The non-germinal center B-cell (GCB) molecular subtype of diffuse large B-cell lymphoma (DLBCL) is associated with chronic activation of B-cell receptor (BCR) signaling [Citation1], has poorer prognosis than GCB subtype DLBCL [Citation2], and treatment with Bruton tyrosine kinase (BTK) and phosphoinositide-3 kinase (PI3K) inhibitors has resulted in greater anti-tumor activity than in GCB DLBCL [Citation3,Citation4]. Acalabrutinib is a selective, potent, covalent inhibitor of BTK with minimal off-target activity and ACP-319 is a second-generation PI3Kδ inhibitor [Citation5–7]. ACP-319 tolerability as monotherapy was previously evaluated in a small clinical trial of 28 patients with relapsed or refractory lymphoid malignancies; hemolytic anemia in 1 patient was the only dose-limiting toxicity and the drug also demonstrated dose-dependent antitumor activity [Citation7]. Preclinical evaluations in BTK and/or PI3K knockout mice [Citation8] and of DLBCL cell lines [Citation9] have suggested synergistic effects of BTK and PI3K signaling inhibition.
In this 2-part study, we evaluated the safety, efficacy, pharmacokinetics (PK), and pharmacodynamics (PD) of acalabrutinib in combination with ACP-319 in patients with R/R B-cell NHL.
This was a phase 1/2 trial (NCT02328014) consisting of a dose-escalation portion (part 1) and a dose-expansion portion (part 2) enrolling adults with R/R B-cell NHL to be administered 1 of 3 dose levels. Full inclusion/exclusion criteria can be found in Supplemental Table 1. Patients received acalabrutinib 100 mg orally twice daily (BID) in combination with either ACP-319 25 mg (cohort 1), 50 mg (cohort 2), or 100 mg (cohort 3) orally BID until disease progression or unacceptable toxicity. The maximum tolerated dose (MTD) was determined using a 3 + 3 dose-escalation design. Based on the MTD of ACP-319 determined in part 1 (50 mg BID), patients in part 2 received acalabrutinib 100 mg BID and ACP-319 50 mg BID, 1 group with non-GCB DLBCL and 1 group with GCB DLBCL.
The primary objective of the study was to characterize safety and identify the MTD. Secondary objectives included overall response rate (ORR), PFS and evaluation of pharmacokinetics, phospho-AKT inhibition, and BTK occupancy. ORR was assessed using criteria from the International Workshop on Chronic Lymphocytic Leukemia, the 6th International Workshop on WM, and Lugano criteria [Citation10–12]. Immunohistochemistry for MYC, BCL-2, and BCL-6 expression were performed at the individual sites. Fluorescence in situ hybridization for gene rearrangements was not routinely performed at the time of trial initiation and patient enrollment. Plasma samples were analyzed to determine PK parameters of ACP-319 and acalabrutinib using a validated liquid chromatography/tandem mass spectrometry. BTK occupancy by acalabrutinib was measured in peripheral blood mononuclear cells and bone marrow using a biotin-tagged acalabrutinib analogue probe. All data were summarized using descriptive statistics.
The protocol was reviewed and approved by an Institutional Review Board at each study site. All participants provided written informed consent.
Forty patients were included in the study. Part 1 consisted of 18 patients across 3 dose levels, which were enrolled sequentially with 6 patients in each. Median (range) durations of acalabrutinib and ACP-319 exposure in patients enrolled in part 1 were 8.8 (0.9–63.4) and 1.3 (0.3–38.7) months, respectively. Twenty-five patients with R/R DLBCL were included in the analysis for part 2, including 9 patients with GCB and 16 with non-GCB DLBCL (3 patients with non-GCB DLBCL were from part 1). Demographics and characteristics for all patients by histology are described in Supplemental Table 2. Median (range) durations of acalabrutinib and ACP-319 exposure in patients enrolled in part 2 were 1.7 (0.5–58.0) and 1.4 (0.3–38.7) months, respectively. Twenty of the 40 patients (50%) discontinued acalabrutinib or both drugs simultaneously due to progressive disease (PD). One additional patient stopped acalabrutinib at the end of cycle 1 due to drug-related thrombocytopenia and then 1 month later stopped ACP-319 and left the study due to PD. Nine patients (23%) discontinued acalabrutinib and the study due to adverse events caused by study drug. Thirteen patients (33%) discontinued ACP-319 due to an adverse event. With the exception of the patient mentioned previously, all of these patients discontinued ACP-319 before acalabrutinib. Six patients (15%) discontinued acalabrutinib and the trial only upon termination of the trial by the sponsor. All six patients had discontinued ACP-319 approximately 2 to 5 years before leaving the trial. Of the remaining 4 patients, 1 withdrew consent, 1 had a complete response at the end of cycle 2 and went on to receive a stem cell transplant, 1 developed melanoma and was withdrawn by the investigator, and 1 developed study drug-related erythema and neutropenia along with non–drug-related cellulitis requiring transfer to a rehabilitation facility and discontinuation from the study.
