Abstract
The phase 1b multicohort KEYNOTE-013 study assessed the safety and antitumor activity of pembrolizumab given at 10 mg/kg/day every 2 weeks for up to 2 years in hematologic malignancies, including myelodysplastic syndromes (MDS) refractory to a hypomethylating agent (HMA). Primary outcomes were safety and objective response rate per International Working Group 2006 criteria. By June 26, 2020, 28 patients were enrolled; median duration of follow-up was 5.6 months (range, 1–78), and 25 patients (89%) had died. Treatment-related adverse events occurred in 10 patients (36%), including 2 (7%) treatment-related discontinuations. No patient achieved complete or partial response. Five patients (19%) had bone marrow complete response, 12 (44%) stable disease, 10 (37%) progressive disease, 6 (22%) cytogenetic response, and 5 (19%) hematologic improvement. Median overall survival (OS) was 6.0 months (95% CI, 4–12); the overall 2-year OS rate was 17%. Pembrolizumab had manageable safety and clinical activity in patients with HMA-refractory MDS.
This trial was registered at www.clinicaltrials.gov as #NCT01953692.
Introduction
Myelodysplastic syndromes (MDS) arise from the clonal proliferation of a transformed myeloid progenitor or a hematopoietic stem cell and is characterized by dysplasia, cytopenia, and increased risk for transformation to acute myeloid leukemia (AML) [Citation1–3]. MDS is most common in patients of advanced age, with disease incidence increasing up to 5 times from the ages of 60 to ≥80 years [Citation3]. Although most cases are idiopathic, MDS predominantly affects men, and disease risk increases from chronic exposure to chromosome-damaging agents, including chemotherapy, radiation, and pesticides [Citation1,Citation3,Citation4].
Patients with MDS are commonly stratified into 2 major risk groups based on International Prognostic Symptom Score (IPSS) criteria: patients at lower risk (IPSS low, intermediate-1) and patients at higher risk (IPSS high, intermediate-2) [Citation5]. Major therapeutic goals for MDS vary based on risk group; the primary aim for patients at lower risk is hematologic improvement, and the primary aim for patients at higher risk is alteration of the natural disease course [Citation5]. Patients with lower-risk MDS and accompanying symptomatic anemia, thrombocytopenia, or recurrent infections are treated with supportive care and low-intensity therapies [Citation5]. In addition, it is recommended that patients with lower-risk MDS and deletion of the long arm of chromosome 5 (del5q) and symptomatic anemia receive lenalidomide [Citation5]. Treatment options for higher-risk MDS are more complex because they depend on the patient’s ability to tolerate intensive therapies such as allogeneic hematopoietic stem cell transplantation (HSCT) and intensive chemotherapy in addition to supportive care [Citation2,Citation5]. In patients with higher-risk MDS who are not candidates for HSCT or high-intensity chemotherapy, however, hypomethylating agents (HMAs) such as azacitidine and decitabine are considered standard of care and have been shown to improve survival compared with conventional therapy [Citation5–8]. However, less than half of patients with IPSS high-risk MDS respond to HMAs, and the estimated median overall survival (OS) is 4 months [Citation6,Citation9]. Furthermore, disease progression transforming to AML has been reported in subsets of patients who have experienced HMA treatment failure [Citation6]. In sum, effective treatment options for patients with high-risk MDS progressing after HMA treatment failure remain an unmet need.
Pembrolizumab is a humanized monoclonal antibody against programmed death 1 (PD-1) that blocks interactions between PD-1 and its ligands, PD-L1 and PD-L2, and it can restore antitumor immune activity in a range of hematologic malignancies [Citation10–13]. Upregulation of PD-1, PD-L1, and PD-L2 has been demonstrated in the peripheral blood mononuclear cells of patients with MDS [Citation14,Citation15]. This upregulation is enhanced by epigenetic modifiers such as azacitidine and has been associated with poor survival [Citation14,Citation15]. The PD-1/PD-L1 pathway thus represents an attractive target in patients with MDS who have experienced failed first-line treatment with an HMA. We present data from the MDS cohort of the KEYNOTE-013 study of pembrolizumab in patients with hematologic malignancies.
