Abstract
Due to the evolving use of haploidentical donor grafts in hematopoietic cell transplantation, there is increased need to better understand the risks and benefits of using bone marrow versus peripheral blood grafts, as well as how specific pre-transplantation conditioning regimens impact patient safety and treatment outcomes. We performed a retrospective analysis of 38 patients at two centers who underwent haploidentical hematopoietic cell transplantation using fludarabine plus melphalan-based conditioning regimens with post-transplant cyclophosphamide and peripheral blood donor grafts. We observed an unexpectedly high rate of early non-relapse mortality and severe cytokine release syndrome. The poor outcomes with 1-year overall survival of 34%, disease-free survival of 29%, and non-relapse mortality of 34% motivate us to reconsider the appropriateness of the combination of fludarabine and melphalan conditioning with T-cell replete peripheral blood grafts in the setting of haploidentical hematopoietic cell transplant with post-transplant cyclophosphamide.
Haploidentical hematopoietic cell transplantation (haplo-HCT) is a readily available and potentially curative therapy for patients with hematologic malignancies. Initial reports of haplo-HCT with post-transplant cyclophosphamide (PTCy) for graft versus host disease (GvHD) prophylaxis utilized bone marrow (BM) grafts and a reduced-intensity conditioning (RIC) regimen with fludarabine, cyclophosphamide, and total body irradiation (Flu/Cy/TBI) with encouraging outcomes [Citation1]. Protocols incorporating myeloablative conditioning and peripheral blood stem cell (PBSC) grafts have since been established and are now widely used [Citation2,Citation3]. One complication of PBSC haplo-HCT with PTCy is cytokine release syndrome (CRS), which occurs in ∼90% of patients [Citation4,Citation5]. Severe CRS occurs in ∼20% of patients and is associated with high rates (∼50%) of non-relapse mortality [Citation4,Citation5].
In other transplant settings, increased intensity RIC regimens with fludarabine and melphalan (FluMel) appear to balance decreased relapse risk with acceptable rates of non-relapse mortality (NRM) for older patients with malignant hematologic conditions [Citation6]. Small studies have demonstrated the feasibility of FluMel with or without thiotepa, anti-thymocyte globulin (ATG), and/or low-dose TBI with BM grafts in the haplo-HCT PTCy platform [Citation7,Citation8]. However, there is a paucity of published data to guide the use of FluMel with PBSC grafts in haplo-HCT.
We performed a two-center, retrospective analysis of all patients receiving a FluMel-based RIC regimen for PBSC haplo-HCT with PTCy between 2015 and 2019. Significance testing for associations between clinical characteristics was assessed by Fisher exact testing. Thirty-eight patients were included in the analysis (). The majority (20; 52.6%) had acute myeloid leukemia (AML), and of these patients four (20%) had active disease at time of transplantation. The median age at date of transplantation was 60 years (range 20–73). All patients received conditioning regimens based on four doses of fludarabine at 30 mg/m2, and a single dose of melphalan on either day −2 or day −1, followed by a haplo-HCT with PTCy and a PBSC graft per institutional protocols. Thirty-six patients (94.7%) received melphalan at 140 mg/m2 (two of whom also received anti-thymocyte globulin), and two patients (5.3%) received melphalan at 100 mg/m2.
Table 1. Patient and treatment characteristics.
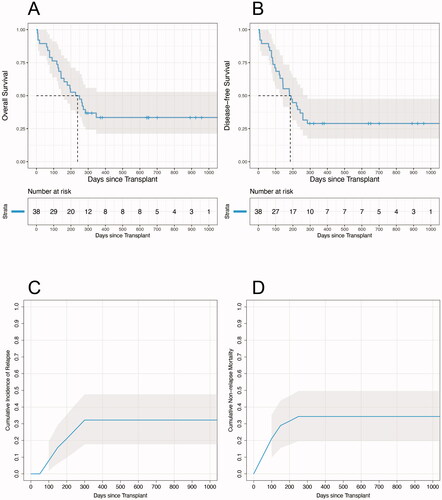
At date of last analysis, 13 (34.2%) patients remained alive, and median follow-up for these surviving patients was 646 days (range 288–1086). All patients with follow-up of less than 100 days died prior to day +100. Median overall survival was 7.8 months from transplant (95% confidence interval 4.7–11.4 months; ). Overall survival rates at days +100 and +365 were 76.3% and 33.5%, respectively. Median disease-free survival, defined as the time from day 0 of stem cell transplantation until date of documented evidence of relapsed disease or death from any cause, whichever occurred first, was 6.1 months (95% confidence interval 4.0–8.5 months; ). The disease-free survival rates at days +100 and +365 were 71.1% and 28.9%, respectively. With all patients mature for analysis at day +100, nine (23.7%) patients suffered early mortality, defined as having died prior to day +100. Eight (88.9%) of these nine patients died without experiencing disease relapse. Cumulative relapse incidences at days +100 and +365 were 7.9% and 32.2%, respectively (). NRM rates at days +100 and +365 were 21.1% and 34.4%, respectively ().
