Abstract
Joint and muscle pain, including arthralgia, myalgia, and musculoskeletal pain, are among the common adverse events (AEs) reported for ibrutinib, a once-daily Bruton’s tyrosine kinase inhibitor approved for the treatment of various B-cell malignancies, including chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). This pooled analysis from nine clinical trials of ibrutinib in CLL and MCL (N = 1178) evaluated patterns of these AEs. Any grade arthralgia, myalgia, and musculoskeletal pain occurred in 18%, 10%, and 6% of patients, respectively. AEs were primarily low-grade (grade 1/2: 97‒99%) and occurred during the first year of treatment; most resolved (67%–80%) at first occurrence. Few (<5%) patients required ibrutinib dose modification; no patients discontinued ibrutinib due to these AEs. Among patients evaluated for concomitant medication use, all those receiving concomitant medications after the first AE occurrence experienced AE resolution. These data suggest that these AEs were not treatment-limiting during ibrutinib therapy.
Introduction
Ibrutinib is an oral, once-daily Bruton’s tyrosine kinase (BTK) inhibitor approved, among other indications, for the treatment of patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) in both first-line and relapsed/refractory settings and for patients with mantle cell lymphoma (MCL) who have received at least one prior therapy [Citation1]. In patients with CLL/SLL, extended treatment with single-agent ibrutinib was well-tolerated and showed sustained efficacy in long-term follow-up (median 5–8 years) for both the first-line and relapsed/refractory settings [Citation2–5]. In a pooled analysis of patients with relapsed/refractory MCL (n = 370), ibrutinib-based therapy (median duration of treatment: 11 months; 31% had ≥2 years of treatment) showed durable responses with no notable emerging toxicity with extended follow-up of 7.5 years [Citation6]. Because patients are treated with once-daily ibrutinib until unacceptable toxicity or progressive disease (PD), safety and tolerability are key for patients expected to receive treatment for an extended duration. The availability of long-term data from multiple clinical studies of ibrutinib is a unique advantage among the available BTK inhibitors and allows for a more complete evaluation of tolerability in a large cohort of patients.
To date, most adverse events (AEs) reported with ibrutinib in clinical trials were of mild to moderate severity (i.e. low-grade; grade 1/2) [Citation7–9]. Dose hold followed by reinitiation of treatment is recommended for grade 1/2 AEs, and most resolve within the first 2 months of treatment, thus facilitating continued ibrutinib therapy.
Joint and/or muscle pain, such as arthralgias, myalgias, and musculoskeletal pains, has been described by patients as AEs that may affect their well-being or lead to ibrutinib treatment discontinuation in the clinical practice setting [Citation10,Citation11]. Similar rates of arthralgia and myalgia have also been reported in acalabrutinib or zanubrutinib [Citation12–14], and these AEs may be a class effect of BTK inhibitors. Although these AEs are typically low-grade and mild to moderate in severity, we aimed to better characterize the pattern (including frequency, time to onset, resolution, and duration) and management of low-grade arthralgia, myalgia, and musculoskeletal pain in patients with CLL/SLL or MCL treated with ibrutinib-based therapy in clinical trials.
Materials and methods
Safety data from the following phase 1 through phase 3 study populations were pooled: CLL3001 (HELIOS), MCL2001 (SPARK), MCL3001 (RAY), PCYC-1102, PCYC-1104, PCYC-1108, PCYC-1109, PCYC-1112 (RESONATE), and PCYC-1115 (RESONATE-2) (Supplemental Table S1). This analysis was limited to patients originally assigned to ibrutinib-based therapy at study entry. For studies in which patients were eligible to roll over to extension studies that did not routinely collect grade 1/2 AEs, the analysis was limited to the follow-up time on the original protocol. In addition, patients receiving different doses of ibrutinib that are not approved and patients who were assigned to control arms were not included in the analysis. See Supplemental Table S1 for a summary of all data sets. Full details of the individual study designs included in the pooled population have been reported previously [Citation8,Citation15–24].
