Abstract
Diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) treatments have been rapidly evolving for patients treated in later lines of therapy (LoT). Country-specific cancer registry data for the US and Western Europe (WE) were combined with physician survey results to project the incidence, prevalence, and number of DLBCL and FL patients eligible for and treated by LoT between 2020 and 2025. The total number of incidents and prevalent cases of DLBCL and FL is expected to increase between 2020 and 2025 in the US and WE. 56% and 53% of the third line plus (3L+) eligible DLBCL patients and 60% and 55% of eligible FL patients initiated treatment in the US and WE, respectively. Further research is warranted to understand the reasons behind the high proportion of treatment eligible patients who do not initiate treatment, and potential differences between countries, especially in the 3L + settings.
Introduction
Diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) are two of several heterogeneous neoplasms that originate in lymphoid tissue comprising non-Hodgkin lymphoma (NHL). The incidence rates of NHL in the United States (US) and Western Europe (WE), including France, Germany, Italy, Spain and the United Kingdom (UK), are among the highest worldwide with an estimated 73,652 new cases for the US and 72,035 estimated for WE combined in 2020 [Citation1]. DLBCL and FL are the most common NHL subtypes in both the US and WE [Citation2].
R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) has been the standard of care in the first line for DLBCL globally [Citation3,Citation4]. Yet, 20% to 50% of DLBCL patients experience relapse or are refractory to first-line and become eligible for second-line and subsequent treatment [Citation5,Citation6]. Unfortunately, once a patient reaches the third line few treatments have been available historically and overall survival is less than 7 months [Citation7]. Recent approvals for DLBCL treatment have primarily occurred in the relapsed setting, including 3 CAR-T therapies (axicabtagene ciloeucel, FDA 2017; EMA 2018; tisagenlecleucel, FDA 2018; EMA 2018; lisocabtagene maraleucel, FDA 2021), tafasitamab-cxix (FDA, 2020), and selinexor (FDA, 2020).
With FL being an indolent disease, some patients delay treatment until they become symptomatic and are put in ‘watchful waiting’ (WW). Rituximab-based regimens are first-line standard of care globally [Citation3,Citation6]. Similarly, rituximab-based regimens are the standard of care in subsequent lines for FL in both the US and WE. Once FL patients become relapsed or are refractory, their overall survival, while much longer than for DLBCL, diminishes with each subsequent LoT [Citation8]. Recently approved agents for the treatment of relapsed/refractory FL the combination of lenalidomide and rituximab (FDA 2019), tazemetostat (FDA 2020), umbralisib (FDA 2021), and the CAR-T therapy axicabtagene ciloeucel (FDA 2021). The approval of CAR-T therapy for multiply relapsed FL patients may redefine the field.
As more new anti-neoplastic agents are introduced and survival duration for DLBCL and FL increase, patients will live longer and take longer to progress, thus able to receive additional lines of therapy. However, information from real-world clinical practice, such as the number of patients eligible for a given LoT, is limited. In similar situations, different researchers [Citation9,Citation10] have utilized the strategy of combining data from various sources, such as publicly available registry-based epidemiology sources and surveys, to estimate the incidence, prevalence, and treated patient populations by lines of therapy. The purpose of this analysis was to use a combination of country-specific cancer registry data and recent physician survey data, to project the incidence, prevalence, and the number of DLBCL and FL patients eligible for and treated by LoT between 2020 and 2025 in the US and WE.
Materials and methods
Data sources
This study was a retrospective data analysis of DLBCL and FL in the US and WE, utilizing combined data from: a) country-specific cancer registries for the US, France, Germany, Italy, Spain, and the UK, b) peer-reviewed literature, and c) results of a physician survey conducted by Kantar Health (CancerMPact® Treatment Architecture). CancerMPact (CMP) is a proprietary data source from Kantar Health, containing data on cancer epidemiology and treatment [Citation11]. This data source comprises different modules of data, of which Treatment Architecture was used for this study.
Cancer registry data
Annual age and sex-specific incidence rates for DLBCL and FL have first identified from country-specific databases [Citation12–17]. The available data from the registries and the approach for deriving missing data are shown in . Where annual incidence rates were not available, as for Spain, or data from the publicly available registry was not as recent as data reported to IARC, as for Italy, comparisons were made to data from other countries and annual rates were applied from comparable countries as indicated.
