Abstract
Glucocorticoids, including dexamethasone, have been a mainstay of treatment for multiple myeloma (MM) for decades. In current treatment protocols and NCCN clinical practice guidelines, dexamethasone is included in all phases of MM treatment as a key adjunct to novel therapies within all preferred therapy regimen, augmenting clinical response rates to these agents. The inclusion of dexamethasone in MM treatment regimens, combined with novel agents, continues to deliver good response rates. Further understanding of drug combinations and dose modifications is anticipated to enhance clinical care, mitigate toxicities and optimize outcomes. New formulations are providing the opportunity for a reduction in pill burden and potential for medication errors, whereby improving treatment adherence. Here, we summarize and discuss the role of dexamethasone in the treatment of MM, its mechanism of action and doses used, and provide a critical appraisal current evidence and its clinical implications.
Introduction
Advancements in the treatment of multiple myeloma (MM) have steadily improved the median duration of survival over the last two decades. However, despite the introduction of therapeutic advances, including proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies and CAR-T cell therapy into the treatment arsenal, the disease remains incurable and therapeutic efficacy is especially limited in refractory and relapsed patients. Several agents have gained clinical approval and treatment strategies may include two or three drug combinations. The choice of therapy is influenced by numerous factors, including age, comorbidities, and stem cell transplantation eligibility.
Glucocorticoids, including prednisone and dexamethasone, have been a mainstay of MM treatment regimens since the 1960s. The 2021 National Comprehensive Cancer Network (NCCN) clinical practice guidelines for MM include dexamethasone as an adjunct within all of the preferred therapy regimens. In current treatment protocols, dexamethasone is included in all phases of MM treatment as a key adjunct to novel therapies, augmenting clinical response rates to these agents [Citation1]. In this narrative review, we summarize and discuss the role of dexamethasone in the treatment of MM, its mechanism of action and doses used, and provide a critical appraisal current evidence and its clinical implications.
Dexamethasone in combination therapy for multiple myeloma treatment and mechanism of action
Multiagent therapy has been recognized as a foundation of MM treatment. There is an inter– and intra-tumoral heterogeneity in MM and although patients may respond well to initial therapy, eventual relapses are inevitable, and successive lines of therapy are often characterized by limited duration responses, potentially leading to treatment resistance. Advancements in MM treatment have included the approval and clinical application of agents, some in combination with each other and all of which include dexamethasone as part of their label. ()
Table 1. Approved agents for treatment of Multiple Myeloma that include dexamethasone in product label.
Recently, Sudalagunta and colleagues studied treatment effect of 46 combinations of MM agents and developed a tool to estimate the clinical synergy or clinical benefit of combination therapy in MM [Citation2]. During the conduct of the study, they noted a wide range of the timing of cell-kill effects of the agents; proteasome inhibitors can induce LD50 in less than 48 h; while immunomodulator, immunologics and dexamethasone required over 96 h to reduce cell viability to 30% of initial measurements, at maximum solubility levels [Citation2]. Of the 46 drug pairs studied, dexamethasone was noted to have demonstrated clinically and statistically synergistic activity with Selinexor, carfilzomib, bortezomib and venetoclax. These findings align with clinically beneficial results in clinical trials of the combinations, or as part of multiagent studies [Citation3–6].
Mechanism of action of dexamethasone in multiple myeloma treatment
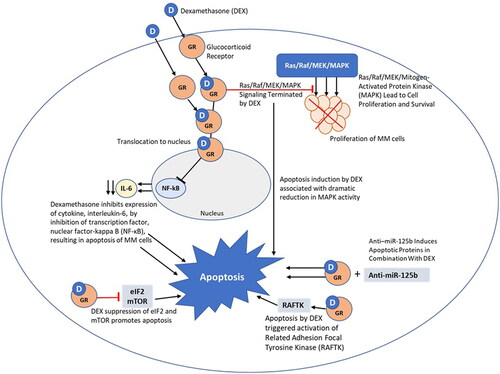
Dexamethasone is a synthetic adrenal corticosteroid drug that is 25-fold more potent than the naturally occurring hormone cortisol in its anti-inflammatory glucocorticoid activity and six times more potent than prednisone [Citation7]. The efficacy of dexamethasone in MM is associated with its ability to induce apoptosis within MM cells. In MM, dexamethasone inhibits the expression of cytokines (e.g. interleukin-6 (IL-6)) as a result of inhibition of transcription factor, nuclear factor-kappa B (NF-κB), which is necessary for the activation of pro-inflammatory cytokines and other genes. ()
IL-6 promotes growth of several tumor types but is a key cytokine in plasma cell disorders. In MM patients, most bone marrow plasma cells produce IL-6 and cells proliferate at a significantly higher level than normal plasma cells. Therefore, inhibition of IL-6 in MM dramatically reduces cell growth. IL-6 expression is regulated by four major transcription factors: AP-1, CREB, C/EBP, and NF-κB. NF-κB is of particular interest in MM as its inhibition reduces IL-6 production, resulting in apoptosis of MM cells.