Table 1. Baseline characteristics and demographics of patients treated with ibrutinib in clinical trials.
Because concomitant medication data availability was inconsistent across the pooled dataset described in Supplemental Table S1 concomitant medication use for these joint/muscle pain AEs were evaluated using a pooled dataset from the phase 3 RESONATE-2 and iLLUMINATE (PCYC-1130; first-line treatment with ibrutinib-obinutuzumab for CLL) [Citation25] studies. This analysis population is referred to as the RESONATE-2/iLLUMINATE dataset hereafter.
All studies were approved by the institutional review board or independent ethics committee at each participating institution and were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent.
Assessments
AE severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, using version 4.0 for RESONATE, PCYC-1102, PCYC-1104, PCYC-1109, and version 4.03 for RESONATE-2, HELIOS, SPARK, RAY, PCYC-1103, and PCYC-1108. The AE collection period was defined from the time of treatment initiation until 30 days after the last dose of the study drug, not including treatment in rollover studies. In contrast to the United States prescribing information, which includes myalgia within the grouped term musculoskeletal pain, the low-grade AE data presented here are based on separate preferred terms of arthralgias, myalgias, and musculoskeletal pains, in accordance with the Medical Dictionary for Regulatory Activities.
In this analysis, AEs that started at grade 1/2 but escalated to grade ≥3 during the first occurrence of the AE were described as the first onset with a worsening grade, as opposed to AEs that started at grade ≥3. Resolution at first occurrence was used to describe cases where the first event (either a single event in a patient or the first of multiple events in a patient) was resolved.
Statistical analysis
Safety data were summarized using descriptive statistics, including proportions for discrete variables and medians for continuous variables.
Results
This pooled analysis included 1178 patients, of whom 808 had CLL/SLL and 370 had MCL (). The median age of patients was 67 years (range, 30–89 years); 61% were aged ≥65 years and 20% were aged ≥75 years. The median number of prior therapies was two (range, 0–13); 38% of patients had ≥3 prior therapies; and 20% (n = 162/808) of patients with CLL/SLL were previously untreated. For patients with CLL/SLL and with MCL, the median duration of ibrutinib exposure was 20.2 months (range, 0.2–71 months) and 11.1 months (range, 0–47 months), respectively.
Arthralgia
Any grade arthralgia occurred in 18% of patients (n = 211), and 99% of these patients (209/211) had grade 1/2 events; only two patients had grade ≥3 events at the first onset. In total, 354 events of any grade occurred in 211 patients (1.68 events per patient); 338 events of grade 1/2 arthralgia were reported among 209 patients. The median time to the first onset of grade 1/2 arthralgia was 4.4 months (range, 0.03–66 months); the first event occurred within the first 6 months of treatment for 58% (121/209) of patients. Incidence of arthralgia decreased after the first year of treatment (). The prevalence of low-grade arthralgia decreased over time (Supplemental Table S2 and Supplemental Figure 1). The median duration of all occurrences of grade 1/2 arthralgia was 4.9 months (range, 0.03–64.8 months).
Figure 1. Percentage of patients with the first onset of low-grade (grade 1/2) arthralgia, myalgia, and musculoskeletal pain over time (N = 1178).
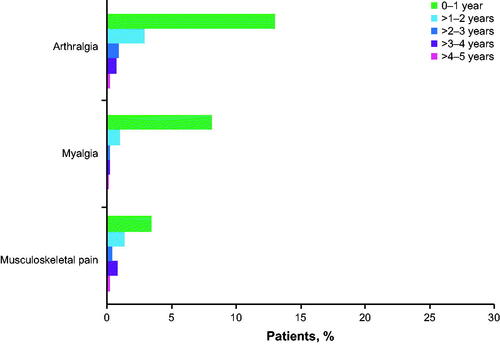
Table 2. Summary of arthralgia, myalgia, and musculoskeletal pain and concomitant medication use for patients treated with ibrutinib (RESONATE-2/iLLUMINATE dataset).
