Abstract
In the primary analysis of LYRA, daratumumab + cyclophosphamide/bortezomib/dexamethasone (DARA + CyBorD) was effective and well tolerated in newly diagnosed multiple myeloma (NDMM) and relapsed multiple myeloma (RMM). We report the final analysis of LYRA (median months of follow-up: NDMM, 35.7; RMM, 35.3) after all patients completed study therapy, were followed for 36 months, or discontinued. Patients received DARA + CyBorD induction, autologous stem cell transplant (if eligible), and 12 months of daratumumab maintenance. Eighty-seven NDMM patients enrolled, 39 underwent transplant, and 63 completed maintenance. Rates of complete response or better were 48.7% and 29.8% for NDMM transplant and NDMM non-transplant patients, respectively, and 36-month progression-free survival rates were 69.3% and 72.6%. Grade 3/4 treatment-emergent adverse events occurred in 61.6% of NDMM patients. Efficacy and safety data are also reported for the smaller RMM cohort (n = 14). DARA + CyBorD followed by daratumumab maintenance was well tolerated and achieved deep, durable responses in NDMM and RMM.
Introduction
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend cyclophosphamide plus bortezomib and dexamethasone (CyBorD) as a treatment regimen ‘useful in certain circumstances’ for patients with transplant-eligible or transplant-ineligible newly diagnosed multiple myeloma (NDMM), and as an ‘other recommended regimen for early relapse (1–3 prior therapies)’ for patients with previously treated myeloma [Citation1]. Daratumumab is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor [Citation2–5] and immunomodulatory [Citation6–8] mechanism of action. Daratumumab is approved as monotherapy for the treatment of relapsed multiple myeloma (RMM) and as combination therapy for RMM and NDMM [Citation9,Citation10]. In vitro studies suggest that cyclophosphamide may enhance daratumumab-mediated killing of multiple myeloma (MM) cells [Citation11]. LYRA was a single-arm phase 2 study which evaluated the efficacy and safety of daratumumab in combination with CyBorD (DARA + CyBorD) in patients with NDMM and RMM. Patients received DARA + CyBorD induction therapy. Those eligible could receive high-dose therapy (HDT) and autologous stem cell transplant (ASCT), and all patients received up to 12 monthly doses of daratumumab monotherapy maintenance treatment. In the previously reported primary analysis of LYRA, DARA + CyBorD demonstrated efficacy and was well tolerated [Citation12]. For the primary endpoint analysis, the rate of very good partial response or better (≥VGPR) after 4 cycles of DARA + CyBorD induction was 44.2% for patients with NDMM and 57.1% for RMM [Citation12]. Following the publication of these primary endpoint results from LYRA, the NCCN Guidelines® included DARA + CyBorD as a treatment option for patients with NDMM or RMM [Citation1]. Here, we present the final end-of-study analysis of LYRA, which supports the primary results and occurred after all patients were followed for 36 months after the start of induction therapy, died, or withdrew from the study.
Methods
Trial design and oversight
This multicenter, single-arm, phase 2 study (ClinicalTrials.gov Identifier: NCT02951819) enrolled patients from 14 November 2016 to 10 January 2018 in community oncology centers in the United States. The study design has been previously published [Citation12] and was conducted in accordance with the principles of the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice guidelines, and the appropriate regulatory and country-specific requirements. Independent ethics committees and institutional review boards at each participating site approved the study protocol and amendments. All patients provided written informed consent.
Patients
The complete eligibility criteria were published previously [Citation12]. Briefly, eligible patients were ≥18 years of age with documented MM as defined by the International Myeloma Working Group (IMWG) criteria [Citation13], an Eastern Cooperative Oncology Group performance status score of 0–2, and previously untreated NDMM or RMM. RMM patients could have received 1 line of therapy including an induction regimen, which may have been followed by HDT and ASCT, and single-agent maintenance therapy; these patients could not be refractory to any proteasome inhibitor (PI) or the combination of PI and immunomodulatory agents. Due to slow recruitment of RMM patients, this cohort was subsequently closed to enrollment.