In part 1, the most common grade ≥3 AEs (occurring in >2 patients) included increased alanine aminotransferase (ALT; 33%), increased aspartate aminotransferase (AST; 28%), diarrhea (22%), and neutropenia (17%) () [Citation13]. SAEs were reported in 61% of patients; 50% experienced grade 3/4 SAEs. AEs led to drug discontinuation in 7 patients. Dose-limiting toxicities were reported in 1 patient receiving ACP-319 50 mg (grade 3 maculopapular rash) and 2 patients receiving 100 mg (grade 3 febrile neutropenia, diarrhea, and pneumonitis, n = 1; grade 3 maculopapular rash, n = 1); therefore, 50 mg BID was the MTD established for ACP-319 and the recommended dose for part 2 in combination with acalabrutinib 100 mg BID.
Table 1. Patients with adverse events from part 1 (patients with R/R B-cell NHL receiving acalabrutinib 100 mg BID and 25, 50, or 100 mg ACP-319 BID) and part 2 (patients with GCB DLBCL or non-GCB DLBCL receiving acalabrutinib 100 mg BID and 50 mg ACP-319 BID).
In part 2, the most common grade ≥3 AEs (in >2 patients) were increased ALT (28%), increased AST (20%), anemia (16%), diarrhea (12%), thrombocytopenia (12%), and rash (12%) (). SAEs were reported in 52% of patients; 40% experienced at least 1 grade 3/4 SAE. AEs led to drug discontinuation in 8 of 25 patients (32%). Reversible hepatotoxicity, presumably immune-related, consisting of grade 3/4 elevations in liver enzymes occurred in 7 patients and was treated with corticosteroids and dose delays. Biopsies were not mandated for evaluation of hepatotoxicity and were not performed. Management of these events is described in Supplemental Table 3.
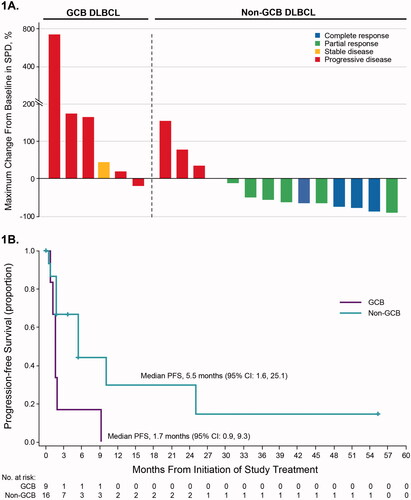
Per investigator assessment, the ORR was 100% (n = 8/8; CR rate 13%) for patients with CLL/SLL. Two of 3 patients with MCL had CRs. The single patient with WM had a PR, and no patients with FL had a response. In patients with non-GCB DLBCL, the ORR was 63% (n = 10/16; CR rate 25%) with a median duration of response of 8.2 months. Of these 10 responders, 6 were double expressors, overexpressing MYC and BCL-2/BCL-6. No responses were observed in the 9 patients with GCB DLBCL (). Median PFS was 5.5 months (95% CI: 1.6, 25.1) in patients with non-GCB DLBCL and 1.7 months (95% CI: 0.9, 9.3) in patients with GCB DLBCL ().