Dexamethasone-induced apoptosis within MM cells is mediated by initial binding to the glucocorticoid receptor. The glucocorticoid receptor is a ligand-activated transcription factor and once activated, down regulation of NF-κB can occur. Dexamethasone provides interference with phosphorylation at RNA polymerase II, which is required to initiate NF-κB induced transcription [Citation8]. It is thought that this reduction in transcription activity of NF-κB leads to MM cell apoptosis as a result of inhibition of antiapoptotic NF-κB–targeted genes [Citation9–13]. Dexamethasone also upregulates IκBα, the nuclear inhibitor of NF-kB, blocking the ability of NF-κB transcription factors to bind to DNA, a requirement for proper NF-κB functioning [Citation14]. Bortezomib was the first proteasome inhibitor, and an NF-kB inhibitor, approved in MM treatment. Richardson et al. evaluated bortezomib treatment, in combination with perifosine, with or without dexamethasone. The addition of dexamethasone for patients in PD or SD improved response in both bortezomib-relapsed and refractory patients with 38% of refractory patient responding [Citation15]. The improved activity from the addition of dexamethasone was similar to that observed in the CREST (Clinical Response and Efficacy Study of Bortezomib in the Treatment of Relapsing Multiple Myeloma) and SUMMIT (Study of Uncontrolled Myeloma Managed With Proteasome Inhibition Therapy) [Citation16]. Later generations of proteasome inhibitors, including carfilzomib, ixazomib, and marizomib also target the NF-κB pathway. Ixazomib, when combined with lenalidomide and dexamethasone for relapsed/refractory MM, effectively inhibits NF-κB activation pathways in MM stromal cells, decreasing cytokine and growth factor production and also inhibition of NF-κB signaling in preosteoclasts, limiting osteoclastogenesis and bone destruction [Citation17,Citation18].
An important mediator of cellular survival, proliferation, angiogenesis and migration are the Ras/Raf/MEK/MAPK/extracellular signal regulated kinase (Erk) signaling pathways. IL-6 induces MM cell proliferation by way of the Ras/Raf/MAPK cascade. Xu et al. reported that at the time of MM diagnosis, Ras mutations may be present in 23–54% and increase to 45–81% in relapsed or refractory patients [Citation19]. The Ras/Raf/MEK/MAPK (ERK) pathway signaling can be terminated by dexamethasone [Citation20].
Protein tyrosine kinases also have an important role in the signaling pathways that control growth, differentiation and apoptosis. Chauhan and colleagues studied the apoptotic pathways in MM cells and reported that one induced by dexamethasone was mediated by Related Adhesion Focal Tyrosine Kinase (RAFTK) and that dexamethasone triggered RAFTK activation and apoptosis in dexamethasone-sensitive MM cells [Citation21].
MM cells express high levels of eIF2α and the inhibition of eIF2 and mTOR translational pathways, by dexamethasone suppression, additively promotes apoptosis [Citation22]. Work from Burwick-Sharma highlighted that dexamethasone treatment of MM cells resulted in phosphorylation of eIF2α, leading to translational upregulation of ATF4, a known eIF2 regulated mRNA, promoting apoptosis [Citation22]. Additionally, they found that dexamethasone supresses protein synthesis and represses mTOR signaling by upregulating REDD1 RNA.
