Abstract
Diffuse large B-cell lymphoma is treated with anti-CD 20 and multi-drug chemotherapy for cure. Positron emission tomography (PET) scans are performed at end of treatment (EOT) to assess response. EOT Deauville scores (DS) are equivocal for treatment response in some situations, requiring physicians to determine the need for further investigations or treatment. Studies have suggested the delta maximum standardised uptake value (ΔSUVmax) to be superior to DS for assessment of metabolic response at interim PET, although its use at EOT PET, especially in cases of equivocal response, has yet to be established. We investigated whether ΔSUVmax could better discriminate prognosis than DS 3 or 4 at EOT. ΔSUVmax did not outperform DS. Combination of DS 3 and International Prognostic Index (IPI) <3 selects for patients with extremely low risk of disease progression (HR 0.06, 95% CI 0.01 to 0.62, p 0.018) compared to DS 4 and IPI ≥3.
Diffuse large B-cell lymphoma (DLBCL) is a common and aggressive non-Hodgkin lymphoma and is classically treated curatively with six cycles of anti-CD 20 in combination with multi-drug chemotherapy regimens [Citation1]. While treatment response at interim F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) scans has been shown to prognosticate for relapse or progression [Citation2,Citation3], the routinely performed end of treatment (EOT) PET scan has not yet been shown to do the same, in situations of equivocal response. In EOT PET scans, the Deauville score (DS) is the most widely used, and an equivocal response is, situationally, represented by a score of 3 or 4 [Citation4]. However, it has been criticized for high false-positive rates and inter-observer variability [Citation5]. The proposed alternative, the delta maximum standardized uptake value (ΔSUVmax) method, has shown greater reproducibility and prognostic performance for overall patient outcomes, albeit only in studies examining interim PET scans [Citation6–8]. To date, no study has directly compared the prognostic values of the DS and the ΔSUVmax method in the EOT setting. Thus, this exploratory study primarily aims to assess if the ΔSUVmax method would better prognosticate relapse/remission in patients with an EOT DS of 3 or 4.
36 patients with histologically proven DLBCL, who had available matching staging and EOT PET scans, and who had completed curative first-line treatment with a subsequent EOT DS 3 or 4, were identified from the national database. All pairs of scans were independently reevaluated for EOT DS and ΔSUVmax by two nuclear medicine specialists. Any discrepancies were resolved with consensus reporting. Deauville scores were reported as per the Lugano classification [Citation4], comparing the most intense FDG uptake at a site of initial disease to both the mediastinal blood pool and the liver. A DS of 5 was defined by uptake of FDG three times that of the liver and/or the presence of any new lesions. ΔSUVmax was calculated by comparing the change in SUVmax of the most FDG-avid lesion of the EOT PET scan with the staging PET scan. Progression was defined by a ΔSUVmax of less than or equal to 66% (ΔSUVmax ≤66%)
The primary endpoint of the study was time-to-progression (TTP), defined from the date of histologically confirmed diagnosis to the date of relapse/progression, confirmed either radiologically or histologically. Overall survival was not used as an endpoint due to the small number of deaths. Univariate survival analysis was performed using the Kaplan–Meier method and the log-rank test. Multivariate analysis was performed using Cox proportional hazard regression. The prognostic performance of the combination of either the DS or the ΔSUVmax with other prognostic factors was evaluated by measurement of discrimination, quantified with Harrell’s c-index. All statistical analyses were carried out in MedCalc statistical software for Windows version 19.5.3, 64-bit (MedCalc Software, Ostend, Belgium) and in STATA version 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
Of the 36 patients, thirteen (36.1%) had EOT PET DS 4, while seven (19.4%) had a ΔSUVmax ≤66% (); three patients fulfilled both EOT DS 4 and ΔSUVmax ≤66%. Six out of 13 patients (46.2%) with DS 4 eventually progressed, compared with five out of 23 (21.7%) patients with DS 3 who eventually relapsed. Median follow-up time was 31.0 months (95% CI: 23.7–48.6 months), beyond the 24 months when the vast majority of DLBCL relapses are known to occur [Citation9].
Table 1. Patient characteristics.
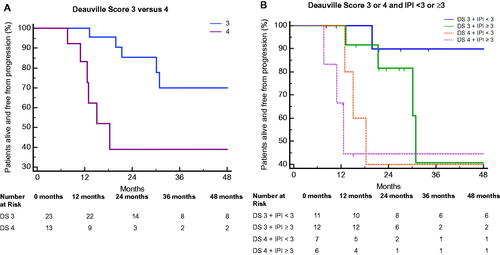
Only DS demonstrated statistical significance for the analysis of time to progression, with EOT DS 4 patients demonstrating a higher risk of progression compared to EOT DS 3 (HR 4.56, 95% CI: 1.36 − 15.28; p 0.014; ). The median TTP for patients with DS 4 was 18.3 months (95% CI 10.9 − 18.3; p 0.0075); median TTP for patients with DS 3 was not reached. On the other hand, ΔSUVmax ≤66% was not associated with a higher risk of progression (HR 1.51, 95% CI: 0.40 − 5.72; p 0.541). Since there is evidence that a ΔSUVmax cutoff of 70% is optimal for interim PET at four cycles of treatment [Citation10], we also attempted this analysis with other ΔSUVmax cutoff values. However, adjusting the ΔSUVmax cutoff value upwards (70%, 75%, 80%) did not improve discrimination. The c-value for prognostication using the DS system was 0.716, while that of the ΔSUVmax method was 0.552.