Trial treatments
Patients received 4–8 cycles (28 days per cycle) of induction therapy of DARA + CyBorD (300 mg/m2 oral cyclophosphamide on days 1, 8, 15, and 22; 1.5 mg/m2 subcutaneous bortezomib on days 1, 8, and 15; 40 mg oral or intravenous [IV] dexamethasone weekly; and 8 mg/kg IV daratumumab on day 1 and 2 of cycle 1, followed by 16 mg/kg IV daratumumab weekly from day 8 of cycle 1 through end of cycle 2, every 2 weeks in cycles 3–6, and every 4 weeks thereafter). After induction, eligible patients received HDT and ASCT at the discretion of the investigator. Following induction and/or ASCT, all patients received up to 12 cycles of daratumumab monotherapy maintenance (16 mg/kg IV daratumumab every 4 weeks). Pre- and post-infusion medications were previously reported [Citation12].
Endpoints and assessments
The primary endpoint was the rate of ≥VGPR, as assessed by a validated computer algorithm to calculate response according to IMWG criteria [Citation13], after 4 cycles of DARA + CyBorD induction therapy. Secondary endpoints included overall response rate, time to ≥VGPR, time to and duration of response (partial response or better), progression-free survival (PFS) and overall survival (OS) at 1 and 3 years, and safety/tolerability. The pre-defined end-of-study analysis occurred when all patients completed the 36-month follow-up visit after the start of induction therapy, died, or withdrew from the study, whichever occurred first.
Statistical analysis
Sample size assumptions and statistical methods have been described previously [Citation12]. Analyses of the primary and secondary endpoints included all enrolled patients who received ≥1 dose of study treatment and recorded ≥1 efficacy evaluation assessment. PFS and OS were analyzed among all enrolled patients (intent to treat population). Safety analyses occurred among all enrolled patients who received ≥1 dose of study treatment. Continuous variables were summarized using descriptive statistics, categorical variables were summarized with frequency tables, and time-to-event variables were analyzed with the Kaplan–Meier method. For response endpoints, the rate and 2-sided 95% confidence interval (CI) were calculated.
Results
Patient characteristics, treatment delivery, and ASCT
A total of 101 patients enrolled (NDMM, n = 87; RMM, n = 14), and 100 received ≥1 dose of study treatment (86 NDMM patients and 14 RMM patients). The RMM cohort had slow recruitment and subsequently closed to enrollment after 14 patients enrolled. Since the RMM cohort is small, this manuscript primarily focuses on the final LYRA analysis of the NDMM cohort. However, for completeness, we include the final analysis outcomes for the RMM patients in the Supplemental Material. Demographic and baseline characteristics were previously reported [Citation12] and are summarized for NDMM in and for RMM in Supplemental Table 1. The median time from diagnosis to enrollment for NDMM patients was 0.08 (range, 0.0–3.1) years and for RMM patients was 2.2 (range, 0.4–5.8) years. Among NDMM patients with cytogenetic data, 36.9% (n = 31) of evaluable patients had high-risk cytogenetics, and 29.9% (n = 26) of NDMM patients had International Staging System stage III disease.
Table 1. Demographic and baseline characteristics.
For NDMM patients, the median follow-up duration at the end of the study was 35.7 months. Eighty-one NDMM patients completed at least 4 cycles of induction, and 61 patients completed 5–8 cycles of induction. Although all patients with NDMM were of eligible age for transplant, transplant decisions were at the discretion of the physician. The median number of induction cycles was 5.0 (range, 4–8) for patients who underwent ASCT and 8.0 (range, 2–8) for patients who did not undergo ASCT. Of the 39 patients who underwent stem cell mobilization and ASCT, 9 underwent chemotherapy mobilization with cyclophosphamide and 19 received plerixafor. The median stem cell yield was 6.2 (range, 2–15) × 106 CD34+ cells/kg, and median time to engraftment was 2.0 (range, 0–6) weeks. All patients, regardless of ASCT status, then received monthly daratumumab maintenance, with 63 completing all 12 cycles of maintenance.