Table 1. Country-specific cancer registry data and peer-reviewed literature sources used to derive the number of incident patients with DLBCL and FL between 2020 and 2025 in the US and WE.
Peer-reviewed literature
For Germany, Italy, Spain, and the UK, incidence rates were available by total NHL only. Therefore, a targeted literature review was conducted to identify the proportion of patients with DLBCL and FL among the total NHL within each of these countries. Country-specific proportions of DLBCL and FL from the peer-reviewed literature were identified for Germany, Spain and the UK and applied to the country-specific total NHL incidence. No studies were identified for Italy, thus the weighted average of the NHL subtype proportions from all articles identified with European patient populations was applied to the total Italy NHL incidence (See Supplemental Material).
Physician survey data
The Treatment Architecture (TA) data within CMP contains findings from an annual survey of physicians in multiple countries, designed to collect information regarding their clinical experience in different tumor types and to identify the unmet needs in the treatment for these tumors (including DLBCL and FL). The NHL surveys in the US and WE conducted in 2018 and 2019 were used for this study. To participate, physicians must have been board-certified medical and/or hematologic oncologists in practice between 3 and 30 years and have been treating a minimum of 30 NHL patients per month.
The questionnaire asked physicians about their experience and medical practice characteristics (years in practice, practice type, etc.) and how they had treated their patients with DLBCL and FL within the past 6 months at the time of the survey. Questions (see Supplemental Material) covered treatment across all LOTs, modality of treatment (radiation, systemic therapy, etc.), systemic therapy regimens used and their duration. Definition of relapsed/refractory disease or LoT was not provided in the survey but was based on the physicians’ own definition from their clinical practice and experience. The survey did not ask for information beyond a fourth line or clinical trial participation.
Analysis
Incidence
The annual age and sex-specific incidence rates for DLBCL and FL for each country were obtained or derived according to the approach provided in . Using the observed historical trend in incidence for each NHL subtype and country, the incidence rates between 2020 and 2025 were projected. The future incidence rates were estimated using the most recent historical age and sex-specific rates and following the observed trend forward for up to 5 years, then the incidence rates were projected across remaining years at a constant rate since the trends far into the future are unknown. In cases of a flat historical trend, the most recent historical incidence rates were used across all future years. Then the projected annual age- and sex-specific rates were multiplied by the respective age- and sex-specific projected population for each country [Citation18–20] to obtain the annual number of incident cases (see Supplemental Material for Equations).
Prevalence
The annual 10-year prevalence of DLBCL and FL for the US and WE was defined as the number of patients diagnosed within the past 10 years (given year and prior 9 years) who survived to the given year. The most recent observed annual survival rates for DLBCL and FL were obtained from SEER for the US [Citation17] and for NHL from EUROCARE-5 for WE [Citation21]. Survival rates for DLBCL and FL in WE were derived from the NHL survival rates and ratios from SEER. Using the country-specific annual total incidence and country-specific observed annual survival rates, the annual 10-year prevalence for DLBCL and FL was calculated (See Supplemental Material for Equations). The annual 20-year prevalence of DLBCL and FL for the US and WE were also calculated using the same methodology and sources (See Supplemental Material).
Treatment
Treatment data were derived by averaging the results of the two most recent annual physician surveys (2018, 2019) available at the time of this study. For each annual survey, the responses for each question are calculated as an unweighted average of all responding physicians to account for variation in clinical practice. These treatment data (e.g. proportion of patients treated in a given LoT) were applied as a constant for all years projected due to the lack of reliable prediction of treatment paradigm in the future.
DLBCL patients eligible for 1 L treatment was defined as including total incident DLBCL. Watchful waiting (WW), i.e. not receiving any treatment, is not significant in DLBCL as patients tend to be treated within the same year. Thus, 1 L treated DLBCL was defined as the number of all newly diagnosed DLBCL patients initiating systemic therapy.
FL patients eligible for 1 L treatment was defined as total incident FL and WW patients from the previous 6 years who survived to the given year. 1 L treated FL was defined as the number of newly diagnosed FL patients initiating treatment in the given year plus a proportion of patients, as reported by the responding physicians, who were under WW and initiated treatment in the given year. When calculating the number of WW patients who initiated treatment in a given year, we took into consideration the time between initial diagnosis and treatment initiation. The surveys did not ask physicians for the time between WW and initiating treatment, thus data on time to treatment were obtained from the peer-reviewed literature [Citation22,Citation23]. Literature showed that few to no patients initiated treatment beyond 6 years on WW.