Patients and methods
Study design and participants
KEYNOTE-013 (NCT01953692) is an open-label, nonrandomized, multicohort, phase 1b study of pembrolizumab in patients with hematologic malignancies. The cohort described here includes patients with MDS. Eligible patients were aged ≥18 years and had primary or secondary MDS with an IPSS score of intermediate-1, intermediate-2, or high-risk that failed to respond to ≥4 previous cycles of HMA treatment (defined as worsened cytopenia, increased percentage of bone marrow blasts, or disease progression [per International Working Group (IWG) criteria] to a more advanced MDS French-American-British subtype than at pretreatment). Patients had to have Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, life expectancy long enough to be assessed for response to or benefit from therapy, bone marrow biopsy/aspirate material for biomarker analysis or newly obtained bone marrow biopsy/aspirate, and adequate organ function.
Patients were ineligible if they previously received or experienced any of the following: treatment with an investigational agent or a device within 4 weeks of study start; treatment with any colony-stimulating factor (CSF) including granulocyte-CSF, granulocyte-macrophage-CSF, erythropoietin, or other hematopoietic cytokine within 2 weeks of study enrollment; received monoclonal antibody or had not recovered from adverse events (AEs) after administration of monoclonal antibody within 4 weeks of study day 1; chemotherapy, targeted small molecule therapy, or radiation therapy within 2 weeks of study day 1; allogeneic HSCT within 5 years of study start; live vaccine within 30 days of study start; active autoimmune disease that required systemic treatment in the past 2 years, known malignancy, clinically active central nervous system involvement, active noninfectious pneumonitis, or active infection necessitating systemic intravenous treatment; known HIV or hepatitis B or C infection; cardiovascular disease; or treatment with an anti–PD-1, anti–PD-L1/L2, anti-CD137, or anti–cytotoxic T-lymphocyte–associated protein-4 antibody, including ipilimumab, or any other agent targeting T-cell costimulation or checkpoint pathways.
The study protocol was approved by the institutional review board or ethics review committee at each study site, and the study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent. All authors had full access to the data and were involved in writing, reviewing, or editing drafts of the manuscript and vouch for the accuracy and completeness of the data analyses.
Enrolled patients received intravenous pembrolizumab 10 mg/kg/day every 2 weeks. Treatment began as close as possible to the day of treatment allocation and continued until confirmed disease progression, unacceptable AEs, or withdrawal because of patient or investigator decision. Disease response assessments were performed every 6 weeks and continued until disease progression, initiation of new anticancer treatment, withdrawal of consent, or completion of 2 years of treatment. Survival was assessed every 12 weeks after disease progression or start of new anticancer treatment.
The primary end points were safety and tolerability and investigator-assessed objective response rate (ORR), based on IWG 2006 criteria for MDS [Citation16], in patients treated with pembrolizumab. ORR was defined as the proportion of patients who achieved complete response (CR) or partial response (PR) per IWG 2006 criteria. Secondary end points included OS, duration of response, investigator-assessed bone marrow response (CR or PR per IWG 2006 criteria), hematologic improvement, and cytogenetic response per IWG 2006 criteria. Safety and tolerability were assessed by clinical review of all adverse experiences, laboratory test results, and vital signs. AEs were graded using the Common Terminology Criteria for Adverse Events, version 4.0 [Citation17].