Efficacy
As previously reported in the primary analysis, the ≥VGPR rate after 4 cycles of DARA + CyBorD induction was 44.2% for NDMM patients [Citation12]. Response rates increased with additional cycles of induction as well as with daratumumab maintenance (). Among the 39 patients who underwent transplant, the rate of ≥VGPR by the end of induction was 64.1% and rose to 82.1% by the end of the study. Similarly, for the 47 patients who did not receive transplant, the rate of ≥VGPR by end of induction was 63.8% and rose to 70.2% by the end of the study. Daratumumab maintenance therapy increased the rates of overall response as well as complete response or better (≥CR). In the 39 transplanted patients, the ≥CR rate was 5.1% by the end of induction and increased to 48.7% by the end of the study. Similarly, for the 47 non-transplant patients, the rate of ≥CR rose from 17.0% by the end of induction to 29.8% by the end of the study. Median duration of response was not reached regardless of transplant status.
Figure 1. Summary of response rates over time. Data for induction cycles 1–4 are from the primary endpoint analysis [Citation12,Citation39]. Response data are also shown for the end of induction and end of study, occurring after all patients completed 1 year of maintenance therapy and were followed for 36 months after the start of induction therapy, died, or withdrew from the study (median follow-up at end of study: NDMM, 35.7 months). Percentages may not add to 100% due to rounding. NDMM: newly diagnosed multiple myeloma; ORR: overall response rate; VGPR: very good partial response; CR: complete response; PR: partial response; sCR: stringent complete response.
![Figure 1. Summary of response rates over time. Data for induction cycles 1–4 are from the primary endpoint analysis [Citation12,Citation39]. Response data are also shown for the end of induction and end of study, occurring after all patients completed 1 year of maintenance therapy and were followed for 36 months after the start of induction therapy, died, or withdrew from the study (median follow-up at end of study: NDMM, 35.7 months). Percentages may not add to 100% due to rounding. NDMM: newly diagnosed multiple myeloma; ORR: overall response rate; VGPR: very good partial response; CR: complete response; PR: partial response; sCR: stringent complete response.](/cms/asset/e6b9a47b-283d-4c5b-b7f1-ca060029e3b6/ilal_a_2076847_f0001_c.jpg)
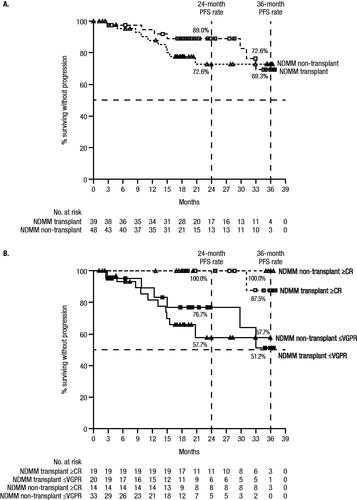
Median PFS was not reached among NDMM patients, regardless of transplant status (). Estimated 36-month PFS rates were 69.3% (95% CI, 43.0–85.3) for transplanted patients and 72.6% (95% CI, 54.0–84.7) for non-transplant patients. NDMM patients who attained ≥CR had improved PFS, with only 1 ≥CR patient progressing by the end of the study. Estimated 36-month PFS rates were higher for NDMM patients who achieved ≥CR versus those achieving VGPR or less (≤VGPR) for both transplanted (87.5% vs 51.2%) and non-transplant patients (100.0% vs 57.7%; ). Estimated 36-month OS rates were 94.9% (95% CI, 81.0–98.7) for transplanted patients and 84.3% (95% CI, 69.8–92.2) for non-transplant patients.