Figure 1. Efficacy outcomes in patients with non-GCB or GCB subtype DLBCL (part 2). (A) Responses and percentage change in tumor burden in individual patients (n = 20). Data are not shown for 3 patients with GCB subtype DLBCL and 2 patients with non-GCB DLBCL, who were not on study long enough to complete response assessment and were thus not evaluable. These patients were treated for ≤42 days. (B) Progression-free survival. Abbreviations: CR: complete response; DLBCL: diffuse large B-cell lymphoma; GCB: germinal center B-cell; PD: progressive disease; PFS: progression-free survival; PR: partial response; SD: stable disease; SPD: sum of product diameters.
In part 1 of the current study, ACP-319 exposure at day 8 (steady-state) increased dose proportionally in a relatively linear manner, similar to PI3Kδ-targeting inhibitors copanlisib and umbralisib [Citation14,Citation15]. Conversely, duvelisib and idelalisib exposures increase in a less than dose-proportional manner [Citation16,Citation17]. Acalabrutinib exposure increased minimally with increasing dose of ACP-319 (Supplemental Table 4). Median BTK occupancy was 95% at steady-state trough in both parts 1 and 2, demonstrating that ACP-319 had no effect on BTK occupancy. Phospho-AKT inhibition at day 8 from ACP-319 in part 1 was dose dependent and supported the ACP-319 50 mg BID dose (Supplemental Figure 1). Phospho-AKT has been rapidly inhibited with duvelisib and reduced to activation levels similar to normal B cells with idelalisib, although not always in a dose-dependent manner [Citation16,Citation18].
Single-agent clinical trials using BTK and PI3K inhibitors have demonstrated preferential activity in non-GCB DLBCL, consistent with its dependence on BCR signaling [Citation3,Citation4], and in preclinical studies, dual inhibition demonstrated additive cytotoxicity, which could overcome single-agent resistance [Citation19–21]. Our results demonstrating clinical activity in non-GCB DLBCL are consistent with these data. While the 63% ORR and 25% CR rate appear improved compared with single-agent results [Citation3], the median response duration was modest at 8 months. However, similar to other PI3K inhibitor trials, and of particular potential concern when combined with other agents that target the BCR [Citation22], we observed grade 3/4 transaminase elevations (33% of patients in this report) with less frequent events of rash, diarrhea, and pneumonitis. A shorter duration of therapy with ACP-319 than with acalabrutinib is likely due to the greater toxicity leading to discontinuation. It is notable that combining umbralisib, a possibly more selective PI3Kδ inhibitor than ACP-319, with ibrutinib did not lead to the same degree of toxicity [Citation23].
Overall, the combination of acalabrutinib 100 mg BID and ACP-319 50 mg BID led to a CR in 25% of non-GCB DLBCL patients, although frequent treatment-limiting hepatotoxicity was observed. Considering evolving research on molecular subtyping of DLBCL that has identified distinct pathogenic mechanisms and outcomes [Citation24], future research should focus on targeting these subtypes of DLBCL with appropriate therapy. While further development of ACP-319 is not planned, these results support ongoing studies of acalabrutinib-based therapy in non-GCB DLBCL.
GLAL-2021-1043-File003.pdf
Download PDF (150.1 KB)Disclosure statement
P.M.B. has received consulting fees from AbbVie/Pharmacyclics, AstraZeneca, BeiGene, Seattle Genetics, Janssen, Genentech, Celgene/BMS, TG Therapeutics, Bayer, and Adaptive; and has participated on a Data Safety Monitoring Board or Advisory Board for TG Therapeutics.
S.D.S. has received grants or contracts from Acerta Pharma BV, AstraZeneca, Bayer, BeiGene, De Novo Biopharma, Genentech, Incyte, Merck Sharp and Dohme, and Portola; and has received consulting fees from AstraZeneca, Millennium/Takeda, BeiGene, Karyopharm, Kite, Incyte, and ADC Therapeutics. His spouse has received grants or contracts from Ayala, Bristol Myers Squibb, and Ignyta.
M.R. declares no competing financial interests.
S.M.O. has been a consultant for Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose, Vaniam, AbbVie, Alexion, Verastem, Juno, Vida Ventures, Autolus, Johnson & Johnson, Merck, Bristol Myers Squibb, and NOVA Research; has received research support from Kite, Regeneron, Acerta, and Caribou; and has been a consultant for or received research support from Gilead, Pharmacyclics, TG Therapeutics, Pfizer, and Sunesis.
