Abstract
Identifying risk factors for intensive care unit (ICU) admission in acute leukemia (AL) patients may guide decision-making and improve prognosis. We included all adult AL patients receiving high-intensive chemotherapy in Denmark from 2005 to 2016. We examined risk factors [crude and adjusted (a) relative risks (RRs) with 95% confidence intervals (CI)] and calculated RRs of death after 1-, 3-, and 5-years in ICU-admitted patients compared with matched cohorts. In 1417 AML and 306 ALL patients, the 1-year risk of ICU admission was 28.1% for AML and 26.4% for ALL patients, with the majority related to the first course of chemotherapy. Performance status >1 was associated with increased risk. The 1-year mortality was higher in ICU-admitted patients (AML: 69.7 vs. 35.0% [aRR 2.74;CI = 2.17–3.47]; ALL 65.0 vs. 20.0% [aRR 3.04;CI = 1.54–6.02]). The excess mortality decreased with time. In this study, performance status was associated with increased risk of ICU admission and identifies high-risk patients. ICU admission was associated with high mortality, especially within the first year.
Introduction
Acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) are aggressive blood cancers affecting all age groups. AML constitutes the majority of adult cases of acute leukemia (AL) whereas ALL is most common in children. AML and ALL differ in terms of treatment and prognosis, but high-intensity long-term chemotherapy is required to obtain complete remission and cure in AML as well as ALL patients [Citation1]. In past decades, treatment of AL has evolved, with intensified treatment regimens resulting in higher survival rates [Citation2–5]. Although generally well tolerated, high-intensity regimens may increase the risk of severe complications. Both high-intensity chemotherapy and allogeneic hematopoietic stem cell transplantation (HSCT) may result in life-threatening complications requiring supportive care in intensive care units (ICU).
The current literature on risk factors for ICU admission in AL patients is sparse [Citation6–8]. Few studies have found comorbidities to be associated with a greater risk of ICU admission in patients with AL and other hematological diseases [Citation6,Citation9], while others reported no such association [Citation7]. A recent study using the Danish Acute Leukemia Registry (DNLR) found that comorbidities had only a small impact on survival in AML patients compared with WHO performance status (PS) in patients selected for high-intensity chemotherapy. However, this study did not examine the risk of ICU admission [Citation10,Citation11], and associations between comorbidities, performance status, and risk of ICU admission need to be further explored. Identification of risk factors for ICU admission may help improve outcomes through increased early monitoring and admission of high-risk AL patients and may help guide discussion with patients and relatives about expected course and prognosis.
Complications requiring ICU admission are associated with high in-hospital and 1-year mortality in AL patients and may lead to treatment delays or dose de-escalation, which may in turn affect long-term prognosis through impaired disease control [Citation9,Citation12–15]. Existing studies were mostly performed in the last century and do not reflect current treatment standards with regard to high-intensity chemotherapy regimens and ICU care [Citation16,Citation17]. A recent Austrian single-center study of patients with AML found a 6-year mortality rate of 80% for ICU patients and 72% for non-ICU-admitted patients [Citation7]. A better understanding of the current mortality after ICU admission in AL patients compared to AL patients without the need for ICU admissions is necessary to provide informed and optimized management of complications.
In this nationwide study, we examined potential patient-, disease-, and treatment-related risk factors for ICU admission in AML and ALL patients. We compared the mortality of all Danish AL patients treated with high-intensity remission-induction chemotherapy with and without ICU admission. Finally, we examined the long-term prognosis in the subgroup of patients receiving dialysis or mechanical ventilation (MV).
Methods
Settings
The study was performed using Danish national registries. The Danish population consists of ∼5.8 million people, with equal access to a universal health care system [Citation18]. Cancer treatment and intensive care are exclusively provided by a public government-supported health care system. All hospital visits and treatments are registered using the civil registration (CPR) number, assigned to all residents at birth or immigration [Citation19]. The CPR number allows individual-level linkage of information between the different data sources.
Data sources
The DNLR was established by the Danish Society of Hematology and contains detailed information on demographics, disease-related, and treatment-related factors in >99% of AL patients (AML since 2000, ALL since 2005). The positive predictive values exceed 95% for the most important variables [Citation20,Citation21]. The Danish Intensive Care Database (DID) includes data on virtually all ICU admissions in Denmark since 2005. The data includes among others information on organ support therapies and the severity of illness [Citation22]. The Civil Registration System (CRS) tracks interaction with the health and social services of all legal Danish residents [Citation19,Citation23]. The Danish National Registry of Patients (DNRP) includes diagnosis and procedure data for all hospital admissions and visits [Citation24].