Statistical analysis
All patients who were treated with ≥1 dose of pembrolizumab were included in the safety analyses. Summary statistics were provided for safety end points as appropriate. Patients with ≥1 baseline efficacy evaluation and ≥1 postbaseline efficacy evaluation (full analysis set) were included in the efficacy analyses. For ORR, the point estimate, 90% CI, and P value for testing whether response rate was greater than the historical control (ORR, 10% with decitabine [N = 13]) [Citation18] were calculated based on the exact binomial distribution. Enrollment of 25 patients was required for this study to have approximately 80% power to detect a 20% improvement in ORR under the null hypothesis that the ORR is 10% with a 1-sided type I error rate of 5% if the true ORR was 30%. At least 6 of 25 responses were required for success of this hypothesis.
Results
Patients
Twenty-eight patients in the MDS cohort were included in the safety analysis population. The median age was 73 years (range, 38–84 years), 23 patients (82%) were 65 or older, and 26 patients (93%) previously received 1 to 2 therapies. Eleven patients (39%) had IPSS intermediate-1, 8 (29%) had intermediate-2, and 7 (25%) had high-risk MDS. In addition, 15 patients (54%) had refractory anemia with excess blasts per French-American-British classification, 3 (11%) had refractory anemia with excess blasts in transformation, and 4 (14%) had chronic myelomonocytic leukemia at baseline (). Two patients (7%) completed 2 years of study treatment, and 26 (93%) discontinued treatment, including 13 (46%) because of progressive disease, 5 (18%) because of AEs, 4 (14%) because of patient withdrawal, and 3 (11%) because of physician decision. By the database cutoff date of June 26, 2020, 25 patients (89%) had died; 9 deaths (32%) resulted from disease progression. The median duration of follow-up, defined as the time from the first dose to the date of death or the database cutoff date, was 5.6 months (range, 1–78 months). Three patients (11%) were on study for ≥1 year ().
Figure 1. Exposure and best response in all evaluable patients (A) and transfusion requirements (B). mCR: bone marrow complete response; PD: progressive disease.
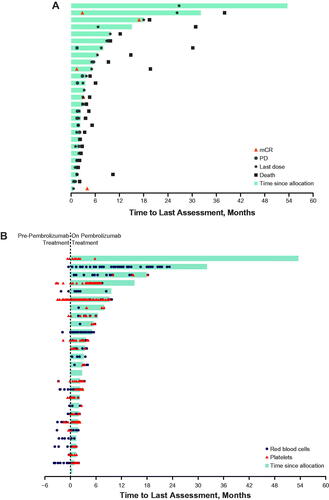
Table 1. Baseline characteristics of all patients as treated.
Safety
Twenty-seven patients (96%) had an any-grade AE, 10 (36%) had treatment-related adverse events (TRAEs), 2 (7%) had serious TRAEs, and 2 (7%) had grade 3/4 TRAEs. The most common TRAEs were hypothyroidism in 4 patients (14%), fatigue in 4 patients (14%), and peripheral edema and decreased appetite in 2 patients each (7%). Of grade 3/4 TRAEs, 1 patient (4%) each experienced gastroenteritis, pain in extremity, and tumor lysis syndrome (). Two patients had 4 TRAEs that led to study discontinuation (musculoskeletal stiffness, peripheral edema, arthralgia, tumor lysis syndrome). There were no treatment-related deaths.
Table 2. Treatment-related adverse events in all patients as treated.
Efficacy
This study did not meet the protocol-specified criteria for a positive outcome. Twenty-seven patients were included in the efficacy analyses because 1 patient did not have ≥1 postbaseline evaluation. No patient achieved CR or PR as best response to treatment with pembrolizumab per IWG 2006 criteria for MDS; 5 patients achieved bone marrow complete response (mCR) (, ). The median duration of response was 2.6 months (95% CI, not reported-not reported). Five patients (19%) experienced hematologic improvement; 2 (7%) had erythroid response, 2 (7%) had platelet response, and 1 (4%) had neutrophil response per IWG 2006 criteria. Six patients had cytogenetic response per IWG 2006 criteria, with 3 (11%) experiencing CR and 3 (11%) experiencing PR. In addition, 12 patients (44%) had stable disease and 10 (37%) had progressive disease (), defined as ≥50% increase in blasts per IWG criteria. One patient who achieved mCR and 1 patient who was on study for >1 year had a diminished requirement for transfusion at the end of the study, and 1 patient who completed ≥2 years of study treatment became transfusion independent (). Assessment of blast levels showed that the patients with mCR had the lowest percentages of blasts and blasts/leukocytes in the bone marrow ().