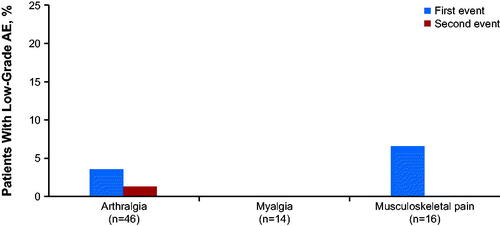
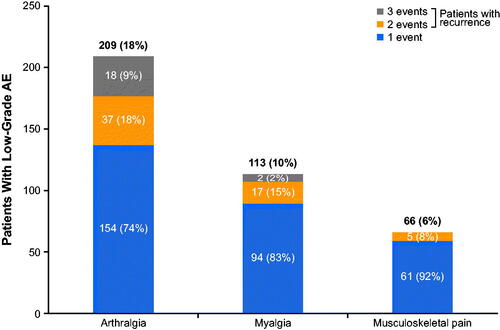
Among patients with grade 1/2 arthralgia, 74% (154/209) had one event, 18% (37/209) had two events, and 9% (18/209) had three or more events (). In the total pooled population (N = 1178), very few patients (0.7%; n = 8) had a grade 1/2 arthralgia event that escalated to a grade ≥3 AE at the first onset.
Figure 2. The proportion of patients with one, two, or three events of low-grade (grade 1/2) arthralgia, myalgia, and musculoskeletal pain in those treated with ibrutinib in clinical trials (N = 1178) AE: adverse event.
Among 209 patients with grade 1/2 arthralgia, 67% (n = 141/209) had resolution at first occurrence, with 43% (n = 61/141) and 58% (n = 82/141) who recovered or had symptoms resolve within 30 and 60 days, respectively. The median time to resolution (for the first occurrence) was 1.3 months (range, 0.03–44 months). No patients discontinued ibrutinib treatment due to grade 1/2 arthralgia. The median duration of symptoms for those with unresolved arthralgia from onset date to the end of the study was 15.0 months (range, 0.03‒67 months).
Seven patients (3%) had a dose hold for ibrutinib and two patients (1%) had an ibrutinib dose reduction due to grade 1/2 arthralgia. All dose reductions due to arthralgia occurred at the first instance of the AE in accordance with the protocol.
Among 248 patients with CLL/SLL treated with first-line ibrutinib or ibrutinib-obinutuzumab in the RESONATE-2/iLLUMINATE dataset, any grade arthralgia occurred in 19% of patients (n = 48/248); 96% of these patients (n = 46/48) had grade 1/2 arthralgia, which was consistent with the overall pooled dataset of 1178 patients with CLL/SLL or MCL. Among the RESONATE-2/iLLUMINATE concomitant medication dataset (N = 248), one patient (0.4%) had a grade 1/2 arthralgia that escalated to a grade ≥3 AE. Patients reporting only grade ≥3 arthralgia at first onset were rare (1%; n = 3/248). Of the 46 patients who experienced grade 1/2 arthralgia, five patients received concomitant medications prior to the onset of arthralgia. Nine of 46 patients (20%) received concomitant medication to manage the first reported event (). Of these patients, 2/9 (1%) received steroids, 6/9 (2%) received NSAIDs, and 2/9 (1%) received opioids or phenylpiperidine derivatives; the AEs resolved in all nine patients. Three of 46 patients (7%) experienced a second arthralgia event, and for this second event, all three patients received NSAIDs along with steroids (n = 1), opioids or phenylpiperidine derivatives (n = 1), or other analgesics (n = 1) (). All three patients had symptoms resolve.
