Abstract
This study evaluated ofatumumab (Ofa), an anti-CD20 monoclonal antibody, alone or with bendamustine (Benda), in transplant-ineligible patients with mantle cell lymphoma. Low-risk patients received Ofa monotherapy. Non-responders received subsequent treatment with Benda-Ofa. Six patients received Ofa monotherapy and 3 patients crossed over to Bend-Ofa. Twenty-four high-risk patients were initially treated with Benda-Ofa. The overall response rate for patients treated with Ofa monotherapy was 1/6 (17%) and 23/25 (92%) for patients treated with Benda-Ofa. With a median follow-up of 8.6 years, all Ofa patients progressed with a median progression-free survival (PFS) of 0.6 years (95% CI 0.31-NR) and remain alive. With a median follow-up of 6.3 years, Bend-Ofa treated patients had median PFS 2.5 years (95% CI 1.8-NR) and a median overall survival of 7.4 years (95% CI 5.8-NR). Benda-Ofa had a favorable adverse event profile and efficacy similar, but not clearly superior, to those reported for Benda-Rituximab.
Introduction
Mantle cell lymphoma (MCL) is a rare subtype of B-cell Non-Hodgkin lymphoma, characterized by increased expression of cyclin D1 driven by the chromosomal translocation juxtaposing the immunoglobulin heavy chain gene with CCND1, t(11;14). Despite this common genetic hallmark, the disease is clinically and biologically heterogeneous, and there is no single established standard of care for the frontline therapy of older MCL patients. Bendamustine plus rituximab (BR) has emerged as one of the preferred treatment regimens for transplant-ineligible MCL patients given its efficacy and tolerability. Two randomized trials demonstrated that induction therapy with BR improves progression-free survival (PFS) compared with RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) and is associated with a more favorable toxicity profile, sparing exposure to anthracycline therapy [Citation1,Citation2].
Ofatumumab is a novel, fully human anti-CD20 monoclonal antibody with demonstrated activity in CLL and non-Hodgkin lymphoma. Ofatumumab recognizes a CD20 epitope distinct from rituximab with a slower dissociation rate and is associated with similar antibody dependent cell mediated cytotoxicity (ADCC) and increased complement dependent cytotoxicity (CDC), but does not directly trigger apoptosis [Citation3]. Thus, it was hypothesized that ofatumumab may have an advantage over rituximab given more efficient complement activation and complement-dependent cytotoxicity. The FDA granted ofatumumab accelerated approval in 2009 for the treatment of CLL refractory to fludarabine and alemtuzumab based on the Hx-CD20-406 multicenter trial and ofatumumab has also been studied in various histologic subsets of B-cell Non-Hodgkin lymphoma [Citation4–6]. The use of ofatumumab in the treatment of mantle cell lymphoma has not been assessed. We hypothesized that the combination of bendamustine with ofatumumab might further improve outcomes for older, transplant-ineligible mantle cell lymphoma patients.
We designed a phase II risk-stratified study of ofatumumab monotherapy or in combination with bendamustine as first line treatment for elderly MCL. Ofatumumab therapy monotherapy was tested in a low-risk group of patients. Here, we report the final analysis of all patients included in this study.
Methods
Patients
In this study, non-transplant eligible adult patients with Karnofsky performance status of ≥70%, histologically confirmed MCL, and evaluable disease on CT or on bone marrow evaluation were enrolled. Subjects were deemed to be ineligible for intensive high-dose chemotherapy with or without autologous stem cell transplant if they met one of the following factors: age ≥65 years, patient refusal, or comorbidities such as coronary artery disease, congestive heart failure (EF < 45%), pulmonary dysfunction (impaired pulmonary function test with DLCO < 50%), liver or kidney dysfunction, or poor performance status. No prior treatment may have been received for MCL, with exception of corticosteroids for ≤7 days or one course of involved-field radiation. Additional eligibility criteria are provided in the Data Supplement.
This phase II study was conducted at Memorial Sloan Kettering Cancer Center (MSKCC) (ClinicalTrials.gov, trial No. NCT01437709). The study was approved by the institutional review board and written informed consent was obtained for all patients before enrollment.