Population
We included all adult Danish patients (15 years or older) registered in DNLR with an AML or ALL diagnosis who were diagnosed and initiated remission-induction chemotherapy between 2005 and 2016 allowing for sufficient follow up-time for treatment and AL-related outcomes (Flowchart, Supplementary Appendix Figure A1).
ICU-admitted patients were defined as patients with ICU admissions between 10 days before and three years after the date of AL diagnosis, assuming that the reason for ICU admission likely was related to leukemia in this time period. For the mortality analysis, we compared AL patients with and without ICU admission.
Risk factors
Detailed baseline patient and disease characteristics, as well as treatment information, were obtained from the DNLR (). Treatment information included time and type of chemotherapy and receipt of HSCT. The number of comorbidities was acquired using diagnoses from hospital admissions, outpatient clinic visits, and emergency room visits as registered in the DNRP up to 10 years before AL diagnosis. ICU admission dates and information on ICU treatments (dialysis and mechanical ventilation [MV]) were obtained from the DID. Based on existing literature, we included the following potential risk factors: age, sex, comorbidity, PS, cytogenetic risk group (MRC) [Citation25], type of AML [de novo, secondary or therapy-related AML (s-AML/t-AML)], ALL phenotype, and Philadelphia chromosome status (for ALL) as potential risk factors for ICU admission.
Table 1. Characteristics of 1417 AML and 306 ALL patients overall and by ICU admission in the matched cohort.
ICU admission and mortality
Primary outcomes were ICU admission and overall mortality. Patients were followed until death or end-of-follow-up (20 August 2018). ICU admitted patients were defined as patients with ICU admissions between 10 days before and three years after the date of AL diagnosis, assuming that the reason for ICU admission likely was related to leukemia in this time period. For the mortality analysis, we compared AL patients with and without ICU admission. Additionally, we compared mortality after ICU admission with a specific type of organ support (dialysis and MV).
Statistical analysis
Descriptive analyses
We described patient and disease characteristics for AML and ALL patients overall and stratified by ICU admission. Also, we described the distribution of ICU admission in relation to AL diagnosis, chemotherapy, complete remission (CR), and relapse rates as well as HSCT performance. The 3-year risk of ICU admission was calculated from the time of diagnosis accounting for competing risk by death. To focus on the risk of ICU admission related to induction and consolidation chemotherapy, we repeated the analyses restricted to 6 months from diagnosis. For patients with ICU admission 1–10 days before AL diagnosis, the ICU admission was considered related to the AL and the admission date was defined as the date of AL diagnosis. Only the first ICU admission after AL diagnosis was used in the analyses.
For AML patients, the cytogenetic risk group was missing for 10% and the white blood cell (WBC) count was missing for 2%. With the assumption of random missingness, we used multiple imputations using chained equations (MICE) with 10 imputations to impute all missing values with the predicted mean matching (pmm) algorithm based on the following variables: sex, age, PS, type of AL, platelets, WBC, blast counts in blood and bone marrow, LDH, and cytogenetics [Citation26,Citation27].
Risk of ICU admission
We examined potential predictors for ICU admission using a pseudo-value approach to compare the relative risk of ICU admission (RR) 1 and 3 years after AL diagnosis [Citation28,Citation29]. For both cohorts; age, sex, comorbidities, and PS were examined as risk factors. Age was categorized in the following intervals: 15–40, 41–50, 51–60, and >60 years. For AML, we additionally examined the effects of cytogenetic risk group, and type of AML (s-AML/t-AML). For ALL, ALL phenotype, and the effect of Philadelphia chromosome status were examined.