Myalgia
Any grade myalgia events occurred in 10% of patients (n = 116), with most of these patients (97%; n = 113/116) experiencing grade 1/2 events; three patients had only grade ≥3 events at the first onset. In total, 152 events of any grade occurred in 116 patients (1.31 events per patient); 147 events of grade 1/2 myalgia were reported among 113 patients (1.30 events per patient). The median time to the first onset of grade 1/2 myalgia was 2.1 months (range, 0.03–60.5 months). Among patients who experienced grade 1/2 myalgia while on ibrutinib, most experienced an event within the first 6 months (73% [83/113 patients]), and 84% (95/113) of patients who had grade 1/2 myalgia experienced the first onset by month 12. Incidence of myalgia decreased after the first year of treatment (). The prevalence of low-grade myalgia decreased over time (Supplemental Table 2 and Supplemental Figure 1). The median duration of all occurrences of grade 1/2 myalgia was 3.2 months (range, 0–65.0 months).
Among the 113 patients with grade 1/2 myalgia, 83% (94/113) had one event, 15% (17/113) had two events, and 2% (2/113) had three or more events (). Only two patients had a grade 1/2 myalgia event that escalated to grade ≥3 at first onset; 67% (n = 76) had resolution at first occurrence, with 55% (n = 42/76) and 70% (n = 53/76) who recovered or had symptoms resolve within 30 and 60 days, respectively. The median time to resolution (for the first occurrence) was 1.0 months (range, 0.03–42 months). No patients discontinued ibrutinib treatment due to grade 1/2 myalgia. The median duration of symptoms for those with unresolved myalgia from the onset date to the end of the study was 17.8 months (range, 0.16‒63).
Two patients (2%) had a dose hold for ibrutinib and one patient (0.9%) had an ibrutinib dose reduction. All dose reductions due to myalgia occurred for the first instance of the AE. As with patients who experienced arthralgia, none of the patients with dose holds for grade 1/2 myalgia had treatment discontinuation due to escalation of the AE.
In the RESONATE-2/iLLUMINATE dataset for evaluation of concomitant medication use, any grade myalgia occurred in 6% of patients (n = 15/248), and 93% of these patients (n = 14/15) experienced grade 1/2 symptoms. No patients had a grade 1/2 myalgia event that escalated to a grade ≥3 AE. One patient reported only grade ≥3 myalgia (0.4%). Among the 14 patients (6%) who experienced grade 1/2 myalgia at the first onset, no patients received concomitant medication to manage the AE after any reported event (). Myalgia symptoms resolved in 9 of 12 patients (75%) with one myalgia event. Three of 14 patients (21%) experienced a second myalgia event, all of whom achieved AE resolution. One patient experienced a third event that did not resolve.
Musculoskeletal pain
Any grade musculoskeletal pain events occurred in 6% of patients (n = 68), with most (66/68; 97%) experiencing grade 1/2 events; two patients had only grade ≥3 events at the first onset. A total of 77 events of any grade occurred in 68 patients (1.13 events per patient); 73 events of grade 1/2 musculoskeletal pain were reported in 66 patients (1.11 events per patient). The median time to the first onset of the grade 1/2 events was 6.8 months (range, 0.3–56.2 months). Among patients who experienced grade 1/2 musculoskeletal pain while on ibrutinib, almost half experienced an event within the first 6 months (45% [30/66 patients]); 61% of patients (40/66) with grade 1/2 musculoskeletal pain experienced the first onset of grade 1/2 musculoskeletal pain by month 12. The incidence of musculoskeletal pain decreased after the first year of treatment (). The prevalence of low-grade musculoskeletal pain remained consistent over time (<4%) (Supplemental Table 2 and Supplemental Figure 1). The median duration of all occurrences of grade 1/2 musculoskeletal pain was 1.8 months (range, 0.03–54.0 months).
Among patients with grade 1/2 musculoskeletal pain, 92% (61/66) had one event, 8% (5/66) had two events, and no patients (0/66) had three or more events (). Only two patients had a grade 1/2 musculoskeletal pain event that escalated to a grade ≥3 AE at the first onset.