Study design and treatment
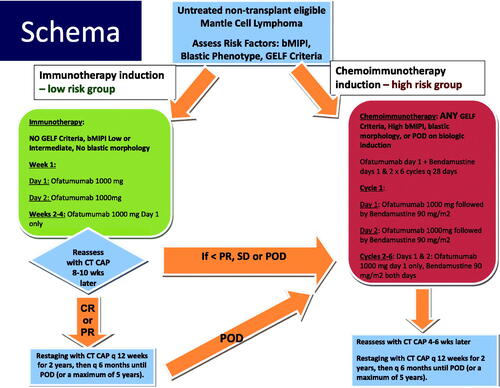
The phase II study schema is summarized in . Patients with low-risk disease initially received ofatumumab monotherapy whereas high-risk patients received bendamustine and ofatumumab (BendOfa). Low risk was defined as no GELF/NCCN criteria for therapy, biologic MIPI (bMIPI) low or intermediate, and absence of blastic or blastoid histology. Low-risk patients received ofatumumab weekly at a dose of 1000 mg on day 1, 2, 8, 15 and 22. Patients failing to achieve at least a partial response (PR) (i.e. with < 50% reduction in nodal masses, stable disease (SD) or progressive disease (PD)), were allowed to cross over and receive additional treatment with bendamustine and ofatumumab. High-risk patients received six planned 28-day cycles of bendamustine (90 mg/m2 on days 1 and 2 of each cycle) and ofatumumab (1000 mg on day 1 and 2 of cycle 1 and on day 1 of subsequent cycles). Of note, for patients with baseline elevated lymphocytosis, the initial day 1 dose of ofatumumab was 300 mg (not 1000 mg) to minimize risk for infusion reaction.
Study assessments
All patients underwent standard staging tests including diagnostic CT scans of neck (if evidence of disease on clinical exam), chest, abdomen, and pelvis, colonoscopy with blind biopsy if clinically indicated, and unilateral bone marrow biopsy and aspirate.
Patients receiving ofatumumab monotherapy were evaluated 8–10 weeks after completion of therapy with restaging CT scans and those receiving BendOfa had restaging CT scans 4–6 weeks after completion of therapy. Responses were assessed according to revised International Working Group response criteria for non-Hodgkin lymphomas [Citation7]. Adverse events (AEs) were assessed per Common Terminology Criteria of Adverse Events (v4.0).
Outcomes and statistical analyses
The primary objective of the study was to assess overall and complete response rates with therapy. Secondary objectives included assessment of overall survival, progression free survival, and response duration. Among low-risk patients, the primary objective was to test whether ofatumumab induction would have an improved overall response rate compared to single-agent rituximab (based on historical controls with ORR ranging between 22 and 37%) [Citation8–10]. A single-agent ORR of ≥60% (20% improvement) with ofatumumab was considered promising. Among patients receiving BendOfa, the primary objective was to test whether the end-of-treatment CR rate was improved compared to previous reports of BR therapy, demonstrating ORR of 75–92%, with a CR rate of 42–50% [Citation1,Citation11]. A CR rate of ≥ 60% (20% improvement) was considered promising. An optimal Simon 2-stage design was used for both cohorts and if there was response in ≥6 of the 12 patients included in the first stage, then enrollment would be extended to a total of 38 patients with type I error rate of 0.10 and type II error rate of 0.20. If 19 or more patients responded overall, the treatment would be considered efficacious and worthy of further testing.
The PFS and OS were estimated using the Kaplan-Meier method. Toxicity was monitored continuously throughout the study, with descriptive statistics provided of observed events. Database from this study was frozen June 1, 2021.
Results
Patient characteristics
From October 2011 to November 2015, 30 patients were enrolled onto the study. The baseline characteristics for patients treated with ofatumumab monotherapy, BendOfa, and all patients are summarized in . In brief, across both treatment cohorts, median age was 72 years with male predominance, 83% were conventional MCL, 57% had high-risk biologic MIPI, and 43% had Ki-67 ≥ 30%. For the low-risk group, per eligibility criteria, 100% had low or intermediate-risk biologic MIPI and 67% had Ki-67 < 30%.