Programmed cell death 5 (PDCD5) is an important apoptosis-related gene that has been found to be downregulated in many types of cancers. A study from Bao et al. found that in MM samples PDCD5 expression was decreased, noting that it may function as a tumor suppressor gene and have an important role in MM pathogenesis [Citation23]. This group had previously reported that recombinant human PDCD5 (rhPDCD5) plus dexamethasone induced apoptosis in a MM cell line.
The activation of the canonical Wnt/β-catenin signaling pathway has been shown to contribute to the progression of MM. β-catenin can translocate into the nucleus, associating with T-cell factor 4 (TCF4) and regulate the subsequent expressions of downstream genes, including c-Myc, survivin and cyclin D1, therefore, it has been suggested that inhibition of Wnt/β-catenin could suppress MM progression. Liu et al. reported that the combination treatment of rhPDCD5 and dexamethasone in MM substantially decreased the expression of surviving protein [Citation24]. The group further studied the combination of rhPDCD5 and dexamethasone on the proliferation of MM cells by regulating the Wnt pathway. They found that rhPDCD5 alone had no substantial effect, while the combination with dexamethasone delivered significant inhibition of MM growth and suggested the Wnt pathway as the potential mechanism [Citation25].
Deregulation of cyclin-dependent kinase 4 and 6 (CDK4/6) is requisite for the loss of cell cycle control in MM. Palbociclib is a selective inhibitor of CDK4/6 and has been studied in sequential administration combination with bortezomib at a reduced dose plus dexamethasone, in patients with relapsed/refractory MM.
Many signaling pathways have been shown to be involved in regulating MM survival and dexamethasone targets several different signal transduction pathways. Understanding the pathway of dexamethasone-induced apoptosis is crucial to understanding treatment resistance and development of new targets in MM. As an example, IL-6 secretion is directly associated with the resistance to spontaneous or dexamethasone-induced apoptosis, indicating that the combination of immunomodulatory drugs with dexamethasone delivers a synergistic cytotoxicity and elimination of NF-κB activity, resulting in improved patient survival.
Toxicities and side-effects of dexamethasone
The inclusion of dexamethasone in many MM treatment regimens has been shown to be synergistic or additive, leading to improved therapeutic benefit. However, glucocorticoids can cause a range of mild to life-threatening side effects, in multiple systems throughout the body, including the immune system, musculoskeletal, gastrointestinal, dermatologic, psychiatric, cardiovascular, ophthalmic and endocrine [Citation26] (). The National Cancer Institute provides common terminology and criteria that can be applied for steroid-related toxicity, which includes, Grade 1 (mild), Grade 2 (moderate), Grade 3 (severe), Grade 4 (life threatening or disabling) and Grade 5 (death) [Citation27].
Table 2. Toxicities associated with dexamethasone.
The pan-immunosuppressive effects of dexamethasone and associated increased risk for infections, especially when used in certain patient populations such as the elderly, add complexity to treatment [Citation28]. Therefore, it is important to weigh the potential benefits of the potent action of dexamethasone against the possibility of toxicities for the patient and mitigate with dose reductions. Patients with MM are at a far greater risk of bacterial and viral infections, which has been shown to be a significant mortality risk, contributing to 22% of MM patient deaths within the first year of diagnosis [Citation29]. When comparing high to low-dose dexamethasone in a treatment regimen combined with lenalidomide, high-dose dexamethasone was associated with higher rate of infections (18%), compared to 9% in those newly diagnosed MM patients receiving the low dose regimen [Citation28]. Although during initial therapy, bacterial infections occur more often, viral infections have been shown to occur more frequently in patients treated with proteasome inhibitors and dexamethasone. More specifically, dexamethasone has been shown to have an association to depressed cell-mediated immunity against cytomegalovirus and varicella-zoster [Citation30].
MM patients are at a very high risk of developing issues related to poor bone health, including osteolytic bone lesions from increased osteoclastic and decreased osteoblastic activity, further compounded by comorbid conditions and as a side effect of steroid treatment [Citation31]. Glucocorticoid-induced osteoporosis is a well described side effect of chronic steroid use, related to the effects of steroids on osteoblasts and osteocytes. Steroids can impair the replication, differentiation and function of osteoblasts and stimulate apoptosis of mature osteoblasts and osteocytes, inhibiting bone formation. Bisphosphonates are often used to mitigate the steroid-induced effects to bone health.