Figure 2. Percentage by best response in all evaluable patients. (A) Bone marrow blasts. (B) Blasts/leukocytes. mCR, bone marrow complete response; PD: progressive disease; SD: stable disease.
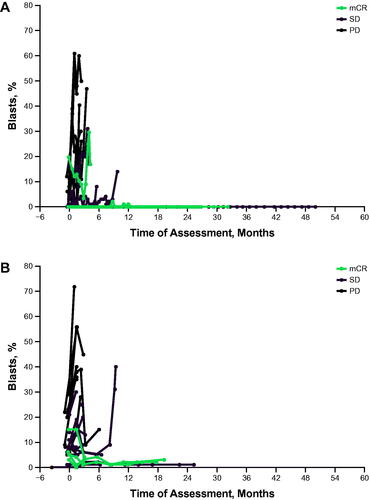
Table 3. Response and hematologic improvement in all evaluable patients per IWG 2006 criteriaa.
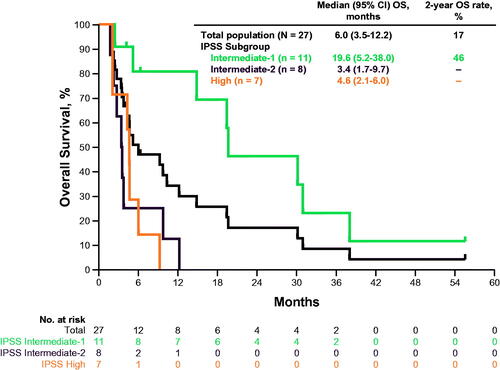
Median OS was 6.0 months (95% CI, 3.5–12.2) in the overall population and 19.6 months (95% CI, 5.2–38.0), 3.4 months (95% CI, 1.7–9.7), and 4.6 months (95% CI, 2.1–6.0) in the IPSS-intermediate-1, intermediate-2, and high-risk MDS subgroups, respectively. The estimated 2-year OS rate was 17% in the overall population and 46% in patients with IPSS intermediate-1 MDS. Patients in the intermediate-2 and the high-risk MDS subgroups did not achieve OS to 2 years (). Nine participants achieved either a CR/PR for cytogenetic response or bone marrow CR as best overall response (2 patients achieved both a cytogenic and bone marrow response); the median (descriptive) was 19.4 months (range: 0.03+, 53.6+) for PFS and 19.4 months (range: 3.5, 55.5+) for OS, where + represents a censored observation.
Discussion
Limited available therapeutic modalities and lack of effective treatment for a substantial proportion of patients with MDS highlight the unmet need for novel strategies. Specifically, treatment with azacitidine has been shown to enhance the expression of PD-1 and its ligands, PD-L1 and PD-L2, and has been posited to adversely affect survival in patients with MDS [Citation14,Citation15]. Therefore, targeting PD-1, PD-L1, and PD-L2 represents a novel strategy for patients with MDS whose first-line treatment with an HMA such as azacytidine failed.
In this study, treatment with pembrolizumab resulted in a manageable safety profile and clinical activity in patients with MDS that progressed after HMA treatment failure. TRAEs were comparable or lower in the MDS subgroup than in patient groups with other hematologic malignancies in phase 1/2 pembrolizumab clinical studies: 40% in patients with multiple myeloma, 71% to 73% in patients with Hodgkin’s lymphoma, and 23% to 24% in patients with primary mediastinal large B-cell lymphoma [Citation11,Citation19,Citation20].