Table 1. Baseline characteristics.
Efficacy
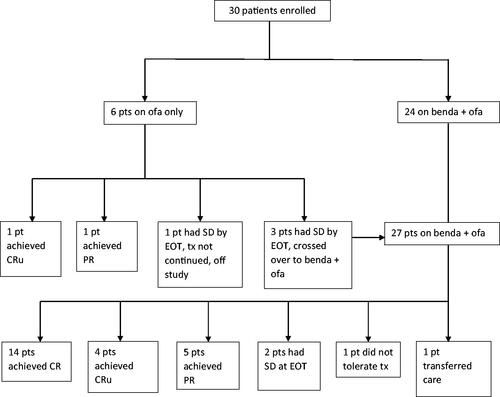
Six patients were enrolled in Ofa group and evaluable for efficacy. Three of these patients crossed over to the BendOfa group. Twenty-four patients were initially enrolled in the BendOfa arm. With the addition of the three crossed-over patients, 27 total patients were treated with BendOfa among which 25 were evaluable for efficacy (). One efficacy inevaluable patient did not tolerate BendOfa due to infusion reactions and came off study after 2 cycles and another inevaluable patient transferred their care after 1 cycle of BendOfa to an alternate institution and came off study.
Among the patients treated with Ofa monotherapy, the ORR rate was 1/6 (17%), 1 CRu. Thus the primary endpoint was not met for Ofa monotherapy arm. The only patient with a CR had response duration of 4.4 years. Among the patients treated with BendOfa, the ORR rate was 23/25 (92%) with 76% CR/CRu (19/25) and 16% PR (4/25). Thus, the primary endpoint was met for the BendOfa arm.
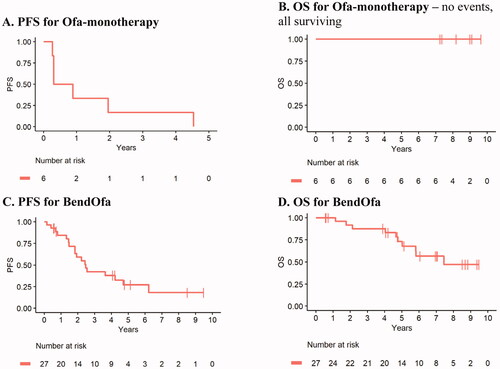
With a median follow up of 8.6 years, all Ofa monotherapy treated patients progressed (n = 6) with a median progression-free survival of 0.6 years (95% CI 0.31-NR) and all patients remain alive (). With a median follow up of 6.3 years among survivors, BendOfa treated patients had median progression-free survival of 2.5 years (95% CI 1.8-NR) and a median overall survival of 7.4 years (95% CI 5.8-NR). Three patients received maintenance rituximab after completing 6 cycles of BendOfa and were censored at the time they initiated maintenance therapy. For the 3 patients who received rituximab maintenance, all 3 patients are alive at this time. Two of the three patients remain in remission with the length of failure-free survival of 7.34 and 6.83 years (defined from initiation of therapy to last visit date). One patient relapsed 6.48 years after initiation of therapy. Given the small number of patients who received maintenance and the potential selection bias for the receipt of maintenance, it is not possible to robustly compare outcomes for patients who did and did not receive maintenance therapy.
Safety
All 30 patients were evaluated for AEs and the data are shown in . The most common AEs were thrombocytopenia (n = 13), fatigue (n = 13), infusion-related reaction (n = 12), neutropenia (n = 7), and constipation (n = 6). Most AEs were grade 1 or 2. The few grade 3 AEs included thrombocytopenia (n = 3), neutropenia (n = 4), hyperglycemia (n = 1), and lung infection (n = 1). One patient had grade 4 neutropenia. Patients who developed neutropenia were all treated with growth-factor support. All twelve infusion-related reactions were related to ofatumumab. One patient came off study due to ofatumumab-related infusion reactions after 2 cycles of BendOfa despite steroid pretreatment and was switched to Rituximab-Bendamustine. There were two patients who had dose reductions of bendamustine to 70 mg/m2 for 1–2 cycles due to neutropenia. There were three patients who had treatment-related serious adverse events including admission for clostridium difficile colitis that resolved with antibiotics (the patient had a pretreatment history of clostridium difficile), supraventricular tachycardia that improved with beta-blocker therapy, and 2-day hospitalization for a low-grade fever with negative infectious work up.