Figure 2. PFS in NDMM patients by (A) transplant status and (B) by transplant status and response. Results of the Kaplan–Meier estimates of PFS at end of study are shown by (A) transplant status among patients who received ≥1 dose of study treatment, and by (B) transplant status and response among the response evaluable population. Median PFS was not reached for any group, and estimated 24- and 36-month PFS rates are provided in the figures. The symbols for panel A are as follows: empty squares, NDMM transplant group; empty triangles, NDMM non-transplant group. For Panel B, the symbols are: empty squares, NDMM transplant with ≥CR group; filled squares, NDMM transplant with ≤VGPR group; empty triangles, NDMM non-transplant with ≥CR group; filled triangles, NDMM non-transplant with ≤VGPR group.
In a subgroup analysis of NDMM patients by cytogenetic risk, response rates also deepened from induction therapy through the end of the study. Among evaluable patients, (standard risk, n = 52; high risk, n = 31), the rate of ≥VGPR after induction was 61.5% for standard-risk patients and 67.7% for high-risk patients, and ≥CR rates were 9.6% and 16.1%, respectively. By the end of the study, ≥VGPR rates were 75.0% for standard-risk patients and 77.4% for high-risk patients, and ≥CR rates rose to 42.3% and 29.0%, respectively. An analysis of disease progression by cytogenetic risk showed that among evaluable NDMM patients (standard risk, n = 53; high risk, n = 31), median PFS was not reached among those with standard risk and was 33.1 months among those with high cytogenetic risk. Estimated 36-month PFS rates were 87.5% for standard-risk patients and 45.2% for high-risk patients.
For the RMM cohort (n = 14), response rates over time and PFS outcomes are shown in Supplemental Figures 1 and 2.
Safety
The most common treatment-emergent adverse events (TEAEs) of any grade in the NDMM cohort are summarized in . The most frequent any-grade TEAE was fatigue (68.6%). Grade 3/4 TEAEs occurred in 62.8% of patients, with the most frequent being neutropenia (12.8%). Serious TEAEs were reported in 32.6% of patients; the most common were pneumonia and pulmonary embolism (2.3% each). Cardiac TEAEs occurred in 16.3% of patients; 4 (4.7%) patients had grade 3 cardiac events (atrial fibrillation, n = 4; atrial flutter, n = 1), all of which were considered serious and not related to treatment. TEAEs leading to treatment discontinuation occurred in 7 patients (anemia [n = 2], atrial fibrillation, hip fracture, nephrotic syndrome, laryngeal edema, and rash [n = 1 each]); 1 patient had a TEAE leading to death (nephrotic syndrome; unrelated to study treatment).
Table 2. Most common TEAEs of any grade (≥25%) and grade 3/4 (≥10%) in the safety analysis set.a
A corresponding summary of the most common TEAEs occurring specifically during the maintenance phase is provided in . Serious TEAEs occurred in 16.7% (n = 6) of NDMM patients who received transplant and 23.1% (n = 9) of those who did not, with atrial fibrillation being the only serious event to occur in more than 1 patient, occurring in 5.1% (n = 2) of NDMM non-transplant patients. TEAEs led to treatment discontinuation in 5.6% (laryngeal edema and rash [n = 1 each]) and 2.6% (hip fracture, n = 1) of NDMM patients who did and did not receive transplant, respectively.
Table 3. Most common TEAEs of any grade (≥20%) and grade 3/4 (≥5%) occurring during the maintenance phase of study therapy in the safety analysis set.a,b
Infusion-related reactions (IRRs) occurred in 55.8% of NDMM patients. Three IRRs were grade 3 (chest discomfort, hypertension, anaphylactic reaction [n = 1 each]), and 1 was grade 4 (laryngeal edema). One patient discontinued study treatment due to an IRR. The majority of IRRs occurred in the first cycle of study therapy. However, 9 of 36 (25.0%) transplanted patients who received maintenance experienced ≥1 IRR during the first maintenance cycle. No IRRs were reported during maintenance for NDMM patients who did not receive transplant. The majority of post-ASCT IRRs were grade 1/2, although 1 patient experienced grade 3 chest discomfort and another developed grade 4 laryngeal edema.
For the RMM cohort, safety data are provided in the Supplemental Material (Supplemental Tables 2 and 3).