Figure 1. (A–B) Kaplan-Meier survival estimates showing overall survival and disease-free survival, respectively, after transplantation for the whole cohort. (C–D) Cumulative incidences of relapse and NRM after transplantation for the whole cohort. Gray shaded areas indicate 95% confidence intervals.
Fifteen (39.5%) of 38 patients had severe (grade 3–5) CRS per the 2018 American Society for Transplantation and Cellular Therapy consensus grading system [Citation9]. Eleven (73.3%) of these 15 patients with severe CRS received treatment with tocilizumab. Acute kidney injury (AKI) occurred at a high rate in patients with severe CRS (10/15 patients, 66.7%) as compared to patients with CRS grade 0–2 (7/23, 30.4%, p = 0.046). Six (85.7%) of the seven patients from the entire cohort who required renal replacement therapy (RRT) had severe CRS. Early NRM was strongly associated with severe CRS, with NRM prior to day +100 occurring in seven (46.7%) of 15 patients with severe CRS, compared to only one (4.3%) of 23 patients with CRS grade 0–2 (p = 0.003).
Of the eight patients with early NRM, seven (87.5%) had creatinine clearance <90 mL/min prior to transplantation, six (75%) had hemoglobin <9.5 g/dL prior to transplantation, seven (87.5%) developed AKI post-transplant, and four (50%) needed RRT post-transplantation. This was compared to 36.6%, 33.3%, 33.3%, and 10%, respectively, of the patients not experiencing early NRM. The outcome of early NRM was significantly associated with each of these four pre- or post-transplantation clinical features (p = 0.016, p = 0.050, p = 0.013, and p = 0.025). Early NRM was not significantly associated with age at transplantation, HCT-CI score, first versus second transplant, disease status at transplant, melphalan dosing, volume overload or diuretic use, or post-transplant infection.
Overall, we observed very poor outcomes with FluMel conditioning and PBSC grafts for haplo-HCT with PTCy. One-year OS in our cohort was 34%, driven by unexpectedly high rates of NRM due to early toxicity after transplantation. Our reported NRM rates of 21% and 34% at day +100 and 1-year, respectively, are much higher than the rates observed in other haplo-HCT studies utilizing RIC platforms with similar patient populations. A somewhat older report using FluMel conditioning with melphalan 140 mg/m2 on day −8 for haplo-HCT with BM grafts found NRM rates at day +100 and 1-year to be 12% and 16%, respectively [Citation10]. A subsequent cohort of similarly treated patients also showed a lower NRM (19% at 1-year) [Citation8]. Both of these studies observed impressive 1-year OS rates of approximately 60%. Besides our patients receiving PBSC grafts, our patient characteristics were comparable to both cohorts.
The high incidence of early NRM observed in our study was strongly associated with severe CRS, with 47% of these patients dying prior to day +100. Patients with early mortality appeared to suffer from similar clinical syndromes characterized by severe CRS and/or high fevers, capillary leak, volume overload, significant edema and anasarca, weight gain, and oliguria. This was exacerbated by IV fluids administered with PTCy, often progressing to AKI, anuria, and metabolic acidosis requiring ICU admission and RRT; some patients suffered further clinical decompensation and death. Anecdotally, while treatment with the anti-IL6R monoclonal antibody tocilizumab would rapidly ameliorate fevers, it did not appear to have an effect on patients’ overall clinical course. These poor outcomes in patients with severe CRS are consistent with previous reports [Citation4,Citation5,Citation11]. Despite the excessive toxicity, this regimen did not appear particularly effective, with 33% of surviving patients relapsing.