Table 2. Treatment-related adverse events observed in 5% or more patients and all grade 3 events.
Discussion
This study represents the first trial of ofatumumab monotherapy or in combination with bendamustine in older untreated MCL patients. The results demonstrate that ofatumumab, similar to rituximab, has limited efficacy as a single-agent in MCL, even among patients with low-risk disease features [Citation9]. However, ofatumumab in combination with bendamustine was associated with high overall and complete response rates and the study met its primary endpoint for this treatment arm with CR/CRu rate of 76% (greater than 60%). Ofatumumab in combination with bendamustine was well-tolerated with predominantly low-grade and manageable adverse effects.
In our study, single-agent ofatumumab had limited efficacy among low-risk patients with an ORR of 17%. The one patient who achieved a CR had a durable response of 4.4 years. In the GELTAMO ICML-2015 study, rituximab in combination with ibrutuinib in indolent MCL was associated with superior ORR of 84% [Citation12]. Despite the high overall response rate associated with rituximab-ibrutinib in indolent MCL, it is unclear if early initiation of therapy in low-risk MCL is associated with an improvement in overall survival. Many low-risk mantle cell lymphoma patients (who do not meet GELF/NCCN criteria for therapy, have low or intermediate biologic MIPI (bMIPI), and do not have evidence of blastic or blastoid histology) are candidates for expectant monitoring and do not require immediate initiation of therapy [Citation13–15]. Low-risk MCL patients generally have more favorable overall survival outcomes when compared to patients who require immediate therapy. All patients in our study with low-risk features at presentation remain alive and it is possible the overall survival of this subset of patients would have been excellent regardless of the initial management approach.
Despite the favorable overall and complete response rates observed with BendaOfa in this study, the long-term outcomes with this combination are not clearly superior when compared to historical outcomes for rituximab and bendamustine. The median PFS associated with BendaOfa of 2.5 years (95% CI 1.8-NR) is similar to the median PFS associated with rituximab and bendamustine, approximately ∼24–35 months [Citation1,Citation2]. In general, ofatumumab has not clearly demonstrated superior efficacy over rituximab in other lymphoma studies. For example, the ORCHARRD study was negative and found no difference in efficacy between ofatumumab versus rituximab in combination with DHAP as salvage treatment of relapsed or refractory DLBCL [Citation16]. In rituximab-refractory indolent B-cell Non-Hodgkin lymphoma (not including MCL), ofatumumab and bendamustine was compared to single-agent bendamustine and no significant improvement in PFS or OS was observed with the combination [Citation17]. Given the relatively small number of patients included in this study and the lack of a randomized comparison, it is not possible to definitively compare ofatumumab versus rituximab in combination with bendamustine.
The safety profile for ofatumumab and bendamustine was consistent with prior experience from other studies and remains acceptable. The most common treatment-related adverse events, predominantly grade 1–2, were thrombocytopenia (43%), fatigue (43%), infusion reactions (30%), and neutropenia (23%). There were no new safety signals identified in mantle cell lymphoma patients. Overall BendaOfa was well-tolerated among this older patient population with a median age of 73 years, including patients up to 90 years of age. There were very few grade 3 or higher toxicities, few serious adverse events, and few dose modifications required.