Prognosis after ICU admission
ICU admitted patients were matched 1:1 to patients alive and not ICU admitted on the date of ICU admission of the matched case. Patients were matched on age (5-year intervals), sex, and time since diagnosis using risk-set sampling. We computed 1-, 3-, and 5-year mortality after the index date. To examine the mortality adjusted for disease-related factors not accounted for in the matching, we used a pseudo-value approach to compute RRs for mortality for ICU-admitted patients and matched comparison cohort patients adjusted for potential confounders in AML (PS, year of diagnosis, MRC cytogenetic risk group, and s-AML/t-AML) in ALL (PS, ALL phenotype, secondary ALL, and Philadelphia chromosome status) [Citation28–30]. We computed RR for 0–30 days after ICU, 31 days −1 year after ICU, and 1–3 years after ICU. Additionally, we examined the prognosis after the use of dialysis and MV. We created matched comparison groups for dialysis and MV patients, respectively, as described in the previous matching. Patients were matched 1:3 due to the smaller number of cases. Again, we estimated 1-, 3-, and 5-year mortality rates after dialysis and MV and estimated adjusted RR of death after 1, 3, and 5 years.
For all analyses, we present crude and adjusted results for AML and ALL patients separately with 95% confidence intervals (CI). All analysis was performed using STATA version 16 (StataCorp LLC, College Station, TX, USA). The study was registered at the Danish Data Protection Agency (Central Denmark record no. 1-16-02-321-18).
Results
Patient characteristics
The final study population included 1417 AML [median age 61 (Interquartile range (IQR) 50–68)] and 306 ALL patients [median age 43 (IQR 25–61)]. Of these, 384 (27.9%) AML and 80 (26.1%) ALL patients were admitted to an ICU within 3 years of diagnosis. Patient characteristics and events during follow-up overall and for the ICU and matched cohort are presented in . Overall, 68.5% of AML and 72.2% of ALL patients achieved a CR. Relapse during follow-up occurred in 29.8% of AML and 12.7% of ALL patients. The total follow-up time was 5559 person-years [median: AML 1.63 (IQR: 0.60–4.36) years; ALL 3.19 (IQR: 1.11–7.26) years]. During follow-up, 960 AML (69.7%) and 144 ALL (47.1%) patients died. Multiple ICU admissions occurred for 8.5% (n = 121) and 10.1% (n = 31) of AML and ALL patients, respectively. Of ICU admitted patients, 19.7% of AML patients and 31.2% of ALL patients were admitted more than once. Overall, the median time to first ICU admission for all AML patients was 64 days (IQR: 15–269). For ALL patients the median time to first ICU was 42 days (IQR: 20–403). The median time for ICU admission for patients during the primary treatment for AML patients was 28 days (IQR: 9–101). Few AML patients had a first ICU admission that occurred following relapse [n = 32, 8.3%, median time to ICU 433 days (IQR: 326–597) or following HSCT treatment (n = 58, 14.6%) median time to ICU was 380 days (IQR: 272–511)]. Very few ALL patients had a first-time ICU admission following relapse (N = 0, 0%) or HSCT (N = 12, 15%).
Risk of ICU admission
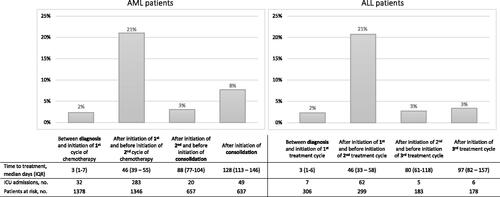
The cumulative risk of ICU admission for AML patients was 22.7% after 1 year and 28.1% after 3 years. The corresponding numbers for ALL were 22.7 and 26.4%. Hence, the majority of ICU admissions occurred within the first year after diagnosis. The highest risk of ICU admission was within the first months after diagnosis and especially during or following the first cycle of remission-induction chemotherapy ().
Figure 1. Prevalence of first time ICU admissions by treatment course for AML and ALL patients. Timing of first time ICU admission according to diagnosis and initiation of treatment cycle 1, 2, and 3 or consolidation for AML and ALL patients.
PS > 1 was associated with an increased risk of ICU admission in both AML [aRR: 1.50 (CI = 1.19–1.90)] and ALL patients [aRR: 1.62 (CI = 0.88–2.99)]. No association was found between the number of comorbidities and ICU risk. These associations are illustrated in Kaplan Meier curves of the ICU admission proportion by time according to PS and number of comorbidities, respectively (). ALL patients between 41 and 50 years of age were more likely to have an ICU admission within 3 years after diagnosis compared to patients <40 years (). Similar effects were seen for ICU admission within 6 months of diagnosis (Supplementary Appendix Table A2).
