Abstract
To describe patient characteristics, treatment patterns, and survival among elderly patients (≥66 years) newly diagnosed with acute myeloid leukemia (AML) meeting ≥1 ineligibility criteria for high-intensity chemotherapy (HIC; i.e. age >75 years, cardiac disease/prior anthracycline therapy, or secondary AML), we analyzed 2007–2017 100% Medicare hematologic cancer data. Patients were stratified based on whether they received HIC or low-intensity chemotherapy (LIC) or best supportive care (BSC) within 60 days after AML diagnosis. Of 4,152 patients, 43.2% received chemotherapy, 33.8% BSC, and 23.1% no therapy. Among chemotherapy-treated patients, HIC was more common than LIC (58.8 vs 41.2%), despite targeting patients meeting ≥1 ineligibility criteria for HIC. Poor overall survival was observed for patients receiving chemotherapy and BSC (median overall survival [interquartile range]: HIC, 1.9 [0.8, 6.6] months; LIC, 3.8 [1.4, 9.3] months; BSC, 1.0 [0.4, 2.5] months). Results highlight the need for additional effective and tolerable treatments for this population.
Introduction
AML is the second most common leukemia in the United States and is associated with the highest number of deaths [Citation1]. AML is particularly common among elderly patients; approximately 75% of patients are ≥65 years and median age at diagnosis is 68 years [Citation2,Citation3]. Notably, the incidence of AML has been gradually increasing over time particularly since 2011 [Citation4]; in contrast, mortality has remained relatively stable [Citation2]. The estimated median overall survival (OS) for patients with AML diagnosed at ≥65 years of age in the United States is 2.7 months, and only approximately 22% of such patients survive one year after diagnosis [Citation2], highlighting the relatively few treatment options for most patients.
Generally, treatment of AML consists of intensive remission induction and postremission/consolidation therapy [Citation5]. Despite the development of novel therapeutics [Citation6], there is currently no optimal treatment approach for elderly patients with AML. As the elderly represent a heterogeneous population, a thorough geriatric assessment is warranted before deciding on a regimen, including assessment of performance status, comorbidities, and age group [Citation7,Citation8]. For patients who are not a candidate for or decline high-intensity chemotherapy (HIC; also referred to as standard induction therapy), low-intensity therapies are recommended. These include low-dose chemotherapy (e.g. cytarabine), single agent low-intensity therapies (e.g. the hypomethylating agent [HMA] azacitidine), targeted therapies (TT; e.g. midostaurin [Citation9]) or best supportive care (BSC) [Citation5]. Alternatively, these patients may be enrolled in clinical trials when available [Citation8].
There is little information available on treatment patterns and outcomes of elderly patients with AML meeting ineligibility criteria for HIC in the real-world setting before the introduction of novel TT combinations (i.e. venetoclax in combination with HMAs) which could be transformative for this population [Citation10]. To understand the types of patients that would be expected to receive these combination therapies, we conducted a retrospective cohort study.
Materials and methods
Data source
This study used the Centers for Medicare & Medicaid Services (CMS) 100% Medicare beneficiaries with hematologic malignancies including AML, from 2007 to 2017. The data included the annual Master Beneficiary Summary File (MBSF), which included the demographic information and Medicare enrollment status, and the annual claims-based standard analytic files, which included Part A institutional, Part B carriers, and Part D pharmacy files [Citation11]. Mortality information (date of death from any cause) was obtained using the date of death field from the MBSF. The study was approved by the Institutional Review Board of the Hennepin Healthcare System, Inc., Office for Human Subjects Research.