J.P.S. has received consulting fees from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, Gilead, Lilly, Pharmacyclics, and TG Therapeutics; has participated on a Data Safety Monitoring Board or Advisory Board for Centessa; and holds stock or stock options in Centessa.
J.M.M. has been a speaker for AstraZeneca, Janssen, and TG Therapeutics.
PP was an employee of Acerta Pharma/AstraZeneca during the time the research was conducted and stock ownership in Acerta Pharma/AstraZeneca.
R.C. and H.Y. are employees of AstraZeneca and have stock or stock options in AstraZeneca.
S.E.S. has received grants or contracts from Acerta, AstraZeneca, BeiGene, Bristol Myers Squibb, Genentech, Gilead, Ionis, Janssen, and Velos Bio; has received consulting fees from Velos Bio, Karyopharm, Genentech, Janssen, and Pharmacyclics; and has participated on a Data Safety Monitoring Board for the Fred Hutchinson Cancer Research Center.
Data-sharing statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92.
- Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947.
- Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926.
- Lenz G, Hawkes E, Verhoef G, et al. Single-agent activity of phosphatidylinositol 3-kinase inhibition with copanlisib in patients with molecularly defined relapsed or refractory diffuse large B-cell lymphoma. Leukemia. 2020;34(8):2184–2197.
- Calquence [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2019.
- Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363(2):240–252.
- Lanasa MC, Glenn M, Mato AR, et al. First-in-human study of AMG 319, a highly selective, small molecule inhibitor of PI3Kδ, in adult patients with relapsed or refractory lymphoid malignancies [abstract. Blood. 2013;122(21):678–678. ].
- Suzuki H, Matsuda S, Terauchi Y, et al. PI3K and btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat Immunol. 2003;4(3):280–286.
- Mathews Griner LA, Guha R, Shinn P, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A. 2014;111(6):2349–2354.
- Owen RG, Kyle RA, Stone MJ, et al. Response assessment in Waldenström macroglobulinaemia: update from the VIth international workshop. Br J Haematol. 2013;160(2):171–176.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the national cancer Institute-Working group 1996 guidelines. Blood. 2008;111(12):5446–5456.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32(27):3059–3068.
- Hsu C, Marshall JL, He AR. Workup and management of immune-mediated hepatobiliary pancreatic toxicities that develop during immune checkpoint inhibitor treatment. Oncologist. 2020;25(2):105–111.
- Patnaik A, Appleman LJ, Tolcher AW, et al. First-in-human phase I study of copanlisib (Bay 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin's lymphomas. Ann Oncol. 2016;27(10):1928–1940.
- Ukoniq [package insert]. Edison, NJ: TG Therapeutics; 2021.
- Flinn IW, O'Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood. 2018;131(8):877–887.
- Ramanathan S, Jin F, Sharma S, et al. Clinical pharmacokinetic and pharmacodynamic profile of idelalisib. Clin Pharmacokinet. 2016;55(1):33–45.
- Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397.
- Spriano F, Tarantelli C, Gaudio E, et al. Single and combined BTK and PI3Kδ inhibition with acalabrutinib and ACP-319 in pre-clinical models of aggressive lymphomas. Br J Haematol. 2019;187(5):595–601.
- Yahiaoui A, Meadows SA, Sorensen RA, et al. PI3Kδ inhibitor idelalisib in combination with BTK inhibitor ONO/GS-4059 in diffuse large B cell lymphoma with acquired resistance to PI3Kδ and BTK inhibitors. PLoS One. 2017;12(2):e0171221.
- Jain N, Singh S, Laliotis G, et al. Targeting phosphatidylinositol 3 kinase-β and -δ for bruton tyrosine kinase resistance in diffuse large B-cell lymphoma. Blood Adv. 2020;4(18):4382–4392.
- Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127(20):2411–2415.
- Davids MS, Kim HT, Nicotra A, et al. Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1-1b study. Lancet Haematol. 2019;6(1):e38–e47.
- Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24(5):679–690.