Figure 2. Kaplan Meier curves of ICU admission risk and survival. Proportion of patients with ICU admission by time according to WHO PS for AML (A) and ALL (B) and according to number of comorbidities for AML (C) and ALL (D). Crude survival from index date by ICU status for AML (E) and ALL patients (F).
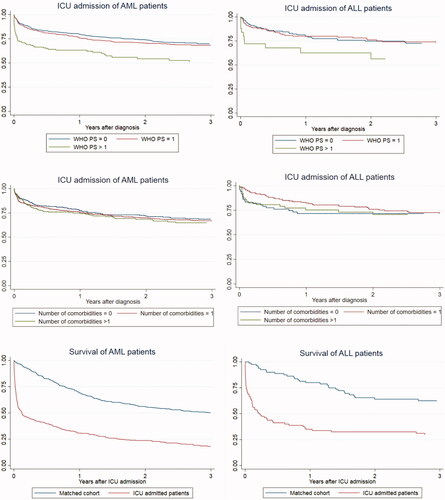
Table 2. Potential risk factors for ICU admission in 1417 AML and 306 ALL patients.
Prognosis after ICU admission
Patients with an ICU admission had increased mortality, especially within the first year (AML, aRR: 2.74; 95% CI = 2.17–3.47; ALL, aRR: 3.04; 95% CI = 1.54–6.02) compared to the matched cohorts of non-ICU AL patients. Over time, the excess mortality declined but was still higher 5 years after ICU admission (AML, aRR: 1.80; 95% CI = 1.42–2.30; ALL, aRR: 2.09; 95% CI = 1.19–3.67). For patients surviving the first year after an ICU admission, the mortality was comparable to the matched cohorts (1–5 years: AML: aRR 0.98; 95% CI = 0.76–1.26) (). Crude survival curves from index date by ICU status is presented in .
Table 3. Mortality after ICU admission in AML and ALL patients.
Risk of ICU admission and prognosis after use of organ support in the ICU
Among ICU patients, 28% of AML and 17.5% of ALL patients received dialysis during their first ICU admission. Dialysis during ICU admission was associated with high 1-year mortality (89.9% for AML and 71.4% for ALL patients) and 5-year mortality (94.3% for AML and 81.0% for ALL) compared to the matched cohort (5-year mortality; 57.8% for AML and 33.9% for ALL).
Similarly, MV was used in 64.7% of AML and 45.0% of ALL patients during the first ICU admission. Patients receiving MV had high 1-year mortality (84.0% for AML and 87.0% for ALL) and 5-year mortality (91.5% for AML and 87.0% for ALL) compared to the matched cohort matched on age, gender, and time since diagnosis (5-year mortality was 54.9% for AML and 30.5% for ALL). RRs of mortality after dialysis and MV, respectively, are listed in .
Table 4. Mortality after dialysis or mechanical ventilation in the ICU for AML and ALL patients.
Discussion
In this nationwide population-based hospital study, more than one-fourth of patients with AL treated with remission-induction chemotherapy were admitted to an ICU within 3 years after diagnosis. Among potential risk factors measured at diagnosis, PS was strongly associated with an increased risk of ICU admission whereas comorbidity and cytogenetics were not. Finally, ICU admission was associated with increased long-term mortality, in particular in patients requiring dialysis or MV. The excess mortality attenuated, but persisted, during the 5-years after the first ICU admission.
In contrast to our findings, previous studies found comorbidity and cytogenetics to be associated with increased risk of ICU admission [Citation6,Citation7] and excess overall mortality [Citation31] in hematological patients. The difference in findings may be explained by our restriction to patients treated with curative intent, who are likely to have less severe comorbidities than those deemed unfit to receive remission-induction chemotherapy [Citation10]. Differences in ICU bed capacity and admission policies may contribute to differing results [Citation32,Citation33]. Finally, the impact of comorbidity may partly also be captured in the PS score, which was not included in the US study.
Age-adjusted regimens may reduce treatment-related complications and partly explain our finding of no effect of age in AML patients [Citation34]. Among ALL patients, age between 41 and 50 years was associated with an increased risk of ICU admission. This age group included the upper limit for treatment with child/adolescent NOPHO protocols for Philadelphia-negative B-ALL [Citation2]. Hence, these patients are the oldest to receive intensified and toxic regimens with higher complication rates, which may explain our findings. The PS score at the time of diagnosis was associated with an increased risk of later ICU admission for patients with AML and possibly ALL. The overall predictive value of baseline PS has previously been found superior to the comorbidity burden in the Danish AML population [Citation10]. We suggest that PS reflects the severity of AL disease and that it is of greater importance for treatment tolerability and patient outcome than comorbidities in AL patients, once they are determined to be eligible for intensive therapy.