Study design
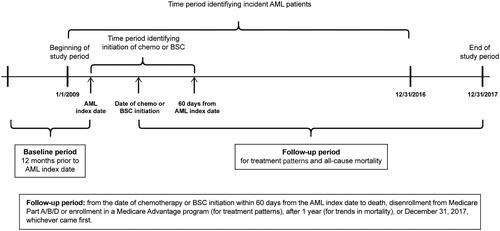
This was a retrospective cohort study among elderly Medicare beneficiaries newly diagnosed with AML who met ≥1 ineligibility criteria for HIC, but were eligible for low intensity therapy and would often be eligible for clinical trials (ECOG PS ≤2) [Citation10]. The case identification period was from January 1 2009 to December 31 2016. The date of first claim qualifying for the definition of incident AML was defined as the index date and was an estimate of the diagnosis date as the exact date may not have been available in the claims data. The baseline period was 12 months before the index date, during which patient demographics and medical history were defined. Up to 60 days after the index date were used as the period to identify initiation of chemotherapy/BSC. The follow-up period for outcomes was from the date of qualifying chemotherapy/BSC initiation to death, disenrollment from any Medicare Part A, B or D or enrollment in a Medicare Advantage program (for treatment patterns), after 1 year (for trends in mortality), or December 31 2017 (end of study period), whichever came first ().
Population
Published algorithms [Citation12,Citation13] were adapted to identify patients newly diagnosed with AML during the case identification period. Details of the algorithm and the eligibility criteria are provided in and the Supplementary Methods. Briefly, we required newly diagnosed patients with a code for not having achieved remission on their first claim for AML and, on the same claim, without codes for remission or relapse (Supplementary Table 1). If the first claim was from Part A outpatient or Part B, presence of a second claim carrying a code for not having achieved remission within 30 days of the first claim was required. We further required patients with ≥1 claim for blood count test AND ≥1 claim for bone marrow test within 30 days before or after the index date [Citation12]. Patients were excluded if they had a claim carrying an AML code any time prior to the index date (i.e. potential prevalent cases), or had acute promyelocytic leukemia or confounding diseases [Citation13].
Figure 2. Patient flow diagram. AML: acute myeloid leukemia; BSC: best supportive care; CML: chronic myeloid leukemia; ECOG: Eastern Cooperative Oncology Group; HIC: high intensity chemotherapy; LIC: low intensity chemotherapy; MDS: myelodysplastic syndrome; MPN: myeloproliferative neoplasm; TT: targeted therapy. (a) The fifth digit of the diagnosis code for AML can be 0 (not having achieved remission), 1 (in remission), or 2 (in relapse). (b) If the first claim is from Part A outpatient or Part B, presence of a second AML claim from any source carrying the diagnosis code for not having achieved remission (.X0) within 30 days of the initial diagnosis is required. (c) Presence of a confounding disease was defined by ≥1 Part A inpatient, skilled nursing facility, home health agency, or hospice claim with specific diagnosis codes for the disease, or ≥2 Part A outpatient or Part B claims with specific diagnosis codes for the disease on different days up to 30 days. This definition was applied for each individual disease during the baseline period and the follow-up period. (d) Other confounding diseases include: neoplasm of uncertain or unspecified behavior, acute lymphoid leukemia, lymphoid leukemia, monocytic leukemia, other leukemia, eosinophilia, gastrointestinal stromal tumor, malignant mast cell tumors. (e) Criteria for ‘ineligible for standard induction chemotherapy’ include: age >75 years at AML diagnosis, cardiac disease or prior anthracycline, secondary AML.
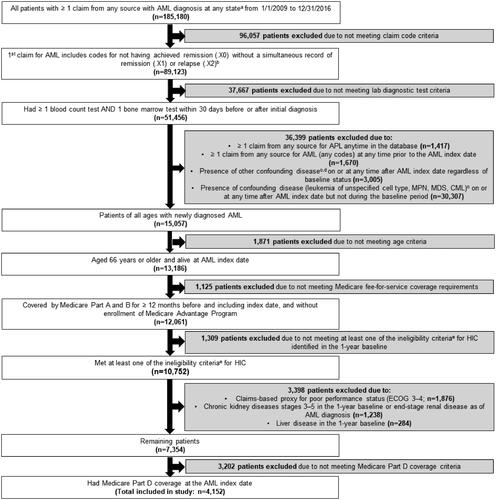
Table 1. Patient characteristics of elderly patients newly diagnosed with AML between 1 January 2009 and 31 December 2016, overall and by treatment status.