Venous thromboembolism (VTE) has been shown to occur commonly during the initial stages of MM treatment, particularly in patients who receive thalidomide or lenalidomide, especially in combination with high-dose dexamethasone, doxorubicin or multiagent chemotherapy, and less frequently at later stages or once the disease is well-controlled [Citation32]. A previous VTE or hyperviscosity have been shown to further increase this risk. As a VTE prophylactic strategy, the International Myeloma Working Group recommends that all patients receiving concurrent high-dose dexamethasone or doxorubicin have low-molecular-weight heparin added to their treatment regimen [Citation32].
Development of acute psychosis has been recognized in patients receiving high-dose glucocorticoids [Citation33]. In a case series by Delforge and Ludwig, the potential mental and psychological side-effects of dexamethasone were highlighted as often underrecognized in MM patients [Citation34]. They reported insomnia and severe mood swings following dexamethasone intake and recommend that making inquiries with the patient’s partner or other family members may be valuable to accurately assess potential steroid-induced psychological and/or social problems that may need to be addressed.
Fortunately, most dexamethasone associated side effects can be managed effectively with careful monitoring; appropriate prophylaxis, pharmacologic, and nonpharmacologic interventions; and patient and caregiver education [Citation26]. In 2008, recognizing a need for specific guidance of steroid therapy side effects in myeloma, the International Myeloma Foundation’s Nurse Leadership Board developed a consensus statement for management for healthcare providers [Citation35]. The consensus statement provides recommendations related to dose reduction of high-dose dexamethasone (40 mg PO) at the first presentation of Grade 2 or 3 toxicities, including reducing dose frequency, or considering tapering to dexamethasone 20 mg [Citation26]. Further, in 2017, King and Faiman published an update related to steroid-associated side effects, patient education tool and corresponding recommendations for management and dose modification. [Citation36] They highlight the importance of tailoring steroid dosing according to patient characteristics (frailty, comorbidities). Advancements in MM therapies, with new mechanisms of action are introducing a shift toward greater flexibility of steroid dosing, with minimal impact to outcomes. Importantly, Rajkumar and colleagues evaluated lenalidomide treatment with either high or low-dose dexamethasone and found that diminishing the high-dose dexamethasone regimen to a once-weekly or low-dose regimen could significantly reduce side effects without losing therapeutic efficacy [Citation28].
While practitioners may taper off dexamethasone, this practice has been understudied and is worthy of future research. One notable example is a randomized trial in newly diagnosed MM patients with intermediate frailty scores not proceeding to transplant were randomized to continuous lenalidomide and dexamethasone or fixed duration lenalidomide and dexamethasone followed by reduced dose lenalidomide maintenance [Citation37]. In that study overall response rates, depth of response and progression free time were similar in both arms. Event free survival, which included grade 4 hematologic adverse events, grade 3-4 nonhematologic events, lenalidomide discontinuation along with progression or death, favored the fixed duration and maintenance arm. This study supports the concept, that at least in this patient population, continuous therapy with dexamethasone does not improve outcomes and is associated with higher toxicity. In the relapsed refractory setting, only belantamab, mafodotin and the recently approved CAR-T cell products have not relied on combinations with dexamethasone for their approval.
Dexamethasone resistance
Despite the extensive use of dexamethasone in MM treatment regimens, up to half of patients do not respond and it is not uncommon for patients to develop a resistance to it following prolonged exposure to high doses [Citation38]. Unfortunately, resistance development is associated with poorer outcomes [Citation39].
Several mechanisms have been implicated in dexamethasone resistance in MM, as well as the identification of subgroups that are more susceptible.
Glucocorticoid receptor expression is critical for the sensitivity of MM cells to dexamethasone and as expected, reduced expression leads to weakened cytotoxic effect. Decreased glucocorticoid receptor expression or receptor loss is a common cause of dexamethasone resistance [Citation40]. Researchers are actively developing novel therapeutics a that can be used to overcome glucocorticoid resistance in the absence of glucocorticoid receptors.
IL-6 secretion is directly associated with resistance to dexamethasone-induced apoptosis as it activates JAK/STAT signaling and upregulates antiapoptotic proteins, BCL-XL and myeloid cell leukemia sequence-1. (MCL1) [Citation41]. Additionally, IL-6 activates SRC-homology tyrosine phosphatase 2 (SHP2), which blocks activation of RAFTK by dexamethasone and resulting apoptosis.