For the second line (2 L) and 3 L + therapy, patients whose disease progressed after receiving the prior line were defined as eligible for the next line. The proportion of 2 L or 3 L + patients who were considered eligible for the next line was applied to the patients treated in the previous line. DLBCL and FL patients treated in 2 L and 3 L + were defined as the number of DLBCL or FL patients who initiated a new systemic treatment upon having relapsed or were refractory to the prior line therapy.
All analyses were descriptive and no formal statistical testing was performed.
The database used for this research (CMP) does not collect or use patient-level data, or any data involving people, medical records, or human samples. All information is retrieved from online physician surveys regarding factual information around overall treatment patterns in NHL in the US and WE. Therefore, no Institutional Review Board approval was necessary.
Results
Incidence and prevalence
The number of incident DLBCL cases in the US is projected to increase from 29,108 to 32,443 and from 26,078 to 27,981 in WE from 2020 to 2025 (), with a total rate of increase of 11% in the US compared to 7% in the WE. This increase is related to increases in the underlying patient populations with older age groups having higher incidence rates. The total population in the US is slightly higher than the total of the 5 WE countries (e.g. 334.5 million in the US vs. 318.7 million in the WE in 2020), with a higher annual increase in the US compared to the WE in the elderly aged 65+ years (3% vs. 2%; data not shown). The projected 10-year prevalence for DLBCL cases is higher overall in the US () than WE and its total rate of increase was also higher (US: 12% vs WE: 7%). The increase in 10-year prevalence is related to the underlying increasing incidence. The 20-year prevalence of DLBCL increased at a total rate of 14% for the US and 11% for WE between 2020 and 2025 ().
Figure 1. Projected incidence and 10-year prevalence of DLBCL and FL in the US and Western Europe, 2020–2025. (A) DLBCL incidence; (B) DLBCL 10-year prevalence; (C) FL incidence; (D) FL 10-year prevalence. DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; WE: Western Europe; US: United States.
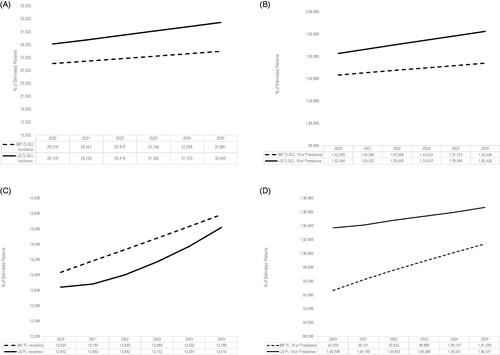
For FL, the number of incident cases in the US is projected to increase from 12,842 to 13,619 and from 13,034 to 13,786 in WE between 2020 and 2025 (), with a total rate of increase of 6% for both the US and WE. The projected 10-year prevalence of FL is higher in the US than WE (). The 10-year prevalence is projected to increase at a lower rate of 3% over the time period for the US compared to 7% for the WE. For the US, the historic incidence rate for FL is declining, resulting in a slower increase in the 10-year prevalence. The 20-year prevalence of FL increased at a total rate of 5% for the US and 12% for WE between 2020 and 2025 ().
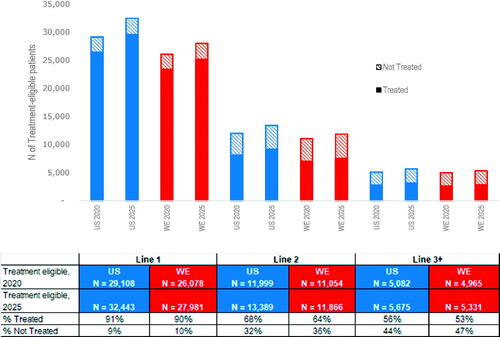
Figure 2. Estimated number of DLBCL treatment-eligible patients and the proportions of treated by line of therapy for the US and Western Europe in 2020 and 2025. Calculations use the average physician response data for the US and WE (2018/2019 surveys) for the proportion of patients who progressed but did not initiate and those who progressed and did initiate 1L-3L + treatment. The proportions of treatment by line of therapy were applied as a constant rate across all years, thus the proportions used to calculate treated and not treated were the same for 2020 and 2025. DLBCL: diffuse large B-cell lymphoma; WE: Western Europe; US: United States.