Patients with newly diagnosed AML were included in the study if they were aged ≥66 years at the index date (to allow for the 12-month baseline period before the index date); were continuously enrolled in Medicare Parts A and B without enrollment in a Medicare Advantage program for ≥12 months before the index date, including the index date; had Medicare Part D coverage at the index date; and met ≥1 of the criteria for ineligibility for HIC (i.e. age >75 years at AML diagnosis, cardiac disease or prior anthracycline use in the 1-year baseline, secondary AML)[Citation10]. Patients were excluded if they had a claims-based proxy for poor Eastern Cooperative Oncology Group performance status (ECOG PS 3–4) [Citation14–16]; had chronic kidney disease at stages 3–5 or liver disease in the baseline period, or had end-stage renal disease at the time of AML diagnosis.
Assessments
Treatment (chemotherapy, BSC, stem cell transplant [SCT]) was identified by the appearance of a Medicare claim with ≥1 relevant billing code (Supplementary Tables 2–5). Details on treatment definition are provided in the Supplementary Methods.
Table 2. Chemotherapy treatment patterns for patients (n = 1,792) receiving chemotherapy within 60 days of AML index date.
Table 3. Unadjusted cumulative incidence of all-cause death and median OS from chemotherapy or BSC initiation.
Type of chemotherapy was defined by the following hierarchy [Citation5]: (1) HIC included chemotherapy given in the inpatient setting and/or outpatient chemotherapy with cladribine, clofarabine, cytarabine (intermediate/high-dose), fludarabine, daunorubicin, idarubicin, mitoxantrone, or etoposide; (2) low-intensity chemotherapy (LIC) included HMAs (decitabine or azacitidine) or low-dose cytarabine without daunorubicin and idarubicin in the absence of HIC; and (3) TT included gemtuzumab ozogamicin, venetoclax, sorafenib, trametinib, midostaurin, and enasidenib in the absence of HIC and LIC. Since <11 patients received regimens containing only TT, the TT group was combined with the LIC group for analyses. Chemotherapy setting was defined based on chemotherapy claim sources in the first course and categorized as inpatient hospital only, outpatient only, and both inpatient and outpatient.
Age was calculated at the index date and categorized into five groups (66–69, 70–74, 75–79, 80–84, and ≥85 years). Presence of antecedent hematological disease (myelodysplastic syndromes or myeloproliferative diseases) and prior cancer (hematologic cancers, solid tumors) was identified during the baseline period. The level of comorbidity was assessed using the Quan-adapted Charlson Comorbidity index (CCI) [Citation17,Citation18] and categorized into 3 groups (0, 1–2, ≥3).
Statistics
This study was descriptive. Categorical variables were reported as number and percentage, and continuous variables as mean (standard deviation [SD]) and median (interquartile range [IQR]). The Chi-squared test was used for comparing categorical variables and t test for continuous variables. Treatment patterns (type, number, duration, and setting) were characterized using the descriptive statistics. The Kaplan-Meier method was used to estimate the cumulative incidence of all-cause death (95% confidence interval [CI]) and calculate median OS (IQR) for all treated patients and by age, CCI, type of treatment (HIC, LIC/TT, or BSC), and year of AML index date. Log-rank test was used for comparing OS between subgroups. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient population
A total of 4,152 patients were included in the study (); of those, 3,194 (76.9%) had chemotherapy or BSC initiated within 60 days of the AML index date and 958 (23.1%) patients were not treated with either therapy. Of patients who received chemotherapy/BSC, 1,053 (33.0%) received HIC, 739 (23.1%) received LIC/TT, and 1,402 (43.9%) received BSC only.