In summary, single-agent ofatumumab does not represent a promising treatment strategy for low-risk MCL given limited efficacy, but the combination of ofatumumab and bendamustine was efficacious and well-tolerated in elderly, untreated MCL patients. The ofatumumab and bendamustine treatment combination represents a viable alternative treatment approach for patients who are intolerant (or allergic) to rituximab given the lack of cross-reactivity between rituximab and ofatumumab. Currently there are multiple studies that are building upon the rituximab-bendamustine therapeutic backbone in MCL, adding novel biologically-targeted agents to BR, such as Bruton’s Tyrosine Kinase inhibitor therapy, lenalidomide, bortezomib, or venetoclax (NCT01776840, NCT01415752, NCT03834688) [Citation18]. In addition, the type II glycoengineered antiCD20 monoclonal antibody Obinutuzumab has demonstrated excellent activity in CLL/SLL and is being increasingly studied, in combination with novel agents, in MCL. Randomized phase 3 trials would be required to definitively compare ofatumumab-bendamustine with other anti-CD20 monoclonal antibodies in combination with bendamustine with or without the addition of novel agents. Given these aforementioned advancements in frontline treatment paradigms for MCL, it is unlikely that BendaOfa will be further developed. Additionally, ofatumumab approval in MCL is unlikely given the agent is predominantly now used for treatment of multiple sclerosis and is no longer available for CLL/SLL. Nevertheless, ofatumumab-bendamustine represents a well-tolerated and efficacious frontline treatment of older MCL patients.
Acknowledgements
PH and CC designed the study. AK, AY, CC, CM, AM, PD, PH, JG, AZ, MC, CG, LL, DS, and AJ collected the data. AK and ED analyzed the data. AK wrote the manuscript. All authors reviewed, revised, and approved the final manuscript for publication.
Disclosure statement
AK receives research funding from Abbvie, Adaptive Biotechnologies, Celgene, Pharmacyclics, and Seattle Genetics; member of the advisory board for Celgene, Kite Pharma, AstraZeneca and the Summit Advisory Committee; has served on the steering committee for the MCL Registry with AstraZeneca. JG is currently employed by Janssen Pharmaceuticals. The remaining authors do not have any conflicts of interest to report.
Additional information
Funding
References
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210.
- Flinn IW, van der Jagt R, Kahl B, et al. First-Line treatment of patients with indolent Non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year follow-up study. J Clin Oncol. 2019;37:984–991.
- Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800.
- Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1-2 study. Blood. 2008;111:1094–1100.
- Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755.
- Maloney DG, Ogura M, Fukuhara N, et al. A phase 3 randomized study (HOMER) of ofatumumab vs rituximab in iNHL relapsed after rituximab-containing therapy. Blood Adv. 2020;4:3886–3893.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586.
- Ghielmini M, Schmitz SF, Burki K, et al. The effect of rituximab on patients with follicular and mantle-cell lymphoma. Swiss group for clinical cancer research (SAKK). Ann Oncol. 2000;11(Suppl 1):123–126.
- Ghielmini M, Schmitz SF, Cogliatti S, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss group for clinical cancer research (SAKK). J Clin Oncol. 2005;23:705–711.
- Nguyen DT, Amess JA, Doughty H, et al. IDEC-C2B8 anti-CD20 (rituximab) immunotherapy in patients with low-grade non-Hodgkin's lymphoma and lymphoproliferative disorders: evaluation of response on 48 patients. Eur J Haematol. 1999;62:76–82.
- Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:3383–3389.
- Gine E, de la Cruz F, Jimenez Ubieto A, et al. Ibrutinib in combination with rituximab for indolent clinical forms of mantle cell lymphoma (IMCL-2015): a multicenter, open-label, single-arm, phase II trial. J Clin Oncol. 2022;40(11):1196–1205.
- Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209–1213.
- Kumar A, Ying Z, Alperovich A, et al. Clinical presentation determines selection of patients for initial observation in mantle cell lymphoma. Haematologica. 2019;104:e163–e166.
- Abrisqueta P, Scott DW, Slack GW, et al. Observation as the initial management strategy in patients with mantle cell lymphoma. Ann Oncol. 2017;28:2489–2495.
- van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-Cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35:544–551.
- Rummel MJ, Janssens A, MacDonald D, et al. A phase 3, randomized study of ofatumumab combined with bendamustine in rituximab-refractory iNHL (COMPLEMENT a + B study). Br J Haematol. 2021;193(6):1123–1133.
- Wang ML, Jurczak W, Jerkeman M, et al. Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N Engl J Med. 2022;386:2482–2494.