Country-specific DLBCL and FL results for the WE are also included in Supplemental Tables S1 and S2.
Physician survey results
A total of 234 and 159 physicians responded to the 2018 and 2019 TA surveys, respectively (). The average number of NHL patients treated each month as reported by the surveyed physicians was similar across surveys and regions, ranging between 57 and 62 patients per month. The most common practice setting for responding US physicians was in oncology group practices (35.9% in 2018; 29.6% in 2019) and academic medical centers for the WE physicians (50.4% in 2018; 46.0% in 2019).
Table 2. Characteristics of physicians surveyed, US and Western Europe 2018/2019.
Treatment by line
When projected between 2020 and 2025, the number of patients initiating an LoT for DLBCL increased by year for both the US and WE (). Changes in the patient estimate over time were related to changes in the increasing underlying incident DLBCL patient population. Patients that were not eligible for the next LoT were those who either died or had not progressed from the prior line and were considered in remission according to the responding physicians. The physician-reported proportion of DLBCL patients treated byline was similar for the US and WE. The proportion of eligible patients not treated increased with each additional LoT from 9% in 1 L to 44% in 3 L + for the US, and from 10% in 1 L to 47% in 3 L + for WE ().
The estimated number of prevalent WW FL patients was higher in the US than WE (). This is due to the combination of increasing underlying incidence of FL and a higher proportion of incident FL patients in the US starting WW (21%) than in WE (13%) ().
Figure 3. Estimated number of FL treatment-eligible patients and the proportions of treated by line of therapy for the US and Western Europe in 2020 and 2025. Calculations use the average physician response data for the US and WE (2018/2019 surveys) for the proportion of patients who progressed but did not initiate and those who progressed and did initiate 1L-3L + treatment. First line FL in this figure includes incident patients eligible for treatment and the proportions of treated are among incident FL patient only. Those not treated of the incident cases are starting watchful waiting. The proportions of treatment by line of therapy were applied as a constant rate across all years, thus the proportions used to calculate treated and not treated were the same for 2020 and 2025. FL: follicular lymphoma; WE: Western Europe; US: United States.
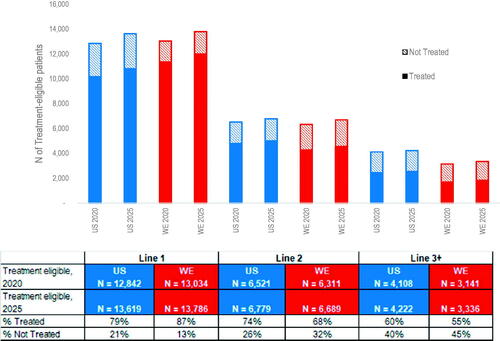
Table 3. Estimated projected total number (N) of FL patients in watchful waiting (WW) and the total number (N) of FL WW patients initiating 1 L treatment in the given year, US and WE 2020–2025.
Similar to DLBCL, the estimated number of FL patients initiating an LoT increased by year for both the US and WE (), and the estimated number of treatment eligible patients decreased with each LoT. The lower 1 L FL treatment rate in the US compared to WE reflects the higher average reported proportion of newly diagnosed FL patients starting WW. The treatment rate of FL decreases with each LoT declining to 60% of eligible FL patients in the US and 55% in WE initiating treatment in 3 L+.
Response rates and average progression-free survival for both the US and WE decreased with an increasing line of therapy (Supplemental Tables S3 and S4).
Discussion
To the best of our knowledge, this is the first study to estimate the number of patients eligible for and initiating treatment by LoT for DLBCL and FL patients in the US and WE. While new therapy options in the relapsed/refractory setting are becoming available for patients with DLBCL and FL, there remains an unmet need for these patients in later lines of therapy, with 60% or less of 3 L eligible patients initiated systemic treatments in both the US and WE. Our study showed that incidence, prevalence and treatment by LoT for DLBCL and FL patients are estimated to continue to increase over 2020–2025 as the underlying aging of the population continues in these regions.