More than 80% of ICU admissions occurred early and during remission-induction chemotherapy. For the minority of patients having a first ICU admission not associated with primary treatment, such as patients undergoing HSCTs in CR1 or rescue treatment to obtain a CR2, the importance of comorbidity or PS at the time of diagnosis is likely of less importance than in patients undergoing up-front treatment. Thus, the results may be less accurate for these groups of patients. Examining ICU admissions within 6 months of AL diagnosis to functionally restrict the cohort to patients undergoing primary induction and consolidation chemotherapy (Supplementary Appendix Table A1) we found similar but slightly stronger associations between PS > 1 and risk of ICU admission, no new association in the sensitivity analysis (Supplementary Appendix Table A2), and no major differences in patient-/disease characteristics.
Similar, the overall prognosis in patients experiencing relapse is poor and the need to keep the pace and intensity of treatment and proceed to HSCT is crucial. Serious and ICU-requiring complications are likely to increase the risk of delayed or reduced-intensity treatment and disease progression. Infections are the main driver of ICU admission regardless of time in the treatment course. However, HSCT-specific complications, such as graft-vs.-host-disease and immunosuppressive therapy may affect the post-ICU mortality in HSCT patients compared to non-HSCT patients.
All patients in the population were treated with curative potential to obtain a CR1 and long-term survival. Overall, we found a lower CR rate in the ICU admitted patients. Whether the inferior response rates can be attributed to changes in AL care (e.g. an enforced treatment pause, dose reductions, or change in treatment plans following ICU admission during induction or consolidation treatment) or due to the nature of the AL cannot be concluded from this study.
We found greatly increased mortality within 30 days and 1 year after ICU admission compared to matched patients not admitted to the ICU. In contrast, a previous study found no difference in long-term mortality in ICU-admitted AML patients compared to non-ICU-admitted patients, when excluding the first 30 days after admission [Citation7]. Our study confirms that the relative risk of death declined with time after ICU admission even when adjusting for potential confounding factors, although the risk remained increased even after 3–5 years.
There may be several reasons for the high mortality in patients treated with dialysis or MV. Risk factors for dialysis-requiring AKI in AL patients include leukostasis, tumor lysis syndrome, infection, and chemotherapy. A Danish study showed that leukemia patients had a 27.5 and 40.0% risk of developing AKI within 1 and 5 years, respectively [Citation35]. Therefore, close monitoring of kidney and lung function in high-risk patients may be a possible strategy to identify life-threatening conditions earlier. Hematological patients with ICU admission due to respiratory failure have better outcomes if the etiology is identified [Citation36] and the time to admission is reduced [Citation37]. The very high mortality in our study stresses the need to optimize the early and improved diagnosis of respiratory failure as suggested in the literature recently [Citation38].
This study provides descriptive, predictive, and prognostic routine clinical care data on critical illness related to ICU admission in AL patients. Our cohort contains complete follow-up data on a large contemporary national cohort from a uniform population-based hospital setting, thereby limiting selection bias. The dataset was created by linkage of high-quality data from national registries, which have been individually validated [Citation20–22,Citation24]. Data were collected independently from the study aims thereby limiting information bias. To avoid the influence of treatment intent (palliative or curative) on ICU admission thresholds and outcomes, we restricted our study to patients eligible for high-intensity chemotherapy. Still, this study has limitations. The study is influenced by ICU triage, as ICU admission was offered to a selected group of patients, who were thought to benefit from and tolerate treatment in an ICU. Hence, ICU admission cannot only be explained by the severity of the critical illness. However, since the study only includes patients, who were chosen to receive high-intensity chemotherapy, the likelihood of withholding from ICU admission in case of critical complications is very small. Furthermore, in Denmark patients are not admitted to the ICU for palliative or end-of-life care, and therefore the increased mortality is unlikely to be at least fully explained by the withholding of ICU treatment options. Health care systems, ICU capacity, and ICU admission policies differ between countries. The United States has 4–6 times the number of ICU beds per capita compared to England and Denmark [Citation39]. This is reflected in ICU admission policies with more elderly and terminal patients for palliative care being admitted to ICUs in the United States compared to the United Kingdom [Citation32,Citation33]. The ICU admission criteria may also differ due to variations in population, pre-ICU treatment possibilities, and financial resources. This may influence the generalizability of our results to other health care systems.