Among 66 patients experiencing grade 1/2 musculoskeletal pain, 80% (n = 53) had resolution at first occurrence, with 55% (n = 29/53) and 72% (n = 38/53) who recovered or had symptoms resolve within 30 and 60 days, respectively. The median time to resolution (for the first occurrence) was 1.0 months (range, 0.03–23 months). No patients discontinued ibrutinib treatment due to musculoskeletal pain. The median duration of symptoms for those with unresolved musculoskeletal pain from the onset date to the end of the study was 15.2 months (0.2‒54 months).
One patient (1.5%) had a dose hold for ibrutinib and one (1.5%) had an ibrutinib dose reduction. As with the other grade 1/2 AEs, all dose reductions due to musculoskeletal pain occurred for the first instance of the AE. None of the patients with dose holds for grade 1/2 musculoskeletal pain had treatment discontinuation due to escalation of the AE.
In the RESONATE-2/iLLUMINATE dataset for evaluation of concomitant medication use, any grade musculoskeletal pain occurred in 7% of patients (n = 17/248), and 94% of these patients (n = 16/17) experienced grade 1/2 symptoms. No patients had a grade 1/2 musculoskeletal pain event that escalated to a grade ≥3 AE. One patient reported only grade ≥3 musculoskeletal pain (0.4%). Five of the 16 patients (31%) in this RESONATE-2/iLLUMINATE dataset who experienced grade 1/2 musculoskeletal pain received concomitant medication to manage the first event and five had symptoms resolve (). As with the other grade 1/2 AEs, concomitant medications were administered for the first reported event, with patients receiving NSAIDs (n = 3; 1%), opioids/phenylpiperidine derivatives (n = 2, 1%), other analgesics (n = 2, 1%), steroids (n = 1, 0.4%), and other medications (n = 1, 0.4%). No patients reported a second event of musculoskeletal pain ().
Among the 1178 patients included in this pooled analysis, 308 (26.1%) had one or more of these events (arthralgia, myalgia, or musculoskeletal pain). Forty-four patients (3.7%) experienced both arthralgia and myalgia; 17 patients (1.4%) experienced both arthralgia and musculoskeletal pain; seven (0.6%) patients experienced both myalgia and musculoskeletal pain; and six patients (0.5%) experienced all three low-grade AEs.
Discussion
This pooled analysis of 1178 patients with CLL/SLL or MCL treated with ibrutinib-based regimens shows that AEs related to joint or muscle pain (in particular, arthralgia) generally manifest as low-grade events during the first year of treatment, with symptoms resolving in the first 2 months in up to 72% of patients, allowing patients to continue ibrutinib treatment. Furthermore, 67% to 80% of these low-grade AEs resolved during the course of ibrutinib treatment and no patients discontinued ibrutinib treatment due to these joint/muscle pain events. In the current analysis with up to 5 years of ibrutinib-based treatment, grade 1/2 arthralgia was reported in fewer than one in five patients across nine clinical trials of ibrutinib, and myalgia and musculoskeletal pain were reported in fewer than one in 10 patients. In our RESONATE-2/iLLUMINATE dataset analysis, 75‒100% of patients experienced resolution of the AE following the first reported event regardless of concomitant medication status. Of the limited number of patients in the RESONATE-2/iLLUMINATE dataset who received concomitant medications for the first reported event, all had symptoms resolve. Importantly, these patients on continuous ibrutinib therapy in the first-line setting experienced similar rates of low-grade AEs compared to the total pooled dataset of patients with CLL/SLL or MCL (arthralgia: 19% in first-line CLL vs 18% in the total pool; myalgia: 6% vs 10%; musculoskeletal pain: 7% vs 6%).