CRS is thought to be primarily mediated by activated T-cells, and PBSC grafts contain nearly 8-fold more T-cells compared to BM grafts. Different allograft T-cell subset contents have been associated with risk of acute and chronic GvHD as well as risk of relapse [Citation12]. Other reports demonstrated increased rates of severe CRS in patients receiving PBSC grafts compared to those receiving BM grafts for haplo-HCT with Flu/Cy/TBI conditioning and PTCy [Citation13]. We hypothesize that a FluMel conditioning regimen may exacerbate this phenomenon. In other transplant types, melphalan is commonly used as part of fludarabine-based RIC regimens that are associated with decreased relapse rates and improved disease-free survival [Citation14]. However, that study of patients with AML and another recent report [Citation15] of patients with non-Hodgkin’s lymphoma found a higher rate of NRM and decreased OS in patients receiving FluMel conditioning for allogeneic HCT compared to fludarabine and busulfan-based conditioning. Further, pharmacokinetic studies find up to a 10-fold interpatient variability in melphalan exposure [Citation16]. Additionally, melphalan has also been shown to have immunomodulatory effects, with evidence of cytokine surges two days after melphalan administration [Citation17]. This would coincide with the timing of PBSC infusion in our cohort, as our patients received melphalan on either day −2 or day −1. The combination of a melphalan-induced proinflammatory environment with T-cell replete haplo-HCT PBSC grafts may have contributed to the unacceptably high rate of severe CRS in our patients.
While our study is limited by its small sample size, heterogenous patient population, and retrospective nature, our institutions no longer use standard FluMel as conditioning for haplo-HCT with PTCy with T-cell replete PBSC grafts. There remains a significant unmet need to better understand the optimal conditioning approach for patients with aggressive hematologic malignancies undergoing haplo-HCT who are older or unfit for standard MAC, and this should be addressed in future randomized controlled trials.
Authorship
L.E., D.A.R.-G., R.A., and E.H. designed research, performed research, collected data, analyzed data, and wrote the manuscript. D.A.R.-G. and E.H. performed statistical analyses. J.F.D., T.F., and J.R.A. reviewed this manuscript and assisted with data interpretation. All authors revised and approved the manuscript before final submission.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all patients for being included in the study.
Disclosure statement
L.E., D.A.R.-G., J.F.D., J.R.A., T.F., R.A., and E.H. have no relevant conflicts to disclose.
Additional information
Funding
References
- Luznik L, O’Donnell PV, Symons HJ, et al. HLA-Haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650.
- Ciurea SO, Zhang M-J, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040.
- Bashey A, Zhang M-J, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-Cell–replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35(26):3002–3009.
- Abboud R, Keller J, Slade M, et al. Severe cytokine-release syndrome after T cell-replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti-IL-6 therapy is safe and well tolerated. Biol Blood Marrow Transplant. 2016;22(10):1851–1860.
- Abboud R, Wan F, Mariotti J, et al. Cytokine release syndrome after haploidentical hematopoietic cell transplantation: an international multicenter analysis. Bone Marrow Transplant (2021). https://doi.org/https://doi.org/10.1038/s41409-021-01403-w
- Ciurea SO, Kongtim P, Varma A, et al. Is there an optimal conditioning for older patients with AML receiving allogeneic hematopoietic cell transplantation? Blood. 2020;135(6):449–452.
- Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118(24):6438–6445.
- Besien K van, Artz A, Champlin RE, Guarneri D, et al. Haploidentical vs haplo-cord transplant in adults under 60 years receiving fludarabine and melphalan conditioning. Blood Adv. 2019;3(12):1858–1867.
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638.
- Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835–1844.
- Imus PH, Blackford AL, Bettinotti M, et al. Severe cytokine release syndrome after haploidentical peripheral blood transplantation. Biol Blood Marrow Transplant. 2019;25(12):2431–2437.
- Vasu S, Geyer S, Bingman A, et al. Granulocyte colony-stimulating factor–mobilized allografts contain activated immune cell subsets associated with risk of acute and chronic graft-versus-Host disease. Biol Blood Marrow Tr. 2016;22(4):658–668.
- Mariotti J, Taurino D, Marino F, et al. Pretransplant active disease status and HLA class II mismatching are associated with increased incidence and severity of cytokine release syndrome after haploidentical transplantation with posttransplant cyclophosphamide. Cancer Med. 2020;9(1):52–61.
- Yamashita T, Takami A, Uchida N, et al. Reduced-intensity stem cell transplantation for acute myeloid leukemia with fludarabine-based conditioning with intravenous busulfan versus melphalan. Bone Marrow Transplant. 2020;55(10):1955–1965.
- Ghosh N, Ahmed S, Ahn KW, et al. Association of reduced-intensity conditioning regimens with overall survival among patients with non-Hodgkin lymphoma undergoing allogeneic transplant. JAMA Oncol. 2020;6(7):1011–1018.
- Nath CE, Shaw PJ, Trotman J, et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Brit J Clin Pharmaco. 2010;69(5):484–497.
- Lu X, Ding Z-C, Cao Y, et al. Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells. J Immunol. 2015;194(4):2011–2021.