Several studies [Citation24–29] reported treatment by LoT in DLBCL patients using data from registries and secondary data sources. Literature-reported treatment rates among 1 L and 2 L eligible DLBCL patients were, in general, lower than those reported by physicians, which might be partly due to improving treatment over time. For instance, 1 L treated DLBCL rates in literature ranged between 65% and 95% compared to 91% and 90% according to the responding physicians in the US and WE, respectively. For Europe [Citation26–28], the 1 L treatment rate ranged between 85% and 95% with the highest treatment rate reported from the Danish National Lymphoma Registry over the years 2000 to 2015 [Citation26]. Treatment rates for 2 L eligible DLBCL patients ranged between 17% and 34% compared to the reported averages of 31% and 29% from the surveyed physicians in the US and WE, respectively [Citation5,Citation24,Citation26,Citation27].
The proportion of incident FL patients initiating WW in the US as reported by the physicians in our study of 21% was the same as reported in the National LymphoCare Study [Citation30] and also similar to the 22% reported in the SEER-Medicare data [Citation31]. The average rate of initiating WW in FL patients reported by WE physicians in the survey of 13% was lower than the 39% reported in the UK HMRN dataset [Citation28]. Potential explanations of these differences included the fact that these studies were based on older data and that about half of the responding physicians in the WE were in academic medical centers, which might encounter more advanced FL patients not eligible for WW. There was also evidence (consistent with our survey data; data not shown) suggesting less use of radiotherapy in FL in the US compared to the WE [Citation32], which might contribute to the higher proportion of WW in the US.
Similarly, a limited number of published literature is available reporting on subsequent lines of FL therapy [Citation30,Citation31,Citation33]. Treatment rate of 2 L eligible FL patients was 37% for patients in the National LymphoCare Study [Citation30] and 61% from SEER-Medicare [Citation31] compared to 44% reported by the US physicians. The study by Wang et al. (2018) reported a rate of 44% for those who received 2 L post 1 L in the HMRN dataset compared to the WE physician reported average rate of 38%. Albarmawi et al. (2020) reported that age is a determinant for receipt of therapy with older groups less likely to receive treatment [Citation31]. Other factors associated with receiving treatment for FL included marital status, stage at diagnosis, and lymphoma grade.
The present study has several limitations. First, the physicians were recruited from a panel and may not be representative of all treating physicians, however, the screening criteria were such that responding physicians had sufficient experience in the treatment of NHL to participate. While recall bias was possible, the survey limited recall to the last six months for most questions to reduce bias. The physicians provided answers based upon the patient pool they treated which may not be representative of the total DLBCL or FL patient population. Definitions were not provided in the survey for LoT or refractory/relapsed disease, thus variation in the responding physicians’ definitions may have an impact on their responses. Factors related to the responding physicians’ institutions such as access to treatment strategies, site-specific practice recommendations, clinical trial participation may have had an impact on the outcomes reported, however, since data for these factors are not captured, we cannot conclude on their impact. In addition, this study is using historical results as a proxy for incidence projections, overall survival, and treatment due to the lack of reliable prediction for future incidence rates or treatment paradigm. Transformation of follicular lymphoma patients to DLBCL in later lines was not estimated since transformation data were not captured. Our limited-duration prevalence estimated is lower than complete prevalence and thus does not represent the total disease burden and does not account for the influence of new therapies. We were also unable to evaluate the impact of the COVID-19 pandemic on our estimates due to the lack of mature data on this matter.
Future incidence and prevalence for both DLBCL and FL are likely to rise in the US and the five WE countries with their growing and aging populations. About 40% or more of 3 L + eligible DLBCL/FL patients did not initiate treatment in both the US and WE; the reasons were not included in the survey. Literature suggests many patients are not eligible for third-line or later therapy secondary to advancing age, cumulative treatment-associated toxicity, the transformation of their lymphoma to more aggressive histology and the absence of effective treatments in the multiply relapsed setting (Citation34]. Outcomes in DLBCL that are refractory to first-line therapy relapses after second-line therapy or after an autologous transplant are very poor, with a complete response rate of 7% and a median survival of 6.3 months (Citation35]. The prognosis for patients with follicular lymphoma who relapse within 24 months of their initial therapy is poor, oftentimes secondary to transformation to more aggressive histology (Citation36]. Recent treatment advances, including the availability of CAR T cells, therapies targeting the B cell receptor signaling pathway and bispecific antibodies may have a significant impact on treatment for both DLBCL and FL, resulting in an increase in the number of patients treated in this setting [Citation37–40].