In the RESONATE/iLLUMINATE studies, patients received NSAIDs, opioids/phenylpiperidine derivatives, other analgesics, or steroids to manage low-grade AEs; this is consistent with suggested management strategies for ibrutinib-related joint and muscle pain AEs recommending the use of low-dose, anti-inflammatory agents, such as acetaminophen or short-term prednisone [Citation10,Citation26,Citation27], low-dose opioids, or anti-epileptic medications [Citation10,Citation26]. Although concomitant medications outside of common NSAIDs may not be uniformly reported for low-grade AEs, in this study we found that these concomitant medications allowed patients to continue ibrutinib therapy and dose modifications were rare for these AEs. No patients discontinued ibrutinib treatment due to any grade arthralgia, myalgia, or musculoskeletal pain.
The mechanisms of treatment-emergent joint or muscle pain AEs, such as arthralgias or myalgias, are unknown; however, these AEs may be a class effect of BTK inhibitors given that similar rates of AE events have been reported in acalabrutinib or zanubrutinib clinical trials. Arthralgia of any grade was reported in 16–21% of patients, and myalgia of any grade was reported in 18% of patients with CLL treated with single-agent acalabrutinib in the first-line and relapsed/refractory settings (median follow-up: 14–28 months) [Citation12–14]. In long-term follow-up (median 41 months) of acalabrutinib in first-line CLL, rates of any grade arthralgia and myalgia were 29% and 12%, respectively [Citation28]. Additionally, arthralgia or myalgia of any grade was reported in 19% of patients with CLL treated with single-agent zanubrutinib (median follow-up: 27 months) [Citation29]. In clinical trials, musculoskeletal pain of any grade was reported in 15–32% of patients with CLL treated with single-agent acalabrutinib and in 19% of patients treated with single-agent zanubrutinib [Citation30,Citation31]. These rates of joint or muscle pain AEs with acalabrutinib or zanubrutinib appear comparable to rates with ibrutinib. Although recent studies reported lower rates of arthralgia with acalabrutinib [Citation32] and zanubrutinib [Citation33], the studies in this pooled analysis have longer-term follow-up and additional time on the study is needed for acalabrutinib and zanubrutinib to fully understand the long-term profile for these agents. This pooled analysis for ibrutinib alongside the recent studies of other BTK inhibitors offers a comprehensive view of joint/muscle pain AEs across this treatment class.
Several potential limitations exist with this current analysis, including variations in treatment regimens (single agent [n = 794; 67% of patients] or in combination with other agents [n = 384; 32.6% of patients]) or ibrutinib doses applied across studies (69% and 31% of patients enrolled in studies with 420 mg and 560 mg doses, respectively).
In conclusion, in this pooled analysis of 1178 patients with CLL or MCL treated with ibrutinib for up to 5 years, joint or muscle pain AEs were reported in 6–18% of patients. In most patients (97‒99%), these AEs were low-grade events and most patients (67–80%) had resolution of the AE at first occurrence. No patients discontinued ibrutinib due to grade 1/2 joint/muscle pain, suggesting that these AEs can be controlled and do not impact a patient’s ability to stay on ibrutinib for the established clinical benefits.
Author contributions
IA-H and AM designed the analysis; TS, SC, DM, PMB, KR, MW, and SO contributed to data collection; AM performed the data analyses; AM and KO-B confirmed the accuracy of the data and compiled it for analysis; and all authors had access to the data and were involved in the interpretation of data, contributed to the manuscript review and revisions, and approved the final version for submission.
GLAL-2021-0948-File002.docx
Download MS Word (2.6 MB)Acknowledgments
We thank all the patients who participated in this trial and their families; David Arthur, PhD, formerly of AbbVie, Inc., for his contributions to data interpretation; and Cindi A. Hoover, PhD, of ApotheCom (San Francisco, CA, USA) for medical writing support, which was funded by Pharmacyclics LLC, an AbbVie Company.