Figure 1. Time to progression (TTP) for (A) patients stratified by Deauville score (DS) 3 versus 4, and (B) patients stratified by DS 3 or 4, combined with IPI. Kaplan–Meier curves are compared against DS 4 and IPI ≥ 3. IPI, International Prognostic Index.
In view of the above findings, we explored whether combining known prognostic variables with DS, rather than ΔSUVmax, could further improve prognostic performance. The following pretreatment prognostic factors were analyzed:
International Prognostic Index (IPI)
Ann-Arbor staging
Molecular subtype, classified according to Han’s algorithm [Citation11]
Hematologic Prognostic Scores – namely the ALC (Absolute Lymphocyte Count)/AMC (Absolute Monocyte Count) prognostic score [Citation12]
Combining the IPI (as a binary variable; IPI <3 vs ≥3) with DS resulted in a bivariate model with an improved c-value of 0.782, from the univariate model using only DS (c = 0.716), indicating better discrimination. In addition, Cox regression demonstrated that patients with DS 3 and IPI < 3 had a statistically significantly lower risk of progression compared with patients with DS 4 and IPI ≥3 [HR (DS 3 and IPI < 3): 0.06 (95% CI 0.01 − 0.62), p 0.018; ]. This suggests that among patients with EOT DS 3–4, patients with both an EOT DS 3 and IPI <3 may have an extremely low risk of disease progression, compared to those with EOT DS 4 and IPI ≥3. Combinations of DS with the other prognostic factors did not yield statistically significant differences in the hazards of progression.
There remains a dearth of evidence regarding the utility of EOT PET in prognosticating survival [Citation13]. EOT PET evaluation in DLBCL patients is important in making further investigative and treatment decisions, particularly in those whose DS does not conclusively reflect disease remission. The high false-positive rates of DS in interim and EOT PET may result in patients, having responded adequately, being erroneously assessed to have viable residual disease instead, and consequently receiving further unnecessary investigations and treatment.
Our study showed that among patients with EOT DS 3 or 4, ΔSUVmax was not a superior prognostic alternative to DS. This corroborates the work of others [Citation14], suggesting that it might be premature to dismiss DS in favor of ΔSUVmax in the evaluation of response to treatment in EOT PET scans. Additionally, unlike the ΔSUVmax method, a logistical advantage of DS is that clinicians only require the stand-alone EOT PET scan, without a pretreatment scan, to evaluate the DS and guide further management [Citation4].
No study has evaluated the factors that, when combined with EOT PET, more clearly discriminate among patients with equivocal EOT PET responses who are more or less likely to experience relapse/progression. To mitigate false positives, we evaluated bivariate combinations of EOT DS with other prognostic indices and found that combining the IPI (binary, ≥3 vs <3) with EOT DS improves Harrell’s c-index and discrimination performance over DS alone – demonstrating improved discrimination between patients with a low and high risk of progression. In particular, the select group of patients who have DS 3 and IPI <3 may have a very low risk of progression and may not require further investigations or treatment. However, the small study population and a low number of events, partly due to our inclusion criteria, resulted in our study not being adequately powered to detect the above findings with greater statistical strength. The result from this limited study is therefore hypothesis-generating. Nevertheless, 23% of our DLBCL patients had an EOT DS of 3 and 4 and would likely form a significant population of patients worldwide with DLBCL. An evaluation with larger numbers of patients across multiple centers would be useful to corroborate our hypothesis that the replacement of DS with the ΔSUVmax method as the choice tool for assessing EOT response currently remains premature. There also remains a need to further test the utility of incorporating multiple variables into a single model to further refine prognostic outcomes.
Additional information
Funding
References
- Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105–116.
- Schmitz C, Hüttmann A, Müller SP, et al. Dynamic risk assessment based on positron emission tomography scanning in diffuse large B-cell lymphoma: post-hoc analysis from the PETAL trial. Eur J Cancer. 2020;124:25–36.
- Duarte S, Afonso C, Marques B, et al. Survival independent predictive value of INTERIM FDG 18‐PET in newly diagnosed diffuse large B cell lymphoma. Hematol Oncol. 2021;39(S2):39.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32(27):3059–3067..
- Han EJ, O JH, Yoon H, et al. FDG PET/CT response in diffuse large B-cell lymphoma: reader variability and association with clinical outcome. Medicine. 2016;95(39):e4983.
- Itti E, Meignan M, Berriolo-Riedinger A, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013;40(9):1312–1320.
- Rekowski J, Hüttmann A, Schmitz C, et al. Interim PET evaluation in diffuse large B-cell lymphoma using published recommendations: comparison of the Deauville 5-point scale and the ΔSUVmax method. J Nucl Med. 2021;62(1):37–42.
- Eertink JJ, Burggraaff CN, Heymans MW, et al. Optimal timing and criteria of interim PET in DLBCL: a comparative study of 1692 patients. Blood Adv. 2021;5(9):2375–2384.
- Maurer MJ, Ghesquières H, Jais J-P, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-Cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066–1073.
- Casasnovas R-O, Meignan M, Berriolo-Riedinger A, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011;118(1):37–43.
- Hans CP. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282.
- Wilcox RA, Ristow K, Habermann TM, et al. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25(9):1502–1509.
- Adams HJA, Kwee TC. Systematic review on the value of end-of-treatment FDG-PET in improving overall survival of lymphoma patients. Ann Hematol. 2020;99(1):1–5.
- Barrington S, Eertink JJ, de Vet HC, et al. Not yet time to abandon the Deauville criteria in diffuse large B cell lymphoma. J Nucl Med. 2021;jnumed.121.262317. https://jnm.snmjournals.org/content/early/2021/04/23/jnumed.121.262317/tab-article-info
