New treatments for Mantle Cell Lymphoma (MCL) have improved outcomes but a majority of patients will have disease relapse after initial therapy and ultimately die from their disease. To date, some of the most effective agents approved in the relapsed and refractory (R/R) setting include the Bruton’s Tyrosine Kinase (BTK) inhibitors – ibrutinib, acalabrutinib, and zanubrutinib. Treatment with BTK inhibitors has resulted in high response rates, good tolerability, and durable responses in MCL [Citation1–4] and they are considered standard of care in the R/R setting. However, in up to a third of patients, the disease will fail to respond to single agent BTK inhibitor treatment and ultimately develop resistance [Citation1,Citation2]. Therefore, combination approaches are being developed to enhance the efficacy of BTK inhibitors.
BTK inhibitors have been studied in combination with CD20 monoclonal antibodies, immunomodulatory drugs, BCL-2 inhibitors, CDK 4/6 inhibitors, and chemo-immunotherapy [Citation5–8]. A phase 2 study of ibrutinib and rituximab has reported favorable efficacy and tolerability and NCCN guidelines include ibrutinib plus rituximab (IR) as one of the preferred regimens for second-line and subsequent therapy in MCL [Citation7]. Additionally, maintenance rituximab after frontline chemotherapy and transplant has become the standard of care [Citation9,Citation10]. Obinutuzumab (GA-101) is a type II antibody with enhanced direct cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) that has shown potent activity across a range of B-cell non-Hodgkin lymphoma (NHL) including CLL and follicular lymphoma [Citation11,Citation12]. The use of obinutuzumab as a single agent in R/R MCL, resulted in an overall response rate (ORR) of 27%. Sixty three percent of trial participants were considered refractory to rituximab [Citation13]. Preclinical data also suggest that ibrutinib may inhibit ADCC of rituximab in MCL cell lines but this effect is not seen with obinutuzumab [Citation14]. Given the impressive activity and tolerability of obinutuzumab and preclinical data to suggest enhanced anti-tumor activity compared to rituximab when combined with ibrutinib, we conducted a Phase 2 study of ibrutinib plus obinutuzumab in R/R MCL.
This single-center investigator-sponsored phase 2 trial (NCT02736617) enrolled patients with R/R MCL from July 2016 to September 2019 at Oregon Health & Science University. The institutional review board approved this study and patients provided informed consent. The study complied with the Declaration of Helskini and the International Conference on Harmonization Guideline for Good Clinical Practice. Key inclusion criteria included: age ≥ 18, histologically confirmed diagnosis of R/R MCL, no active infections or uncontrolled intercurrent illness, no prior history of bleeding, and adequate marrow and organ function. Adequate marrow and organ function was determined based on lab parameters: absolute neutrophil count ≥ 1.0 109/L, platelets ≥ 50 109/L, total bilirubin ≤ 2.5 × institutional limit, AST/ALT ≤ 2.5 × institutional upper limit of normal, and creatinine ≤ 2. The presence of CNS disease was allowed and anticoagulation was permitted but warfarin use was excluded. Prior BTK inhibitor was not allowed; however, subjects were eligible if they had been initiated on ibrutinib less than 14 days prior to enrollment. Patients received ibrutinib 560 mg orally once daily starting on day 1 and obinutuzumab 1000 mg on days 1, 8, and 15 for cycle 1 and day 1 for subsequent cycles. A total of 6 cycles were given for induction, with each cycle lasting 28 days. Treatment was continued until disease progression or unacceptable AEs. Patients obtaining at least partial response (PR) received obinutuzumab maintenance of 1000 mg every 2 months for up to 2 years (12 doses). The primary endpoint of the study was best ORR per Lugano Criteria [Citation15]. Secondary objectives included progression free survival (PFS) and incidence of toxicity defined as any adverse event grade 3 and higher per CTCAE v. 4.0. Subjects who received both study medications and had one response assessment were evaluable for response.