Disclosure statement
TS: consulting or advisory role with Juno Therapeutics, Celgene, Kite Pharma, AstraZeneca, and BeiGene; institutional research funding from Kite Pharma, Juno Therapeutics, Celgene, AstraZeneca, TG Therapeutics, Oncternal, BeiGene, and Pharmacyclics LLC, an AbbVie Company; speakers bureau with AstraZeneca, Janssen, and Pharmacyclics LLC, an AbbVie Company; SC: honoraria from Janssen and Pharmacyclics LLC, an AbbVie Company; consulting or advisory role with AbbVie, Celgene, Pharmacyclics LLC, an AbbVie Company, Janssen, Novartis, Astellas, and AstraZeneca; research funding from AbbVie, Acerta, Celgene, Gilead, Janssen, Pharmacyclics LLC, an AbbVie Company, and Takeda; expert testimony for Genentech; travel accommodations from AbbVie, BeiGene, Celgene, Genentech, Janssen, and Pharmacyclics LLC, an AbbVie Company; other relationship with BeiGene; MM: honoraria from Kite Pharma and Genentech; consulting/advisory role with Kite Pharma/Gilead, Seagen, Molecular Templates, BTG, Pharmacyclics, an AbbVie Company, Verastem, Genentech, and Celgene; research funding from Unum Therapeutics, Molecular Templates, Incyte, BeiGene, Denovo Biopharma, Pharmacyclics, an AbbVie Company, Nordic Nanovector, Bristol Myers Squibb, Genentech, and Celgene; speakers bureau with Kite Pharma; PMB: consulting/advisory role with Celgene, Pharmacyclics, an AbbVie Company, AbbVie, Gilead, Seagen, Merck, Genentech, Bristol Myers Squibb, MEI Pharma, Janssen, MorphoSys, AstraZeneca, and TG Therapeutics; research funding from TG Therapeutics and AstraZeneca; KR: consulting/advisory role with Acerta Pharma, AstraZeneca, Innate Pharma, and Pharmacyclics LLC, an AbbVie Company; research funding from Genentech, AbbVie, Novartis, and Janssen; travel accommodations from AstraZeneca; AM: employment with and stock ownership in AbbVie; RV: employment and leadership with Pharmacyclics LLC, an AbbVie Company; stock ownership in AbbVie and Gilead; AS: employment with Pharmacyclics LLC, an AbbVie Company; stock ownership in AbbVie; SD and AZ: employment with and stock ownership in Janssen; IA-H: employment with Janssen; stock ownership in AbbVie and Bristol Myers Squibb; KO-B: employment with, stock ownership in, and travel/accommodations/expenses from AbbVie; MW: honoraria from Acerta Pharma, China Anti-Cancer Association, BeiGene, Breast and Gynecological International Cancer Society, BioInvent, Chinese American Hematologist and Oncologist Network, Chinese Medical Association, Clinical Care Options, Eastern Virginia Medical School, Epizyme, Hebei Cancer Prevention Federation, Imedex, Kite Pharma, TS Oncology, Miltenyi Biomedicine GmBH, Moffitt Cancer Center, Mumbai Hematology Group, Newbridge Pharmaceuticals, Physicians' Education Resource, Practice Point Communications, Scripps Clinic, The First Affiliated Hospital of Zhejiang University, Pharmacyclics LLC, an AbbVie Company, Janssen, AstraZeneca, OMI, Dava Oncology, and OncLive; consulting/advisory role with Pharmacyclics LLC, an AbbVie Company, Bayer Healthcare, BeiGene, BioInvent, CStone Pharmaceuticals, DTRM Biopharma, Epizyme, Genentech, Miltenyi Biomedicine GmBH, VelosBio, Janssen, AstraZeneca, Kite Pharma, Juno, InnoCare, Oncternal, and Loxo Oncology; research funding from BeiGene, Genentech, InnoCare, Eli Lilly, Pharmacyclics LLC, an AbbVie Company, Janssen, AstraZeneca, Kite Pharma, Juno, Celgene, Loxo Oncology, VelosBio, Acerta Pharma, Molecular Templates, BioInvent, and Oncternal; travel accommodations from Physicians' Education Resource, Janssen, Pharmacyclics LLC, an AbbVie Company, AstraZeneca, Acerta Pharma, Juno, Celgene, Kite Pharma, Loxo Oncology, VelosBio, Verastem, Molecular Templates, BioInvent, and Oncternal; SO: consulting/advisory role with Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen, Aptose Biosciences, Vaniam Group, AbbVie, Alexion, Verastem, Eisai, Gilead, Pharmacyclics LLC, an AbbVie Company, TG Therapeutics, Pfizer, Juno Therapeutics, and Sunesis; research funding from Kite Pharma, Regeneron, Acerta, Gilead, Pharmacyclics LLC, an AbbVie Company, TG Therapeutics, Pfizer, and Sunesis.