The study did not meet the prespecified clinical response threshold because no patient achieved a response per IWG 2006 criteria (ORR, 0) and the lower bound of the 90% CI for ORR did not exclude the 10% null hypothesis. Five of 27 evaluable patients (19%) achieved mCR. Six patients (3 each) had cytogenetic CR (11%) or PR (11%) based on IWG 2006 criteria. In addition, 5 patients (19%) experienced hematologic improvement, 2 (7%) had an erythroid response, 2 (7%) had a platelet response, and 1 (4%) had neutrophil response based on IWG criteria. All responders in the study had IPSS intermediate-1 MDS, in keeping with previous observations that, typically, patients with IPSS high-risk MDS that progressed after HMA treatment failure have poor prognoses [Citation21]. Most patients in the current study had IPSS high-risk MDS (15 [54%] categorized as IPSS intermediate-2 or high-risk). All were transfusion dependent at baseline and had poorer outcomes than patients with IPSS-intermediate-1 MDS. Thus, the median OS of 6.0 months seen in this study was consistent with the median OS of 4 months previously reported in patients who experienced HMA treatment failure and supports the generally poor prognosis seen in this patient population at higher risk [Citation6,Citation9]. In contrast, the median OS of 19.6 months with a 2-year OS rate of 46% observed in patients with IPSS intermediate-1 MDS is higher than that seen previously in patients with IPSS intermediate-1 MDS after treatment failure with an HMA (14 months), supporting the activity of pembrolizumab in this patient population [Citation6].
Recently, a phase 2 trial was conducted to study combination therapy with azacitidine and pembrolizumab in patients with high-risk MDS who previously experienced failed treatment with an HMA (N = 20) [Citation13]. At a median follow-up of 27.9 months, the ORR was 25% and the median OS was 6.1 months. Combination treatment was well tolerated with no new safety signals [Citation13].
Limitations in this study include the open-label, single arm design and small number of patients enrolled. In addition, cytogenic data were not evaluable for those patients who achieved a cytogenic response. Further analyses of genetic alterations may determine characteristics that predict response to PD-1 inhibitor therapy for MDS. Also, as no patients in the study were PD-L1 positive and PD-l expression in immune cells was not evaluated, the role of PD-1/L1 expression on response could not be evaluated. Future studies in a PD-L1-positive patient population and/or the evaluation of PD-1 expression in immune cells may further elucidate the role of PD-1/PD-L1 expression on response in MDS.
Overall, the data presented here show that treatment with pembrolizumab was associated with a manageable safety profile in all treated patients and with clinical activity in patients with IPSS intermediate-1 MDS that had progressed after failure of first-line treatment with a hypomethylating agent. The clinical activity of pembrolizumab seen in this study supports ongoing investigation of pembrolizumab as a part of a combination therapy regimen.
Author contributions
Contribution: G.G.M. and B.D.S. conceived, designed, and planned the study; G.G.M., M.F., and B.D.S. acquired the data; G.G.M., Y.Z., M.F., P.M., and B.D.S analyzed the data; G.G.M., V.R., Y.Z., M.F., and P.M. interpreted the results; G.G.M., V.R., Y.Z., M.F., P.M., and B.D.S. critically reviewed or revised the manuscript for important intellectual content; G.G.M. and V.R. drafted the manuscript.