Discussion
This end-of-study analysis of the phase 2 LYRA trial demonstrates that the immunomodulatory drug-sparing regimen of DARA + CyBorD induction followed by monthly daratumumab monotherapy maintenance led to deep and durable responses for patients with MM in the community oncology setting in the United States. For NDMM patients, responses deepened with continued therapy, with the highest response rates occurring by the end of the study, resulting in durable PFS. The rate of ≥CR was only 5% at the end of 4 cycles of induction but increased to 49% and 30% for patients who did or did not undergo ASCT, respectively, and estimated 36-month PFS rates were approximately 70% for both groups. Similar deepening of response with extended daratumumab therapy was also observed in the GRIFFIN study of daratumumab plus lenalidomide, bortezomib, and dexamethasone (D-RVd) as induction therapy, followed by ASCT, D-RVd consolidation, and up to 2 years of daratumumab and lenalidomide (D-R) maintenance therapy. In GRIFFIN, the rate of ≥CR for the D-RVd group was 19% at the end of induction and then rose to 27% after ASCT and to 82% after 2 years of maintenance [Citation14]. The results from LYRA demonstrate the benefit of adding daratumumab to CyBorD, which is the most frequently used immunomodulatory drug-sparing alkylator-based regimen for NDMM in the United States [Citation15] and is an attractive regimen worldwide due to improved accessibility in some regions.
The LYRA results in NDMM patients who underwent ASCT are similar to findings from a small European study of DARA + CyBorD in 18 transplant-eligible NDMM patients [Citation16]. After induction, ASCT, and consolidation (median follow-up, 16.8 months), 14 patients were progression-free in the European study, and among response-evaluable patients, ≥VGPR and ≥CR were achieved in 100% and 57% of patients, respectively. Additionally, results among NDMM patients who did not undergo transplant in LYRA were comparable to results from the phase 3 studies ALYCONE [Citation17] and MAIA [Citation18], which both evaluated daratumumab-combination therapies in transplant-ineligible NDMM. In ALCYONE (median follow-up, 40.1 months), daratumumab in combination with bortezomib, melphalan, and prednisone (D-VMP) was associated with an estimated 36-month PFS rate of 50.7% [Citation19]. In MAIA (median follow-up, 47.9 months), daratumumab plus lenalidomide and dexamethasone (D-Rd) led to an estimated 36-month PFS rate of 67.4% [Citation20]. Interestingly, the higher ≥CR rate in NDMM patients in LYRA who underwent ASCT did not result in longer overall PFS compared to patients who did not receive HDT and ASCT. Nonetheless, it is important to note that transplant patients had a higher rate of ≥CR than non-transplant patients, and achievement of ≥CR was associated with longer PFS for both groups; these data suggest that although there was no significant difference in PFS for the transplant and non-transplant groups, transplant continues to have an important role among patients who are eligible. The apparent discrepancy between depth of response and overall PFS for the transplant and non-transplant groups in LYRA may be due to the relatively small sizes (<50 patients) in this non-randomized study, patient selection for transplant, and the excellent PFS of the non-transplant group.
LYRA demonstrated that maintenance therapy with monthly daratumumab monotherapy led to deepening of responses and an estimated 36-month PFS rate of approximately 70%, regardless of transplant status. Of note, daratumumab monotherapy maintenance was administered every 4 weeks for 48 weeks in LYRA, in contrast to the CASSIOPEIA study in transplant-eligible NDMM [Citation21], which gave daratumumab monotherapy maintenance at a reduced intensity of every 8 weeks for 2 years among those achieving partial response or better after consolidation therapy [Citation21,Citation22]. In part 2 of CASSIOPEIA, daratumumab monotherapy maintenance did not improve PFS, compared with observation, in patients who had received induction and consolidation therapy with daratumumab plus bortezomib, thalidomide, and dexamethasone (D-VTd) [Citation22]. It is possible that the daratumumab monotherapy maintenance benefit observed in LYRA was due to monthly dosing, as opposed to every 8 weeks as used in CASSIOPEIA; this is supported by data from the CENTAURUS study, which administered different schedules of daratumumab monotherapy to patients with smoldering MM and indicated that every-4-week dosing provided better CD38 target saturation [Citation23]. To this end, while the CASSIOPEIA study was already too far underway for changes in the daratumumab maintenance schedule to be made, the GRIFFIN study in transplant-eligible NDMM, which gives maintenance therapy with D-R, was amended to change the frequency of daratumumab maintenance from every 8 weeks to every 4 weeks [Citation24]. Analysis of the GRIFFIN study after patients completed 12 months of maintenance therapy indicated that response rates were improved for the daratumumab group compared with the control group [Citation25]. Thus, these findings suggest that the dosing interval (4 weeks vs 8 weeks) of daratumumab maintenance may be critical. Furthermore, the data from LYRA suggest a potential benefit for daratumumab maintenance therapy following induction with DARA + CyBorD; however, larger randomized studies are needed to determine whether daratumumab maintenance benefits patients who receive daratumumab-based induction therapy.