Generally, patients that initiated chemotherapy/BSC within 60 days of AML index date were slightly younger than patients that did not initiate chemotherapy/BSC (mean age [SD]: 78.1 [6.5] vs. 79.9 [7.0] years; p < 0.001; ) and were less likely to have a CCI ≥3 compared with untreated patients (22.9 vs. 30.2%; p < 0.001). Among treated patients, those receiving HIC were much younger than those who received LIC/TT or BSC (mean [SD]: 75.4 [5.8] vs. 79.0 [5.7] vs. 79.8 [6.8] years, respectively; p < 0.001). Furthermore, patients that received HIC or LIC/TT were less likely to have a CCI ≥3 compared with those who received BSC (20.6 vs. 19.8 vs. 26.3%, respectively; p < 0.001).
Significant variations in number of HIC ineligibility criteria met by patients were observed across therapy groups (p < 0.001; ). Meeting one ineligibility criteria was most common in the HIC group than in the other three groups (HIC vs. LIC/TT vs. BSC vs. no treatment: 51.9 vs. 35.7 vs. 38.1 vs. 39.7%, respectively) while meeting all three ineligibility criteria was least common in the HIC group than in the other three groups (9.1 vs. 18.8 vs. 16.3 vs. 15.4%).
Treatment patterns
Less than half of all patients (n = 1,792/4,152; 43.2%) had chemotherapy initiated within 60 days from AML index date. The vast majority of these patients (n = 1,636/1,792; 91.3%) received only one course of chemotherapy (). The mean (SD) duration of the first course of chemotherapy was 15.0 (23.6) weeks. Generally, slightly more chemotherapy-treated patients received HIC versus LIC/TT (HIC, 58.8%; LIC/TT, 41.2%). HIC was typically initiated in hospital; LIC/TT was usually administered in an outpatient setting, and was typically azacitidine (n = 394/739, 53.3%), decitabine (n = 310/739, 41.9%), or low-dose cytarabine (n = 15/739, 2.0%). Furthermore, percentages of patients receiving their first course of chemotherapy in an inpatient or outpatient setting were approximately equal (inpatient only, 42.2%; outpatient only, 40.5%; both settings, 17.3%).
Of the 1,792 patients who received chemotherapy, 85 patients (4.7%) had a SCT. The median time from initiation of chemotherapy to SCT was 106 days (IQR: 59–164). SCT was more common in younger patients (66–69 years) than those ≥70 years old (18.2 vs. 2.2%, respectively), in patients receiving HIC than those receiving LIC/TT (6.9 vs 1.6%, respectively), and in patients receiving chemotherapy in both the inpatient and outpatient setting versus only inpatient and only outpatient settings (9.7 vs. 5.8 vs. 1.5%, respectively).
About one-third of all patients (n = 1,402/4,152; 33.8%) received only BSC within 60 days from AML index date. The most common treatment received as BSC was blood transfusion, alone (n = 1,239/1,402, 88.4%) or in combination with hydroxyurea (n = 112/1,402, 8.0%). Of all patients receiving a blood transfusion as BSC, the most common treatment was red blood cells or whole blood, alone (n = 1,044/1351, 77.3%) or in combination with platelets (n = 279/1,351, 20.7%).
Mortality
The cumulative incidence (95% CI) of all-cause death at the 30-day and 90-day mark was slightly higher for patients who received HIC versus LIC/TT (30-day all-cause death: HIC, 32.4% [29.6, 35.3]; LIC/TT, 18.0% [15.4, 21.0]; 90-day all-cause death: HIC, 60.0% [57.1, 63.0]; LIC/TT, 42.5% [39.0, 46.1]; ). Additionally, patients receiving BSC had an even higher cumulative incidence of all-cause death at the 30-day and 90-day mark: 51.9% (49.3, 54.6) and 78.5% (76.3, 80.6), respectively.
