gilead within the past 2 years. J.M.M. has acted as a consultant for AbbVie and Janssen within the past 2 years. M.M. has acted as a consultant for AbbVie, Janssen, Verastem, and Astra Zeneca within the past 2 years in addition to receiving honoraria from AbbVie, Janssen, Gilead, Roche, and AstraZeneca together with research funding from Roche. T.M. has received honoraria from Roche, AbbVie, Janssen, Gilead, AstraZeneca, Novartis, and Alexion. S.S. has acted as a consultant within the past 2 years for and received research funding and honoraria from AbbVie, AstraZeneca, Celgene, Gilead, Hoffmann La-Roche, Janssen, and Novartis. F.S., M.D-H., J.W., W.B., and P.K. are employees of MorphoSys AG. C-M.W. has acted as a consultant within the past 2 years for and received research funding and honoraria from Hoffmann La-Roche, Janssen, Gilead, AbbVie, and MorphoSys. J.A.W. has acted as a consultant within the past 2 years for Janssen, Pharmacyclics, AbbVie, AstraZeneca, and Arqule and received research funding from Loxo and AbbVie. J.S. has received research funding from Sanofi, Novartis, and AbbVie and lecture fees from Roche, Janssen, AbbVie, and Gilead. P.N. has no conflicts of interest to declare.
Additional information
Funding
Notes
1 About tafasitamab: Tafasitamab is a humanized Fc-modified cytolytic CD19-targeting monoclonal antibody. In 2010, MorphoSys licensed exclusive worldwide rights to develop and commercialize tafasitamab from Xencor, Inc. Tafasitamab incorporates an XmAb® engineered Fc domain, which mediates B-cell lysis through apoptosis and immune effector mechanism including antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). In January 2020, MorphoSys and Incyte entered into a collaboration and licensing agreement to further develop and commercialize tafasitamab globally. Following accelerated approval by the U.S. Food and Drug Administration in July 2020 for tafasitamab in combination with lenalidomide for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low grade lymphoma, who are not eligible for autologous stem cell transplant, tafasitamab is being co-commercialized by MorphoSys and Incyte in the United States. Incyte has exclusive commercialization rights outside the United States. XmAb® is a trademark of Xencor, Inc.
References
- Thompson PA, Burger JA. Bruton's tyrosine kinase inhibitors: first and second generation agents for patients with chronic lymphocytic leukemia (CLL). Expert Opin Investig Drugs. 2018;27(1):31–42.
- Byrd JC, Hillmen P, O’Brien S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;133(19):2031–2042.
- Byrd JC, Furman RR, Coutre S, et al. The Bruton's tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765) promotes high response rate, durable remissions, and is tolerable in treatment naïve (TN) and relapsed or refractory (RR) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) patients including patients with high-risk (HR) disease: new and updated results of 116 patients in a phase Ib/II study. Blood. 2012;120(21):189.
- IMBRUVICA (ibrutinib capsules). Summary of product characteristics, Janssen-Cilag International NV, Beerse, Belgium, 2019.
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291.
- Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067.
- Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528.
- Mato AR, Roeker LE, Eyre TA, et al. A retrospective comparison of venetoclax alone or in combination with an anti-CD20 monoclonal antibody in R/R CLL. Blood Adv. 2019;3(10):1568–1573.
- VENCLEXTA (venetoclax tablets). Highlights of prescribing information, AbbVie Ltd, Maidenhead, UK, 2019.
- VENCLEXTA (venetoclax tablets). Summary of product characteristics, AbbVie Ltd, Maidenhead, UK, 2019.
- CALQUENCE (acalabrutinib capsules). Highlights of prescribing information, AstraZeneca UK Ltd., Luton, UK, 2017.
- ZYDELIG (idelalisib tablets). Highlights of prescribing information, Gilead Sciences Ltd., London, UK, 2020.
- ZYDELIG (idelalisib tablets). Summary of product characteristics, Gilead Sciences Ltd., London, UK, 2020.
- Ghia P, Pluta A, Wach M, et al. ASCEND: phase ill, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–2861.
- Awan FT, Lapalombella R, Trotta R, et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood. 2010;115(6):1204–1213.
- Horna P, Nowakowski G, Endell J, et al. Comparative assessment of surface CD19 and CD20 expression on B-cell lymphomas from clinical biopsies: implications for targeted therapies. Blood. 2019;134(Suppl. 1):5345.
- Horton HM, Bernett MJ, Pong E, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68(19):8049–8057.
- Woyach JA, Awan F, Flinn IW, et al. A phase 1 trial of the Fc-engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood. 2014;124(24):3553–3560.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia Updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456.
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007.
- Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax–rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120.
- MabThera (rituximab injection). Summary of product characteristics, Roche Products Limited, Welwyn Garden City, UK, 2020.
- RITUXAN (rituximab injection). Highlights of prescribing information, Roche Products Limited, Welwyn Garden City, UK, 2020.
- GAZYVA (obinutuzumab injection). Summary of product characteristics, Roche Products Limited, Welwyn Garden City, UK, 2020.
- GAZYVA (obinutuzumab injection). Highlights of prescribing information, Roche Products Limited, Welwyn Garden City, UK, 2020.
- Woyach JA, Ruppert AS, Awan F, et al. A phase II study of the Fc engineered CD19 antibody MOR208 in combination with lenalidomide for patients with chronic lymphocytic leukemia (CLL). Blood. 2015;126(23):2953.
- Woyach JA, Ruppert AS, Awan FT, et al. Updated results from a phase II study of the fc engineered CD19 antibody MOR208 in combination with lenalidomide for patients with chronic lymphocytic leukemia (CLL) and Richter’s transformation or ibrutinib for patients with ibrutinib-resistant clones. Blood. 2016;128(22):4386.
- Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometr Syst Pharmacol. 2017;6(9):576–588.
- Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19(1):65–75.
- Skarzynski M, Niemann CU, Lee YS, et al. Interactions between ibrutinib and anti-CD20 antibodies: competing effects on the outcome of combination therapy. Clin Cancer Res. 2016;22(1):86–95.
- Rawstron A, Munir T, Brock K, et al. Ibrutinib and obinutuzumab in CLL: improved MRD response rates with substantially enhanced MRD depletion for patients with >1 year prior ibrutinib exposure. Blood. 2018;132(Suppl. 1):181.
- Sharman JP, Banerji V, Fogliatto LM, et al. Phase 3 study of acalabrutinib combined with obinutuzumab or alone vs obinutuzumab plus chlorambucil in patients with treatment-naive chronic lymphocytic leukemia: results from ELEVATE TN. Blood. 2019;134(Suppl. 1):31.