This study estimated the number of patients eligible for a given line of therapy which not only includes the number of patients newly diagnosed, but those who are starting a line of therapy who may progress and be eligible for subsequent lines of therapy. The introduction of new therapies may lead to patients living longer and taking longer to progress which could lead to changes in the number of patients eligible for treatment in the future. This paper provides a base for what these patient estimates may look like in the future using current information on treatment from a survey of physicians. Future research using real-world data sources, such as chart review, is necessary to understand changes in treatment patterns given the rapidly evolving field, to better understand the large percentage of patients not treated in the third-line setting.
GLAL-2021-0579-File006.docx
Download MS Word (82.6 KB)GLAL-2021-0579-File005.docx
Download MS Word (42.4 KB)Disclosure statement
WG and RGWQ are employees of and shareholders of Regeneron Pharmaceuticals, Inc. RGWQ is also a shareholder of Amgen, Inc. and Pfizer, Inc.
JA is an employee of Beth Israel Deaconess Medical Center and has consultancy with Regeneron and Juno.
GK, KK, and KN are employed by Kantar, a global consultancy company that acts in the healthcare market, and have clients: pharmaceutical companies, health insurance companies and hospitals.
Additional information
Funding
References
- GLOBOCAN. Cancer today [Internet]. [cited 2021 Jan 19]. Available from: http://gco.iarc.fr/today/home
- SEER Cancer Stat Facts [Internet]. SEER. [cited 2021 Feb 1]. Available from: https://seer.cancer.gov/statfacts/index.html
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.4.2020. ©National Comprehensive Cancer Network, Inc. 2021. All rights reserved. [cited 19 Jan 2021]. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 (Suppl 5):v116–125.
- Purdum A, Tieu R, Reddy SR, et al. Direct costs associated with relapsed diffuse large B-cell lymphoma therapies. Oncologist. 2019;24(9):1229–1236.
- Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(3):298–308.
- Halwani AS, Chien H-C, Morreall DK, et al. Survival patterns in patients with relapsed or refractory diffuse large B cell lymphoma: treatment trajectories and responses after the first relapse. Blood. 2019;134(Supplement_1):1622.
- Batlevi CL, Sha F, Alperovich A, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. 2020;10(7):74.
- Katz SJ, Hawley ST, Bondarenko I, et al. Oncologists’ influence on receipt of adjuvant chemotherapy: does it matter whom you see for treatment of curable breast cancer? Breast Cancer Res Treat. 2017;165(3):751–756.
- Kurian AW, Bondarenko I, Jagsi R, et al. Recent trends in chemotherapy use and oncologists’ treatment recommendations for early-stage breast cancer. JNCI J Natl Cancer Inst. 2018;110(5):493–500.
- CancerMPact. Treatment Architecture [Internet]. 2018. [cited 2019 Nov 15]. Available from: Kantar, www.cancermpact.com
- Le Guyader-Peyrou S, Defossez G, Dantony E, et al. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018 – Hémopathies malignes: Étude à partir des registres des cancers du réseau Francim [Internet]. France: Sante publique; 2019. p. 169. [cited 2021 Mar 2] Available from: https://www.santepubliquefrance.fr/docs/estimations-nationales-de-l-incidence-et-de-la-mortalite-par-cancer-en-france-metropolitaine-entre-1990-et-2018-volume-2-hemopathies-malignes
- AIRTUM ITACAN. Cancer in Italy, Version 2.0. Italian Association of Cancer Registries [Internet]. [cited 2014 Aug 14]. Available from: http://www.registri-tumori.it
- German Centre for Cancer Registry Data, Robert Koch Institute. Database Query with estimates for cancer incidence, prevalence and survival in Germany, based on data of the population-based cancer registries ( Mortality data provided by the Federal Statistical Office. [Internet]. [cited 2019 Dec 17]. Available from: www.krebsdaten.de/database
- Bray F, Colombet M, Mery L, et al. editors. Cancer Incidence in Five Continents. Vol. XI [Internet]. Lyon: International Agency for Research on Cancer; 2017. p. 15. [cited 2021 Mar 2]. Available from: https://ci5.iarc.fr, [cited February 2018]; Available from: https://publications.iarc.fr/Databases/Iarc-Cancerbases/Cancer-Incidence-In-Five-Continents-Vol.-XI-2017.