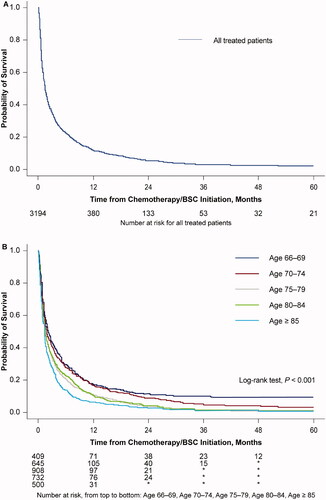
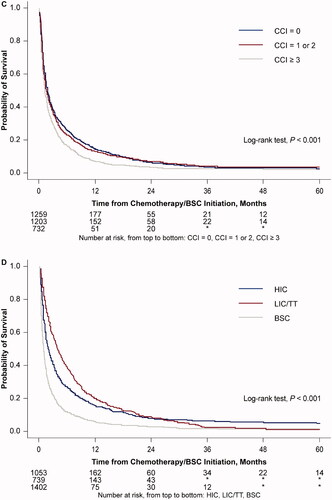
Median OS (IQR) for all patients who received chemotherapy/BSC was 1.6 (0.7, 5.2) months (; ). A decline in OS was observed with increasing age at index date, with the median OS (IQR) being 2.1 (0.8, 8.0) months for patients aged 66–69 years at index date, and 1.3 (0.6, 3.4) months for patients aged ≥85 years (). A similar decline in median OS (IQR) was observed as CCI increased (CCI 0, 1.8 months [0.7, 6.4]; CCI 1–2, 1.6 months [0.6, 5.2]; CCI ≥3, 1.2 months [0.5, 3.7]; ). Patients receiving LIC/TT had the longest median OS (IQR) at 3.8 (1.4, 9.3) months, compared with 1.9 (0.8, 6.6) months for those receiving HIC, and 1.0 (0.4, 2.5) month for those receiving BSC ().
Figure 3. Overall survival probability from treatment initiation: (A) all treated patients, and by (B) age at the AML index date, (C) CCI, and (D) type of therapy. AML: acute myeloid leukemia; BSC: best supportive care; CCI: Quan-Charlson Comorbidity Index; HIC: high intensity chemotherapy; LIC: low intensity chemotherapy; TT: targeted therapy.
When stratifying patients by year of AML index date, there was a decrease in cumulative incidence of all-cause death and a slight increase in median OS over time (Supplementary Table 6; Supplementary Figure 1). More specifically, the cumulative incidence (95% CI) of all-cause death at the 30-day mark was 40.4% (35.4, 45.9) in 2009 and 35.7% (31.2, 40.6) in 2016. Similar trends were observed for the 90-day mark and 1-year mark (90-day mark: 68.3 [63.2, 73.2] and 59.1 [54.3, 63.9] for 2009 and 2016, respectively; 1-year mark: 91.3 [88.0, 94.0] and 81.3 [77.3, 84.9] for 2009 and 2016, respectively). Median OS (IQR) was 1.2 (0.6, 4.2) months in 2009 and 1.9 (0.6, 7.2) months in 2016.