The long-term safety profile of DARA + CyBorD followed by daratumumab maintenance was consistent with previous reports, and no new safety concerns were observed with longer follow-up [Citation12]. The rate of serious cardiac-related TEAEs was substantially lower than previously described for the phase 3 ANDROMEDA study of DARA + CyBorD in light-chain amyloidosis, suggesting that the cardiac toxicity observed with this regimen in ANDROMEDA was largely due to underlying cardiac amyloidosis [Citation26,Citation27]. In LYRA, only 4 patients had grade 3 cardiac events, all of which were atrial fibrillation and flutter and deemed by investigators to be unrelated to study treatment. These findings indicate that DARA + CyBorD has an acceptable cardiac safety profile in MM and are generally consistent with previous reports of CyBorD in myeloma [Citation28–33] as well as prior observations that daratumumab-containing regimens are not associated with cardiac toxicity [Citation17,Citation18,Citation21,Citation34,Citation35]. In particular, the randomized CASSIOPEIA and GRIFFIN studies of daratumumab combination therapy with PIs and immunomodulatory agents as induction therapy, followed by HDT and ASCT, did not demonstrate increased toxicity with the addition of daratumumab [Citation21,Citation24]. The LYRA study also confirmed the finding from CASSIOPEIA and GRIFFIN that stem cell mobilization and ASCT are feasible following daratumumab induction therapy [Citation21,Citation24].
In summary, these data indicate that DARA + CyBorD induction followed by daratumumab maintenance is well tolerated and effective for patients with NDMM, regardless of transplant status. DARA + CyBorD induced robust responses, which deepened with daratumumab maintenance therapy. We also provide follow-up data in the smaller RMM cohort, which support the primary analysis that this regimen is safe and effective; however, larger studies in previously treated myeloma patients should be conducted to confirm these findings. Of note, based upon the publication by Yimer et al. in 2018, which reported the primary endpoint analysis of LYRA [Citation12], the NCCN Guidelines included DARA + CyBorD as a primary treatment option useful in certain circumstances for transplant-eligible NDMM patients, as an other recommended therapy for transplant-ineligible NDMM patients, and as an other recommended treatment for myeloma patients after early relapse (1–3 prior therapies) [Citation1]. This final analysis of LYRA includes approximately 27 months of additional follow-up, demonstrates the benefit of maintenance therapy, provides PFS data for the first time, and supports the treatment recommendations provided in the NCCN Guidelines for MM [Citation1].
GLAL-2022-0011-File008.pdf
Download PDF (341 KB)Acknowledgments
The authors thank the patients who volunteered to participate in this trial, their families, and the staff members at the trial sites who cared for them. J.M.B. and R.M.R. contributed to study design and data acquisition; Y.L, K.Q., M.Q., and T.S.L. contributed to study design; H.Y., J.M., E.F., W.I.B., M.N., D.S., and D.H. contributed to data acquisition; and all authors contributed to data analysis or interpretation, reviewed the manuscript, approved the final version, decided to publish this report, and vouch for the data accuracy and completeness. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
Data sharing statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Disclosure statement
H.Y. has served on a speakers bureau for Janssen, AstraZeneca, BeiGene, Amgen, Sanofi Genzyme, Pharmacyclics, and Karyopharm Therapeutics; and holds equity ownership in Karyopharm Therapeutics.