Deregulated expression of microRNAs (miRs) plays a role in the pathogenesis and progression of multiple myeloma. The upregulation of miR-21 has been associated with the development of dexamethasone resistance in MM cells when bound to bone marrow stromal cells, suggesting that the tumor microenvironment is an important component in cell adhesion mediated resistance [Citation42].
Overexpression of Bcl-2, a regulator of glucocorticoid induced apoptosis, has been associated with dexamethasone resistance in MM cells [Citation43]. Chen and colleagues recently studied the potential of dihydroartemisinin, to overcome dexamethasone resistance in MM. They reported that dihydroartemisinin augmented the dexamethasone effects within MM cells, resulting in increased production of reactive oxygen species (ROS) and translocation of cytochrome C from the mitochondria to the cytoplasm, and importantly, downregulated dexamethasone-induced expression of Bcl-2, overcoming resistance [Citation44].
Another potential mechanism of drug resistance in MM is related to clonal evolution of MM cells. In the study from Avet-Loiseau, relapsed and refractory MM patients treated with lenalidomide and dexamethasone in the presence of del(13) and t(4;14) chromosomal abnormalities exhibited lower response rates and shorter median progression-free survival [Citation45]. B7 homolog 1 (B7-H1), (programmed death ligand-1 (PDL-1) or CD274), an inhibitory molecule of T cells, is upregulated on the surface of cells from MM patients and has been implicated in the protection of tumor cells from immune attack. Tamura et al. compared B7-H1 -/+ human myeloma cells lines (HMCLs) and found that B7-H1+ HMCLs were more proliferative and less susceptible to dexamethasone and melphalan treatment that B7-H1- and were also associated with higher Bcl-2 and FasL expression [Citation46].
Dexamethasone-induced Ras-related protein 1 (RASD1) supresses cell growth and is encoded by the RASD1 gene on chromosome 17. Methylation of RASD1 was found to be associated with its inactivation, correlating with resistance to dexamethasone [Citation47].
Mutations of the NF-κB gene are highly prevalent in MM and have a pivotal role in drug resistance. Keats et al. studied the NF-kB pathway in the pathogenesis of MM and reported results that suggest that constitutive activation of the non-canonical NF-kB pathway by inactivation of TRAF3 is associated with dexamethasone resistance and proteasome inhibitor sensitivity [Citation48]. The authors note that the resistance to dexamethasone was unexpected but associate the finding to one of the mechanisms of action of glucocorticoids is to target only the canonical NFkB pathway.
Although important advancements have been made toward understanding dexamethasone resistance in MM, definitive mechanisms remain unclear and drug development continues to strive to overcome the varying mechanisms of drug resistance.
Dexamethasone dosing and administration in multiple myeloma
As noted, dexamethasone is nearly ubiquitous in MM treatment regimens, commonly dosed orally, 40 mg, once weekly [Citation49]. To our knowledge, this frequently used dose, or a maximum tolerated dose, was not established as the result of early phase clinical trials, but rather based on empirical evidence.
Achieving the desired 40 mg dose of dexamethasone, using commonly available 4 mg tablet, requires the patient to take ten tablets daily, in addition to the other oral agents included in their chemotherapy regimen. The treatment regimens for multiple myeloma patients are complex and can be associated with a heavy pill burden, contributing to increased risk of poor adherence, resulting in less effective dosing which contributes to a negative impact on treatment and survival outcomes [Citation50]. Fewer drugs in an oral regimen may be associated with better adherence, though more robust studies on this topic are warranted [Citation49]. Recently, a 20 mg oral formulation of dexamethasone (Hemady ™, Acrotech Biopharma) was approved by the US FDA to be used in combination with other antimyeloma products, allowing for decreased pill burden [Citation51]. Although there are recognized benefits to fewer tablets, currently there is a significant cost difference per total dose between branded 20 mg dexamethasone tablets and available lower dose generic brands that may be an important consideration for patients.
It is important to recognize that dose reductions of dexamethasone are often needed, due to patient age, tolerability and toxicity. Careful monitoring of mood disorders and immunosuppression is required and dose adjustments made accordingly to improve tolerability. Although steroid dosing is typically reduced in elderly patients (>70yrs) from 40 mg to 20 mg, a standard dosing guideline and consistency among clinicians is lacking.