Acknowledgments
The authors thank the patients and their families and caregivers and all primary investigators and their site personnel. The authors also thank Brooke Burton (employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) for clinical study support and Luana Atherly-Henderson, PhD, CMPP (employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) for medical writing and/or editorial support. Additional medical writing and/or editorial assistance was provided by Dominic Singson, MD, and Matthew Grzywacz, PhD, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Disclosure statement
G.G.M. has received funding for the current manuscript from the NIH Cancer Center; has received research funding from Amphivena, Helsinn, Novartis, AbbVie, Bristol Myers Squibb, Astex, Onconova, H3 Biomedicine, Merck & Co., Inc., Curis, Janssen, Genentech, Forty Seven, and Aprea; and has been a consultant for Bristol Myers Squibb, Astex, Helsinn, and Genentech. VR has received honoraria from AZD and Roche; has received research funding from GlaxoSmithKline, Argen-X, and Astex; and has participated on a data safety monitoring board or an advisory board for AZD, Gilead, Infinity, MSD, Bristol Myers Squibb, Nanostring, Incyte, and Roche. YZ is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. MF and PM are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and are stockholders of Merck & Co., Inc., Kenilworth, NJ, USA. BDS has received funding for the current manuscript from Merck & Co., Inc.; received research funding from Merck & Co., Inc.; has been a consultant for Jazz Pharmaceuticals, Novartis, and Pfizer; and has participated on a data safety monitoring board or an advisory board for Celgene, a subsidiary of Bristol Myers Squibb.
Data availability statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Additional information
Funding
References
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872–1885.
- Hellström-Lindberg E, Tobiasson M, Greenberg P. Myelodysplastic syndromes: moving towards personalized management. Haematologica. 2020;105(7):1765–1779.
- Mohammad AA. Myelodysplastic syndrome from theoretical review to clinical application view. Oncol Rev. 2018;12(2):397.
- Sweeney MR, Applebaum KM, Arem H, et al. Medical conditions and modifiable risk factors for myelodysplastic syndrome: a systematic review. Cancer Epidemiol Biomarkers Prev. 2019;28(9):1502–1517.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): myelodysplatic syndromes (Version 3.2021). 2021. https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf
- Gil-Perez A, Montalban-Bravo G. Management of myelodysplastic syndromes after failure of response to hypomethylating agents. Ther Adv Hematol. 2019;10:2040620719847059.
- Seymour JF, Fenaux P, Silverman LR, et al. Effects of azacitidine compared with conventional care regimens in elderly (>/= 75 years) patients with higher-risk myelodysplastic syndromes. Crit Rev Oncol Hematol. 2010;76(3):218–227.
- Lubbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29(15):1987–1996.
- Jabbour E, Garcia-Manero G, Batty N, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116(16):3830–3834.
- Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016;34(31):3733–3739.
- Armand P, Kuruvilla J, Michot JM, et al. KEYNOTE-013 4-year follow-up of pembrolizumab in classical hodgkin lymphoma after brentuximab vedotin failure. Blood Adv. 2020;4(12):2617–2622.
- Armand P, Chen YB, Redd RA, et al. PD-1 blockade with pembrolizumab for classical hodgkin lymphoma after autologous stem cell transplantation. Blood. 2019;134(1):22–29.
- Chien KS, Borthakur G, Naqvi K, et al. Final results from a phase II study combining azacitidine and pembrolizumab in patients with higher-risk myelodysplastic syndrome after failure of hypomethylating agent therapy. Blood. 2020;136(Supplement 1):23–24.
- Yang H, Bueso-Ramos C, DiNardo C, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280–1288.
- Cheng P, Eksioglu EA, Chen X, et al. S100A9-induced overexpression of PD-1/PD-L1 contributes to ineffective hematopoiesis in myelodysplastic syndromes. Leukemia. 2019;33(8):2034–2046.
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425.
- US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE), version 4.0. 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
- Bhatnagar B, Zandberg DP, Vannorsdall EJ, et al. Lack of response of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) to decitabine after failure of azacitidine. Blood. 2012;120(21):3858–3858.
- Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144–1153.
- Ribrag V, Avigan DE, Green DJ, et al. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br J Haematol. 2019;186(3):e41–e44.
- Zeidan AM, Stahl M, Komrokji R. Emerging biological therapies for the treatment of myelodysplastic syndromes. Expert Opin Emerg Drugs. 2016;21(3):283–300.