- Cancer Research UK. Cancer Statistics for the UK [Internet]. [cited 2021 Mar 2]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk
- SEER*Stat Database: NPCR and SEER Incidence. – U.S. Cancer Statistics Public Use Database, Nov 2018 submission (2001–2016). United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2019, based on the November 2018 submission.
- U.S. Census Bureau. 2014 to 2060 Population Projections based on Census 2010 (released 2014) [Internet]. [cited 2019 Nov 15]. Available from: http://www.census.gov/population/projections/data/national/
- United Nations, Department of Economic and Social Affairs, Population Division. New York: World Population Prospects: The 2015 Revision. 2015.
- Instituto Nacionale de Estadistica. [cited 2016 Sep 15]. Available from: www.ine.es
- EUROCARE-5 Database. Survival of Cancer Patients in Europe The EUROCARE-5 Study [Internet]. [cited 2019 Feb 15]. Available from: https://w3.iss.it/site/EU5Results/
- Solal-Céligny P, Bellei M, Marcheselli L, et al. Watchful waiting in low-tumor burden follicular lymphoma in the rituximab era: results of an F2-study database. J Clin Oncol. 2012;30(31):3848–3853.
- Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol. 2014;15(4):424–435.
- Ionescu-Ittu R, Shang A, Velde NV, et al. Second-line rituximab-bendamustine versus rituximab-gemcitabine-oxaliplatin in diffuse large B-cell lymphoma in the real world. J Comp Eff Res. 2019;8(13):1067–1075.
- Flowers CR, Fedewa SA, Chen AY, et al. Disparities in the early adoption of chemoimmunotherapy for diffuse large B-cell lymphoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1520–1530.
- Arboe B, Olsen MH, Gørløv JS, et al. Treatment intensity and survival in patients with relapsed or refractory diffuse large B-cell lymphoma in Denmark: a real-life population-based study. Clin Epidemiol. 2019;11:207–216.
- Rovira J, Valera A, Colomo L, et al. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann Hematol. 2015;94(5):803–812.
- Patmore R, Roman E, Smith A, et al. Patient’s age and treatment for haematological malignancy: a report from the haematological malignancy research network. University of York: Haematological Malignancy Research Network; 2014.
- Ayers EC, Margolis D, Landsburg DJ. Real world outcomes in patients with relapsed/refractory diffuse large B-cell lymphoma receiving palliative intent therapies. Clin Lymphoma Myeloma Leuk. 2020;20(10):661–667.
- Link BK, Day B-M, Zhou X, et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational National LymphoCare Study. Br J Haematol. 2019;184(4):660–663.
- Albarmawi H, Nagarajan M, Onukwugha E, et al. Follicular lymphoma treatment patterns between 2000 and 2014: a SEER-Medicare analysis of elderly patients. Future Oncol Lond Engl. 2020;16(8):353–365.
- Vargo JA, Gill BS, Balasubramani GK, et al. What is the optimal management of early-stage low-grade follicular lymphoma in the modern era? Cancer. 2015;121(18):3325–3334.
- Wang H-I, Roman E, Crouch S, et al. A generic model for follicular lymphoma: predicting cost, life expectancy, and quality-adjusted-life-year using UK population-based observational data. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2018;21(10):1176–1185.
- Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2017;35(22):2473–2481.
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808.
- Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the national LymphoCare study. J Clin Oncol. 2015;33(23):2516–2522.
- Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018.
- Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–3905.
- Bannerji R, Allan JN, Arnason JE, et al. Odronextamab (REGN1979), a human CD20x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy. Blood. 2020;136(Supplement 1):42–43.
- Phillips TJ, Olszewski AJ, Munoz J, et al. Mosunetuzumab, a novel CD20/CD3 bispecific antibody, in combination with CHOP confers high response rates in patients with diffuse large B-cell lymphoma. Blood. 2020;136(Supplement 1):37–38.