Discussion
The goal of this study was to describe real-world treatment patterns and mortality in a large population-based cohort of elderly patients with newly-diagnosed AML who met ≥1 of the criteria for ineligibility for HIC (i.e. age >75 years at AML diagnosis, cardiac disease or prior anthracycline therapy, or secondary AML), but were likely to tolerate low intensity therapy and would often be eligible for clinical trials (i.e. ECOG PS ≤2, no liver disease, and no advanced stages of renal disease) [Citation10]. The most striking observation in this study is that most patients (56.8%) in the selected population received no chemotherapy treatment for AML. This was an unexpected finding based on the exclusion criteria for the study. Since patients with a claims-based proxy for poor performance status (ECOG PS 3–4) [Citation14,Citation15] or other comorbidities (i.e. liver disease, advanced stages of renal disease), which would typically make them ineligible for chemotherapy treatment, were excluded it was expected that most patients would have been eligible to receive some form of antileukemic therapy. In addition, among patients who did receive chemotherapy treatment, HIC was more common than LIC/TT (58.8 vs. 41.2%) despite the fact that the population selected was unlikely to be suitable for HIC as previously defined [Citation10]. These unexpected findings are likely related to patients’ presentation meeting the inclusion criteria. The study findings showed that patients receiving HIC were more likely to have met only one ineligibility criteria for HIC (HIC vs LIC/TT vs BSC vs no treatment: 51.9 vs. 35.7 vs. 38.1 vs. 39.7%) and least likely to have met all three ineligibility criteria (HIC vs LIC/TT vs BSC vs no treatment: 9.1 vs. 18.8 vs. 16.3 vs. 15.4%). Additionally, patients who received BSC or no treatment were more likely to be older (≥75 years), and/or have cardiac disease or prior exposure to anthracyclines, and have a higher comorbidity burden (CCI ≥3) than those who received chemotherapy. Concerns regarding toxicity of chemotherapy, decreased quality of life, and increased risk of treatment-related morbidity and mortality may have precluded these patients from receiving chemotherapy. Present guidelines recommend selecting treatment based on ECOG PS, comorbid condition, or AML adverse factor (secondary AML, novel AML without unfavorable cytogenetics or molecular markers) rather than relying on chronologic age alone [Citation5].
Despite these unexpected findings, results from this study are in agreement with earlier investigations of treatment patterns in elderly patients with AML, even when populations were not selected for those who typically would not be eligible for intensive therapy. More specifically, in a similar study conducted prior to this analysis, approximately 60% of patients with AML remained untreated [Citation19]; this is similar to the results seen in the current study, where 56.8% of all patients received BSC or were untreated. It is disappointing that 10 years later, these non-treatment trends remained similar [Citation19]. Of treated patients in our analysis, 44% received BSC, reflective of the 37% and 43% of treated patients receiving BSC in earlier studies [Citation20,Citation21].
Additionally, low utilization of SCT in the selected population treated with chemotherapy in the current study (4.7%) is consistent with earlier studies which showed that 8% (mean age 75 years) [Citation19] and 5.5% (61 − 75 years) [Citation22] of treated patients underwent SCT. The potential barriers to SCT in elderly patients with AML are multifactorial [Citation23] and as expected, chronologic age appears to be one of the major driving factors in receiving SCT. Our observation of a much lower receipt of SCT in patients aged ≥70 years is similar to that in an earlier study which showed that patients aged 71–75 years of age had 80% lower odds of receiving SCT compared with those aged 66–70 years of age [Citation22]. Lack of physician referral to a blood and marrow transplant specialist may also contribute to the low rate of SCT. In a national survey of hematologists and medical oncologists in the US, physician’s perception of SCT risks, insurance coverage, age, comorbidity, race, and social support were considered strong factors influencing physician referral for SCT [Citation24]. Efforts are needed to improve access to SCT for appropriate patients. Encouragingly, in a trial of venetoclax and HMAs in older patients with treatment-naive AML ineligible for HIC, approximately 15% of patients withdrew from the trial to undergo SCT, suggesting that the increased complete response rates from that combination therapy may enable more patients to undergo potentially curative SCT [Citation10].
We found that the 30-day and 1-year survival rates were low for both HIC and LIC/TT, and even lower for BSC. This signals an urgent unmet need for more effective therapies for this patient group. Despite this need, elderly patients are highly underrepresented in clinical trials for AML [Citation25]. We also observed a higher early mortality among patients receiving HIC compared to those receiving LIC. One possible explanation for this observation is the likelihood of complications from induction therapy contributing to mortality, particularly in the elderly [Citation26]. This underscores the importance of appropriately selecting the most tolerable regimen for each patient.