This trial was conducted according to Simon’s two-stage optimal design. Stage I enrollment included 6 patients and if 4 or more responses (CR/PR) were seen, the trial would continue to Stage II which could enroll up to 14 more patients. Safety stopping rules utilizing continuous toxicity monitoring and sequential boundaries were included. The boundary is equivalent to testing the null hypothesis that, after each patient, the event rate is significantly higher than 20% using one-sided 10% significance level [Citation16]. Baseline characteristics were described using median (minimum, maximum) for continuous variables and counts and frequencies for categorical variables. Primary and secondary endpoints were reported for efficacy evaluable population using a point and interval estimate (95% confidence interval) of the overall response (CR/PR). Kaplan–Meier method was used to estimate PFS rate and median PFS. OR was estimated using the exact binomial method. Duration of response (DOR), from first CR/PR until progression or last tumor assessment, was estimated using the Kaplan–Meier method. Estimates for median follow up used descriptive statistics as Kaplan–Meier method was not estimable (no events).
The study met the stage 1 endpoint and proceeded to stage 2. However, given logistical challenges, namely an interruption of clinical research operations and decreased accrual due to the COVID pandemic, the decision was made to stop the study early. Here, we report the final results of 10 patients enrolled on this study; 90% were men and median age was 72 (range, 55–81). The median Ki67 was 65% (range, 10–90) and median MIPI score was 6.5 (range 4–8). The median time from diagnosis to enrollment was 21.5 months (). All 10 participants who received at least one dose of either study drug and were included in the safety analysis. Nine participants who received at least one dose of both study drugs and had at least one response assessment were included in the efficacy analysis. Of the 10 patients, 6 completed 6 cycles of induction. One participant who withdrew consent 6 days after starting study therapy was included in the safety analysis only. One participant received 2 cycles of obinutuzumab and only 1 cycle of ibrutinib and was included in the safety and efficacy data set.
Table 1. Baseline demographic and clinical-disease characteristics.
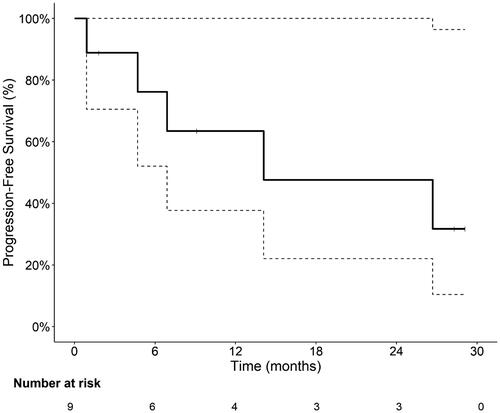
Median study follow up was 14 months (range 1–54 months). No participants died during the study, meaning overall survival was not estimable. In the efficacy evaluable population (n = 9), five patients (55.6%) achieved a CR and three patients (33.3%) a PR leading to an ORR rate of 89% (CI 52–100%). Six of eight patients (67%) continued on to maintenance therapy for a median of 11 doses (range 1–12) received. Median PFS was 14 months (6.9 month to not reached (NR)) (). PFS rate was 76% (CI 52–100%) at 6 months, 63% (CI 38–100%) at 12 months and 48% (CI 22–100%) at 24 months. Notably, one subject with multiply relapsed disease and CNS involvement had a CR lasting > 2 years.
Treatment was well-tolerated. Incidence of toxicity was reported as grade 3 or higher adverse events in the safety population. () There were a total of 18 events occurring in 13 patients. The most common AE included neutropenia and thrombocytopenia. Other events included generalized muscle weakness, infection, infusion related reaction, pain in extremity, somnolence and stroke. These side-effects were comparable to those seen with obinutuzumab monotherapy in NHL and IR in MCL [Citation7,Citation13].