We included several factors potentially associated with the risk of and prognosis after ICU admission but may have missed a few, e.g. patients who had decided to not undergo specific treatments. Furthermore, our cohort of ICU-admitted ALL patients was small (N = 80), which limited the statistical precision of the analyses in this cohort.
Early intervention in high-risk patients has been associated with improved outcomes for patients with hematological malignancies [Citation9,Citation40,Citation41]. Evolving therapies in AL and improvement in intensive care may hamper the use of previous predictors and create a need for new prognostic tools [Citation42], which could optimize prevention strategies and potentially avoid critical complications that could require ICU admission. Based on this study, we suggest PS at diagnosis as a possible factor to identify high-risk patients throughout the treatment course. Since the risk of ICU admission was especially increased after initiation of the first treatment course, risk assessment is relevant and important before initiating treatment. The critical complications leading to ICU admission and excess mortality compared to other AL patients diminished over time, suggesting that an ICU admission earlier in the treatment course in itself should not determine a treatment decision, such as HSCT performance or initiation of relapse treatment in the individual patient.
In conclusion, more than one-fourth of AL patients were admitted to an ICU. Performance status was associated with increased risk of ICU admission and may serve as a tool to identify high-risk patients for early monitoring and management. ICU admission is associated with high mortality in patients with AL, especially increased within the first year after admission and in ICU patients receiving dialysis or MV during the ICU stay.
Ethical approval
According to Danish legislation, no approval from an ethics committee is required for registry-based studies. The study was approved and registered at the Danish Data Protection Agency (Central Denmark record 1-16-02-321-18).
Author contributions
Each of the authors has substantially contributed to conducting the underlying research and drafting of this manuscript. All authors have been involved in conceptualization, visualization, and the writing process. Dr. Cecilie Maeng did the data editing, analyses, and creation of tables/figures under the close supervision of Dr. Lene Østgård, Professor Christian Christiansen, and Professor Kathleen Liu.
Study presentations
Preliminary data was presented at the American Society of Hematology (ASH) conference 2019 in Orlando, Florida.
GLAL-2021-1277-File004.docx
Download MS Word (365.8 KB)Acknowledgments
We wish to thank all the individuals who carefully report the available and relevant clinical data to the Danish National Acute Leukemia Registry and the Danish Intensive Care Database.
Disclosure statement
We have no conflicts of interest to declare.
Additional information
Funding
References
- Swerdlow SC, Harris NL, Jaffe ES, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 24th ed.; 2017.
- Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018;32(3):606–615.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101.
- Othus M, Kantarjian H, Petersdorf S, et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given ‘intense’ induction regimens: a report from SWOG and MD Anderson. Leukemia. 2014;28(2):289–292.
- Halpern AB, Culakova E, Walter RB, et al. Association of risk factors, mortality, and care costs of adults with acute myeloid leukemia with admission to the intensive care unit. JAMA Oncol. 2017;3(3):374–381.
- Schellongowski P, Staudinger T, Kundi M, et al. Prognostic factors for intensive care unit admission, intensive care outcome, and post-intensive care survival in patients with de novo acute myeloid leukemia: a single center experience. Haematologica. 2011;96(2):231–237.
- Keenan T, LeBlanc TW, Traeger L, et al. Outcomes for older patients with acute myeloid leukemia admitted to the intensive care unit. Blood. 2015;126(23):2104–2104.
- Azoulay E, Mokart D, Pene F, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium–a Groupe de Recherche Respiratoire en Reanimation Onco-Hematologique study. J Clin Oncol. 2013;31(22):2810–2818.
- Ostgard LS, Norgaard JM, Sengelov H, et al. Comorbidity and performance status in acute myeloid leukemia patients: a nation-wide population-based cohort study. Leukemia. 2015;29(3):548–555.
- Young J, Badgery-Parker T, Dobbins T, et al. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage. 2015;49(2):258–264.
- Jackson K, Mollee P, Morris K, et al. Outcomes and prognostic factors for patients with acute myeloid leukemia admitted to the intensive care unit. Leuk Lymphoma. 2014;55(1):97–104.