Use of dexamethasone for early disease control, followed by a tapering and discontinuation, rather than chronic steroid use, is becoming more widely practiced. Key trials have provided clinically meaningful data regarding the use of glucocorticoids in the modern era as well as historical studies. Investigations of high versus low dosing of dexamethasone, dose modifications and reductions, and the clinical applications to different populations have improved treatment delivery and outcomes. A decade ago, Rajkumar et al. investigated whether low-dose dexamethasone in combination with lenalidomide is non-inferior to and has lower toxicity than high-dose dexamethasone plus lenalidomide [Citation28]. The treatment regimen included lenalidomide and a high dose dexamethasone group, 40 mg on days 1–4, 9–12, and 17–20 of a 28-day cycle, compared to a low-dose regimen of dexamethasone 40 mg on days 1, 8, 15, and 22 of a 28-day cycle (low dose) with the same lenalidomide dosing. In this study of 445 patients, lenalidomide plus low-dose dexamethasone demonstrated better short-term overall survival and lower toxicity than the high-dose dexamethasone combination in newly diagnosed myeloma [Citation28]. More recently, in a randomized phase III study of lenalidomide-dexamethasone treatment for elderly MM patients, LaRocca et al. assessed the efficacy and feasibility of a dose/schedule-adjusted regimen with or without dexamethasone [Citation37]. Enrolled patients received 20 mg dexamethasone on days 1, 8, 15, and 22 until progression or intolerance. They reported that early interruption of dexamethasone, and lenalidomide dose reduction to 10 mg as maintenance after the initial nine cycles, did not affect the efficacy of this regimen [Citation37]. Additionally, in this elderly cohort, 31% of patients required a reduction in dexamethasone dose due to adverse events, further confirming tolerability issues, which may also negatively impact treatment adherence.
Conclusion
With few exceptions, dexamethasone has been included in combination therapies for patients from first-line therapy and through the relapsed-refractory setting. While resistance has been documented, the ongoing inclusion of dexamethasone persists as it continues to deliver good response rates, due to its activity and non-overlapping toxicity toxicity profile with other therapies. In addition to demonstrated anti-myeloma activity, dexamethasone may also mitigate some side effects, such as nausea and anorexia, seen with other synergistic myeloma therapy. However, it is important to note that dexamethasone also has its own toxicity profile, including hyperglycemia, immunosuppression, insomnia, and agitation/anxiety.
Future studies addressing dexamethasone resistance, and potentially sparing dexamethasone in later line therapies are both lacking and warranted.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sonneveld P, Broijl A. Treatment of relapsed and refractory multiple myeloma. Haematologica. 2016;101(8):995.
- Sudalagunta P, Silva MC, Canevarolo RR, et al. A pharmacodynamic model of clinical synergy in multiple myeloma. EBioMedicine. 2020;54:102716.
- Papadopoulos KP, Siegel DS, Vesole DH, et al. Phase I study of 30-minute infusion of carfilzomib as single agent or in combination with low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. 2015;33(7):732–739.
- Hassan Zafar M, Khan A, Aggarwal S, et al. Efficacy and tolerability of bortezomib and dexamethasone in newly diagnosed multiple myeloma. South Asian J Cancer. 2018;7(1):58–60.
- Moreau P, Chanan-Khan A, Roberts AW, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130(22):2392–2400.
- Burki TK. Selinexor and dexamethasone in multiple myeloma. Lancet Oncol. 2018;19(3):e146.
- Arth GE, Fried J, Johnston DBR, et al. 16-Methylated steroids. II. 16α-methyl analogs of cortisone, a new group of anti-inflammatory steroids. 9α-halo derivatives. J Am Chem Soc. 1958;80(12):3161–3163.
- Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14(18):2314–2329.
- Rowland TL, McHugh SM, Deighton J, et al. Selective down-regulation of T cell- and non-T cell-derived tumour necrosis factor alpha by thalidomide: comparisons with dexamethasone. Immunol Lett. 1999;68(2–3):325–332.
- Scheinman RI, Gualberto A, Jewell CM, et al. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15(2):943–953.