J.M. has served on a speakers bureau for Janssen and AstraZeneca.
E.F. has served as a consultant and/or participated in a Data Safety Monitoring Board or Advisor Board for Amgen, Celgene, Janssen, Juno Therapeutics, AbbVie, Adaptive Biotechnologies, AstraZeneca, Cardinal Health, GlaxoSmithKline, Karyopharm, Sanofi Genzyme, Takeda, and Kite/Gilead.
W.I.B. has served as a consultant or in an advisory role for Amgen, Celgene, and Janssen; has served on a speakers bureau for Amgen, Celgene, Janssen, and Takeda; has provided expert testimony for Celgene and Takeda; has received honoraria from Amgen, Celgene, Janssen, and Takeda; and has received research funding from Acetylon Pharmaceuticals, Bristol Myers Squibb, Celgene, Karyopharm Therapeutics, Merck, Amgen, and Sanofi.
J.M.B. has served as a consultant or in an advisory role for Genentech/Roche, AbbVie, Seattle Genetics, Bayer, AstraZeneca, Adaptive Biotechnologies, Verastem, MorphoSys, Kura Oncology, Epizyme, BeiGene, Kymera, TG Therapeutics, X4 Pharmaceuticals, and Novartis; and served on a speakers bureau for Seattle Genetics and BeiGene.
M.N. has served as a consultant or in an advisory role for Celgene and Bristol Myers Squibb and has served on a speakers bureau for Bristol Myers Squibb.
D.S. has nothing to disclose.
P.B. is an employee of Janssen.
D.H. and Y.L. were employees of Janssen at the time of the study and have equity ownership in Johnson & Johnson.
K.S.G., T.S.L., K.Q., and M.Q. are employees of Janssen and have equity ownership.
R.M.R. has served as a consultant or in an advisory role for Amgen, Bristol Myers Squibb/Celgene, Coherus BioSciences, Fresenius Kabi, and Takeda; participated in a Data Safety Monitoring Board for CARsgen Therapeutics; and has equity ownership in McKesson Specialty Health.
Additional information
Funding
References
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V2.2022.© National Comprehensive Cancer Network, Inc. 2021. All rights reserved. [cited 2021 Oct 22]. To view the most recent and complete version of the guideline, go online to NCCN.org.
- de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848.
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124(21):3474–3474.
- Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–321.
- Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016;197(3):807–813.
- Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394.
- Adams HC III, Stevenaert F, Krejcik J, et al. High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A. 2019;95(3):279–289.
- Casneuf T, Adams HC III, van de Donk NWCJ, et al. Deep immune profiling of patients treated with lenalidomide and dexamethasone with or without daratumumab. Leukemia. 2021;35(2):573–584.
- DARZALEX® (daratumumab) [package insert]. Horsham, PA: Janssen Biotech, Inc.; 2022.
- European Medicines Agency. Darzalex. 2020. [cited 2020 Oct 16] Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/darzalex.
- Naicker SD, Feerick CL, Lynch K, et al. Cyclophosphamide alters the tumor cell secretome to potentiate the anti-myeloma activity of daratumumab through augmentation of macrophage-mediated antibody dependent cellular phagocytosis. Oncoimmunology. 2021;10(1):1859263.
- Yimer H, Melear J, Faber E, et al. Daratumumab, bortezomib, cyclophosphamide and dexamethasone in newly diagnosed and relapsed multiple myeloma: LYRA study. Br J Haematol. 2019;185(3):492–502.
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346.
- Laubach J, Kaufman JL, Sborov DW, et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (RVd) in patients (Pts) with transplant-eligible newly diagnosed multiple myeloma: NDMM: updated analysis of GRIFFIN after 24 months of maintenance. Presented at the 2021 American Society of Hematology Annual Meeting; 2021 December 11.