- Pohlen M, Thoennissen NH, Braess J, et al. Patients with acute myeloid leukemia admitted to intensive care units: outcome analysis and risk prediction. PLOS One. 2016;11(8):e0160871.
- Magid T, Haase N, Andersen JS, et al. Intensive care of haematological patients. Dan Med J. 2012;59(3):A4395.
- Kraguljac AP, Croucher D, Christian M, et al. Outcomes and predictors of mortality for patients with acute leukemia admitted to the intensive care unit. Can Respir J. 2016;2016:3027656.
- Yau E, Rohatiner AZ, Lister TA, et al. Long term prognosis and quality of life following intensive care for life-threatening complications of haematological malignancy. Br J Cancer. 1991;64(5):938–942.
- Lloyd-Thomas AR, Dhaliwal HS, Lister TA, et al. Intensive therapy for life-threatening medical complications of haematological malignancy. Intensive Care Med. 1986;12(4):317–324.
- Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591.
- Schmidt M, Pedersen L, Sorensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549.
- Ostgard LS, Norgaard JM, Raaschou-Jensen KK, et al. The Danish national acute leukemia registry. CLEP. 2016;8:553–560.
- Ostgard LS, Norgaard JM, Severinsen MT, et al. Data quality in the Danish national acute leukemia registry: a hematological data resource. Clin Epidemiol. 2013;5:335–344.
- Christiansen CF, Moller MH, Nielsen H, et al. The Danish intensive care database. Clin Epidemiol. 2016;8:525–530.
- Population in Denmark 2019; 2019.
- Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322–2333.
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213.
- Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
- Parner ET, Andersen PK, Overgaard M. Cumulative risk regression in case-cohort studies using pseudo-observations. Lifetime Data Anal. 2020;26(4):639–658.
- Andersen PK, Syriopoulou E, Parner ET. Causal inference in survival analysis using pseudo-observations. Stat Med. 2017;36(17):2669–2681.
- Kjaersgaard MI, Parner ET. Instrumental variable method for time-to-event data using a pseudo-observation approach. Biometrics. 2016;72(2):463–472.
- Hong WJ, Medeiros BC. Unfavorable-risk cytogenetics in acute myeloid leukemia. Expert Rev Hematol. 2011;4(2):173–184.
- Wunsch H, Linde-Zwirble WT, Harrison DA, et al. Use of intensive care services during terminal hospitalizations in England and the United States. Am J Respir Crit Care Med. 2009;180(9):875–880.
- Prin M, Wunsch H. International comparisons of intensive care: informing outcomes and improving standards. Curr Opin Crit Care. 2012;18(6):700–706.
- Ostgard LS, Norgaard JM, Sengelov H, et al. Impact of chemotherapy delay on short- and long-term survival in younger and older AML patients: a Danish population-based cohort study. Leukemia. 2014;28(9):1926–1929.
- Christiansen CF, Johansen MB, Langeberg WJ, et al. Incidence of acute kidney injury in cancer patients: a Danish population-based cohort study. Eur J Intern Med. 2011;22(4):399–406.
- Contejean A, Lemiale V, Resche-Rigon M, et al. Increased mortality in hematological malignancy patients with acute respiratory failure from undetermined etiology: a Groupe de Recherche en Reanimation Respiratoire en Onco-Hematologique (Grrr-OH) study. Ann Intensive Care. 2016;6(1):102.
- Mokart D, Lambert J, Schnell D, et al. Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure. Leuk Lymphoma. 2013;54(8):1724–1729.
- Azoulay E, Schellongowski P, Darmon M, et al. The intensive care medicine research agenda on critically ill oncology and hematology patients. Intensive Care Med. 2017;43(9):1366–1382.
- Wunsch H, Angus DC, Harrison DA, et al. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36(10):2787–2793, e2781–e2789.
- Cardoso LT, Grion CM, Matsuo T, et al. Impact of delayed admission to intensive care units on mortality of critically ill patients: a cohort study. Crit Care. 2011;15(1):R28.
- Pene F, Aubron C, Azoulay E, et al. Outcome of critically ill allogeneic hematopoietic stem-cell transplantation recipients: a reappraisal of indications for organ failure supports. J Clin Oncol. 2006;24(4):643–649.
- Azoulay E, Pene F, Darmon M, et al. Managing critically ill hematology patients: time to think differently. Blood Rev. 2015;29(6):359–367.