Data availability statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu
Additional information
Funding
References
- Imbruvica (ibrutinib) [prescribing information]. Sunnyvale, CA: Pharmacyclics LLC; 2020.
- Byrd JC, Furman RR, Coutre SE, et al. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin Cancer Res. 2020;26(15):3918–3927.
- Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–798.
- Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–1363.
- Byrd JC, Furman RR, Coutre S, et al. Up to 7 years of follow-up of single-agent ibrutinib in the phase 1b/2 PCYC-1102 trial of first line and relapsed/refractory patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. 2018;132(Supplement 1):3133–3133.
- Rule S, Dreyling MH, Goy A, et al. Long-term outcomes with ibrutinib versus the prior regimen: a pooled analysis in relapsed/refractory (R/R) mantle cell lymphoma (MCL) with up to 7.5 years of extended follow-up. Blood. 2019;134(Supplement_1):1538–1538.
- Coutre SE, Byrd JC, Hillmen P, et al. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;3(12):1799–1807.
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lympho-ma. N Engl J Med. 2013;369(6):507–516.
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126(6):739–745.
- Stephens DM, Byrd JC. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood. 2019;133(12):1298–1307.
- Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–879.
- Awan FT, Schuh A, Brown JR, et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3(9):1553–1562.
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291.
- Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–332.
- O'Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58.
- Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223.
- Jaglowski SM, Jones JA, Nagar V, et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood. 2015;126(7):842–850.
- Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42.
- Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib . Blood. 2015;125(16):2497–2506.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437.
- Coutre SE, Furman RR, Flinn IW, et al. Extended treatment with single-agent ibrutinib at the 420 mg dose leads to durable responses in chronic lymphocytic leukemia/small lymphocytic lymphoma. Clin Cancer Res. 2017;23(5):1149–1155.
- Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211.
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–778.
- Brown JR, Barrientos JC, Barr PM, et al. The Bruton tyrosine kinase inhibitor ibrutinib with chemoimmunotherapy in patients with chronic lymphocytic leukemia. Blood. 2015;125(19):2915–2922.
- Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56.
- Gribben JG, Bosch F, Cymbalista F, et al. Optimising outcomes for patients with chronic lymphocytic leukaemia on ibrutinib therapy: European recommendations for clinical practice. Br J Haematol. 2018;180(5):666–679.
- Lasica M, Tam CS. Management of ibrutinib toxicities: a practical guide. Curr Hematol Malig Rep. 2020;15(3):177–186.
- Byrd JC, Wierda WG, Schuh A, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;135(15):1204–1213.
- Cull G, Simpson D, Opat S, et al. Treatment with the Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) demonstrates high overall response rate and durable responses in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): updated results from a phase 1/2. Trial. Blood. 2019;134(Supplement_1):500–500.
- Calquence (acalabrutinib) capsules, for oral use [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2019.
- Brukinsa (zanubrutinib) capsules, for oral use [prescribing information]. San Mateo, CA: BeiGene USA, Inc.; 2019.
- Byrd JC, Hillmen P, Ghia P, et al. First results of a head-to-head trial of acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2021;39(15_suppl):7500–7500.
- Hillmen P, Eichhorst B, Brown JR, et al. First interim analysis of ALPINE study: Results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Presented at EHA 2021 Virtual; June 9–17, 2021.