- Reeder CB, Reece DE, Kukreti V, et al. Long-term survival with cyclophosphamide, bortezomib and dexamethasone induction therapy in patients with newly diagnosed multiple myeloma. Br J Haematol. 2014;167(4):563–565.
- O’Dwyer M, Henderson R, Naicker SD, et al. CyBorD-DARA is potent initial induction for MM and enhances ADCP: initial results of the 16-BCNI-001/CTRIAL-IE 16-02 study. Blood Adv. 2019;3(12):1815–1825.
- Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–528.
- Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104–2115.
- Mateos MV, Cavo M, Blade J, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395(10218):132–141.
- Kumar SK, Facon T, Usmani SZ, et al. Updated analysis of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with transplant-ineligible newly diagnosed multiple myeloma (NDMM): the phase 3 MAIA study. Presented at: The American Society of Hematology Annual Meeting and Exposition; 2020 December 5–8. Virtual.
- Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29–38.
- Moreau P, Hulin C, Perrot A, et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(10):1378–1390.
- Landgren CO, Chari A, Cohen YC, et al. Daratumumab monotherapy for patients with intermediate-risk or high-risk smoldering multiple myeloma: a randomized, open-label, multicenter, phase 2 study (CENTAURUS). Leukemia. 2020;34(7):1840–1852.
- Voorhees PM, Kaufman JL, Laubach JP, et al. Daratumumab, lenalidomide, bortezomib, & dexamethasone for transplant-eligible newly diagnosed multiple myeloma: GRIFFIN. Blood. 2020;136(8):936–945.
- Kaufman JL, Rodriguez C, Reeves B. editors, et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (RVd) in patients with transplant-eligible newly diagnosed multiple myeloma (NDMM): updated analysis of GRIFFIN after 12 months of maintenance therapy 2020. Paper presented at: The American Society of Hematology Annual Meeting and Exposition; 2020 December 5–8. Virtual.
- DARZALEX FASPRO® (daratumumab and hyaluronidase-fihj) [package insert]. Horsham, PA: Janssen Biotech Inc.; 2022.
- Palladini G, Kastritis E, Maurer MS, et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136(1):71–80.
- Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23(7):1337–1341.
- Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–4382.
- Mai EK, Bertsch U, Durig J, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015;29(8):1721–1729.
- Carreira S, Porta N, Arce-Gallego S, et al. Biomarkers associating with PARP inhibitor benefit in prostate cancer in the TOPARP-B trial. Cancer Discov. 2021;11(11):2812–2827.
- Figueiredo A, Atkins H, Mallick R, et al. Cyclophosphamide-bortezomib-dexamethasone compared with bortezomib-dexamethasone in transplantation-eligible patients with newly diagnosed multiple myeloma. Curr Oncol. 2020;27(2):e81–e85.
- Einsele H, Engelhardt M, Tapprich C, et al. Phase II study of bortezomib, cyclophosphamide and dexamethasone as induction therapy in multiple myeloma: DSMM XI trial. Br J Haematol. 2017;179(4):586–597.
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766.
- Smol T, Dufour A, Tricot S, et al. Combination of t(4;14), del(17p13), del(1p32) and 1q21 gain FISH probes identifies clonal heterogeneity and enhances the detection of adverse cytogenetic profiles in 233 newly diagnosed multiple myeloma. Mol Cytogenet. 2017;10:26.
- Gao W, Du J, Liu J, et al. What multiple myeloma with t(11;14) should be classified into in novel agent era: standard or intermediate risk? Front Oncol. 2020;10:538126.
- Ross FM, Avet-Loiseau H, Ameye G, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012;97(8):1272–1277.
- Rifkin RM, Melear J, Faber E, et al. Daratumumab (DARA) maintenance therapy improves depth of response and results in durable progression-free survival (PFS) following DARA plus cyclophosphamide, bortezomib, and dexamethasone (CyBorD) induction therapy in multiple myeloma (MM): update of the LYRA study. Presented at: The American Society of Hematology Annual Meeting and Exposition; 2019 December 6–10.