A slight improvement of overall survival was detected in patients with more recent AML index dates. A likely explanation for this finding is the recent development and approval of several TT and other drugs for AML, many of which are indicated for newly-diagnosed elderly patients [Citation6,Citation27], and have been associated with increased response rates, longer OS, and generally tolerable risk profiles.
The landscape of AML treatment options has been evolving rapidly in recent years [Citation6]. A new treatment option combining the oral BCL2 inhibitor venetoclax, with either azacitidine or low-dose cytarabine, has been approved for use and has shown promising results with higher complete response rates and longer OS [Citation28–30]. However, real-world outcomes for venetoclax with azacitidine have been inferior to clinical trial results [Citation31], and adverse events (AEs) must be appropriately managed [Citation29]. Therefore, despite the availability of new treatments, maximizing benefit and minimizing toxicity still proves challenging for elderly patients, particularly when attempting to balance potential AEs and therapeutic benefit.
This study has some limitations. Firstly, an algorithm adapted from two published studies [Citation12,Citation13] was used to identify patients with newly diagnosed AML, so there was some potential for misclassification. However, the vast majority of patients had a short survival as would be expected of this population, so it is unlikely there was a significant amount of diagnosis misclassification. Secondly, inpatient treatment data were sparse since the diagnostic-related group system does not require a detailed accounting of inpatient services provided. For this reason, inpatient treatment was used as a proxy for HIC and the specific treatment regimen was unknown. This was an assumption that could also potentially lead to misclassification of treatment. Thirdly, as an administrative claims dataset was used, there are limits to which clinical factors were available. Some additional factors which would have further helped define the population if available include ECOG PS, cytogenetics and bone marrow disease burden, factors commonly used for patient risk assessment and guide treatment selection [Citation8,Citation32]. Although a validated claims-based poor disability status prediction model [Citation14,Citation16] was used to estimate the probability of poor disability status as a proxy measure for ECOG PS of 3–4 at the diagnosis of AML, misclassification of performance status likely exists. Fourthly, the time period evaluating the treatment patterns is before the era of new TT (e.g. the oral BCL2 inhibitor venetoclax). Thus, our analyses could not assess whether the introduction of new TT has changed treatment patterns and overall survival. Lastly, the study findings may not be applied to all older patients diagnosed with AML with Medicare fee-for-service coverage or older patients with Medicare Advantage coverage or patients residing outside the United States. Despite these limitations, this study provides data on overall treatment patterns and survival in elderly patients newly diagnosed with AML who typically do not meet the ineligibility criteria for HIC, and reflects how these patients were managed in routine clinical practice in the United States.
Conclusions
The population identified herein consisted of elderly patients with newly-diagnosed AML, who met ≥1 of the criteria for ineligibility for HIC, but were eligible for low intensity therapy and would often be eligible for clinical trials (ECOG PS ≤2). As might be expected, results indicate that the majority of these patients did not receive HIC or LIC. This highlights the need to develop additional effective and tolerable treatments for elderly patients with AML.
GLAL-2021-0270-File011.docx
Download MS Word (189 KB)Acknowledgments
The authors thank Vicky Kanta, PhD and Lee Hohaia, PharmD (ICON, North Wales, PA), whose work was funded by Amgen Inc., for medical writing assistance in the preparation of this manuscript.
Disclosure statement
Shuling Li, Yuanyuan Ji, and Yi Peng are employees of Chronic Disease Research Group, Hennepin Healthcare Research Institute, which has received project funding from Amgen Inc.
Christopher Kim is an employee and stockholder of Amgen Inc. Vamsi Kota has received honoraria from Novartis and Pfizer, and research funding from Amgen and Incyte.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30.
- Shallis RM, Wang R, Davidoff A, et al. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019;36:70–87.
- Turbeville S, Francis KM, Behm I, et al. Prevalence and incidence of acute myeloid leukemia may be higher than currently accepted estimates among the ≥65 year-old population in the United States. Blood. 2014;124(21):958–958.