To our knowledge, this is the first report on the combination of ibrutinib and obinutuzumab in R/R MCL. We demonstrate high treatment efficacy in a cohort of predominantly high-risk MCL patients. Although this study was limited by a small sample size, these results are consistent with previous reports using IR reporting an ORR of 88% and 44% CR rate in 50 patients [Citation7]. Median event-free survival was 16 months and median duration of response was 46 months. This included maintenance therapy with rituximab for 2 years. Notably, however, the population from the study using IR was relatively low risk. The proportion of patients with high-risk MIPI score was 12% [Citation7].
Our study reveals similar efficacy with the combination of obinutuzumab and ibrutinib with an ORR of 89%. Our study also included a higher risk population with a median Ki67 of 65%, median time since prior therapy < 2 years, and median MIPI score of 6.5 with 70% of patients having a high MIPI score. Of particular interest is the durable CR (27 months) seen in a multiply relapsed patient (4 prior therapies) with parenchymal and leptomeningeal CNS disease prior to study enrollment suggesting this may be of particular interest in future studies.
The combination of ibrutinib and obinutuzumab is an active regimen in a high-risk relapsed mantle cell lymphoma population. Further evaluation of combinations incorporating obinutuzumab and BTK inhibitors, both those currently available and under development, remains an area of interest.
Author contributions
M. S. Kim: Writing-original draft, review, and editing. T. Banerjee: Writing-original draft, review, and editing. A. I. Chen: Enrollment, final review & editing. A. V. Danilov Enrollment, final review & editing. R. MacKinnon: Study Conduct. B. Thurlow: Project administration. S. Thakurta: Project administration. K. Orand: Project administration. C. Degnin: Study design, statistical support, writing original draft, review, and editing. B. Park: Study Design, statistical support. S. E. Spurgeon: Conceptualization, funding acquisition, protocol writing, project administration, supervision, writing – original draft, review & editing.
Disclosure statement
S. E. S: Research Funding: Genentech, Janssen, Pharmacyclics, Bristol Meyers/Celgene, Merck, Gilead Sciences, Acerta Pharma. Consultancy: Flamingo Therapeutics. Advisory Board: Astra Zeneca.
A. V. D. received consulting fees from Abbvie, AstraZeneca, BeiGene, Genentech, Pharmacyclics and TG Therapeutics and has ongoing research funding from AstraZeneca, Bayer Oncology, Bristol Meyers Squibb, Genentech, MEI Pharma, SecuraBio, TG Therapeutics and Takeda Oncology.
Additional information
Funding
References
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126(6):739–745.
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516.
- Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659–667.
- Song Y, Zhou K, Zou D, et al. Treatment of patients with relapsed or refractory Mantle-Cell Lymphoma with Zanubrutinib, a Selective Inhibitor of Bruton's Tyrosine Kinase. Clin Cancer Res. 2020;26(16):4216–4224.
- Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of Mantle-Cell lymphoma. N Engl J Med. 2018;378(13):1211–1223.
- Jain P, Zhao S, Lee HJ, et al. Ibrutinib with rituximab in First-Line treatment of older patients with mantle cell lymphoma. J Clin Oncol. 2022;40(2):202–212. JCO.21.01797.
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):48–56.
- Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol. 2018;5(3):e109–e116.
- Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in Mantle-Cell lymphoma. N Engl J Med. 2017;377(13):1250–1260.
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520–531.
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110.
- Marcus R, Davies A, Ando K, et al. Obinutuzumab for the First-Line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331–1344.
- Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2912–2919.
- Albertsson-Lindblad A, Freiburghaus C, Jerkeman M, et al. Ibrutinib inhibits antibody dependent cellular cytotoxicity induced by rituximab or obinutuzumab in MCL cell lines, not overcome by addition of lenalidomide. Exp Hematol Oncol. 2019;8:16.
- Cheson BD, Fisher RI, Barrington SF, et al.; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of hodgkin and Non-Hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32(27):3059–3067.
- Ivanova A, Qaqish BF, Schell MJ. Continuous toxicity monitoring in phase II trials in oncology. Biometrics. 2005;61(2):540–545.