- Hao T, Li-Talley M, Buck A, et al. An emerging trend of rapid increase of leukemia but not all cancers in the aging population in the United States. Sci Rep. 2019;9(1):12070.
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721–749.
- Cerrano M, Itzykson R. New treatment options for acute myeloid leukemia in 2019. Curr Oncol Rep. 2019;21(2):16.
- Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713.
- Webster JA, Pratz KW. Acute myeloid leukemia in the elderly: therapeutic options and choice. Leuk Lymphoma. 2018;59(2):274–287.
- Tomlinson BK, Gallogly MM, Kane DM, et al. A phase II study of midostaurin and 5-azacitidine for untreated elderly and unfit patients with FLT3 wild-type acute myelogenous leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(4):226–233.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
- Mues KE, Liede A, Liu J, et al. Use of the medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol. 2017;9:267–277.
- Craig BM, Rollison DE, List AF, et al. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol Biomarkers Prev. 2012;21(3):474–481.
- Irish W, Ryan M, Gache L, et al. Acute myeloid leukemia: a retrospective claims analysis of resource utilization and expenditures for newly diagnosed patients from first-line induction to remission and relapse. Curr Med Res Opin. 2017;33(3):519–527.
- Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4(2):157–165.
- Li S, Natwick T, Liu J, et al. Mortality by a proxy performance status as defined by a claims-based measure for disability status in older patients with newly diagnosed multiple myeloma in the United States. J Geriatr Oncol. 2019;10(3):490–496.
- Davidoff AJ, Gardner LD, Zuckerman IH, et al. Validation of disability status, a claims-based measure of functional status for cancer treatment and outcomes studies. Med Care. 2014;52(6):500–510.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Medeiros BC, Satram-Hoang S, Hurst D, et al. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94(7):1127–1138.
- Bories P, Bertoli S, Bérard E, et al. Intensive chemotherapy, azacitidine, or supportive care in older acute myeloid leukemia patients: an analysis from a regional healthcare network. Am J Hematol. 2014;89(12):E244–252.
- Ma E, Bonthapally V, Chawla A, et al. An evaluation of treatment patterns and outcomes in elderly patients newly diagnosed with acute myeloid leukemia: a retrospective analysis of electronic medical records from US community oncology practices. Clin Lymphoma Myeloma Leuk. 2016;16(11):625–636.e623.
- Bhatt VR, Chen B, Gyawali B, et al. Socioeconomic and health system factors associated with lower utilization of hematopoietic cell transplantation in older patients with acute myeloid leukemia. Bone Marrow Transplant. 2018;53(10):1288–1294.
- Lipof JJ, Loh KP, O'Dwyer K, et al. Allogeneic hematopoietic cell transplantation for older adults with acute myeloid leukemia. Cancers. 2018;10(6):179
- Pidala J, Craig BM, Lee SJ, et al. Practice variation in physician referral for allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(1):63–67.
- Kalin B, Pijnappel EN, van Gelder M, et al. Intensive treatment and trial participation in elderly acute myeloid leukemia patients: a population-based analysis in the Netherlands. Cancer Epidemiol. 2018;57:90–96.
- Ho G, Jonas BA, Li Q, et al. Early mortality and complications in hospitalized adult Californians with acute myeloid leukaemia. Br J Haematol. 2017;177(5):791–799.
- Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93(10):1267–1291.
- Venclexta (venetoclax). Full prescribing information., AbbVie Inc., North Chicago, IL, 2020.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629.
- Wei AH, Strickland SA, Jr., Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–1284.
- Winters AC, Gutman JA, Purev E, et al. Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv. 2019;3(20):2911–2919.
- Percival ME, Lai C, Estey E, et al. Bone marrow evaluation for diagnosis and monitoring of acute myeloid leukemia. Blood Rev. 2017;31(4):185–192.