- Tao Y, Williams-Skipp C, Scheinman RI. Mapping of glucocorticoid receptor DNA binding domain surfaces contributing to transrepression of NFkappa B and induction of apoptosis. J Biol Chem. 2001;276(4):2329–2332.
- Helmberg A, Auphan N, Caelles C, et al. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. Embo J. 1995;14(3):452–460.
- Bladh LG, Liden J, Dahlman-Wright K, et al. Identification of endogenous glucocorticoid repressed genes differentially regulated by a glucocorticoid receptor mutant able to separate between nuclear factor-kappaB and activator protein-1 repression. Mol Pharmacol. 2005;67(3):815–826.
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107(2):135–142.
- Richardson PG, Wolf J, Jakubowiak A, et al. Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: results of a multicenter phase I/II trial. J Clin Oncol. 2011;29(32):4243–4249.
- Jagannath S, Richardson PG, Barlogie B, SUMMIT/CREST Investigators, et al. Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica. 2006;91(7):929–934.
- Chauhan D, Tian Z, Zhou B, et al. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin Cancer Res. 2011;17(16):5311–5321.
- Garcia-Gomez AD, Quwaider M, Canavese EM, et al. Preclinical activity of the oral proteasome inhibitor MLN9708 in myeloma bone disease. Clin Cancer Res. 2014;20(6):1542–1554.
- Xu J, Pfarr N, Endris V, et al. Molecular signaling in multiple myeloma: association of RAS/RAF mutations and MEK/ERK pathway activation. Oncogenesis. 2017;6(5):e337.
- Dehghanifard A, Kaviani S, Abroun S, et al. Various signaling pathways in multiple myeloma cells and effects of treatment on these pathways. Clin Lymphoma Myeloma Leuk. 2018;18(5):311–320.
- Chauhan D, Hideshima T, Pandey P, et al. RAFTK/PYK2-dependent and -independent apoptosis in multiple myeloma cells. Oncogene. 1999;18(48):6733–6740.
- Burwick N, Sharma S. Glucocorticoids in multiple myeloma: past, present, and future. Ann Hematol. 2019;98(1):19–28.
- Bao L, Ruan GR, Lu XJ, et al. Abnormal expression of programmed cell death 5 gene in multiple myeloma patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(3):634–637.
- Liu J, Li X, Gui R, et al. Effect of human recombinant PDCD5 protein on cell apoptosis of multiple myeloma KM3 cells induced by dexamethasone and its mechanism. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35(7):725–731.
- Cheng Q, Liu L, Fu Y, et al. RhPDCD5 combined with dexamethasone increases antitumor activity in multiple myeloma partially via inhibiting the WNT signalling pathway. Clin Exp Pharmacol Physiol. 2018;45(2):140–145.
- Faiman B, Bilotti E, Mangan PA, IMF Nurse Leadership Board, et al. Steroid-associated side effects in patients with multiple myeloma: consensus statement of the IMF nurse leadership board. Clin J Oncol Nurs. 2008;12(3 Suppl):53–63.
- National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. NIH publication 09-7473. Published May 29, 2009; Revised June 14, 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf Accessed 09 September 2022
- Rajkumar SV, Jacobus S, Callander NS, Eastern Cooperative Oncology Group, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37.
- Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107–113.
- Shustik C, Belch A, Robinson S, et al. A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7. Br J Haematol. 2007;136(2):203–211.
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s.
- Palumbo A, Rajkumar SV, Dimopoulos MA, International Myeloma Working Group, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22(2):414–423.
- Brown ES, Chandler PA. Mood and cognitive changes during systemic corticosteroid therapy. Prim Care Companion J Clin Psychiatry. 2001;3(1):17–21.
- Delforge M, Ludwig H. How I manage the toxicities of myeloma drugs. Blood. 2017;129(17):2359–2367.
- Bertolotti P, Bilotti E, Colson K, et al. Management of side effects of novel therapies for multiple myeloma: consensus statements developed by the international myeloma foundation’s nurse leadership board. Clin J Oncol Nurs. 2008;12(3 Suppl):9–12.
- King T, Faiman B. Steroid-Associated side effects: a symptom management update on multiple myeloma treatment. Clin J Oncol Nurs. 2017;21(2):240–249.
- Larocca A, Bonello F, Gaidano G, et al. Dose/schedule-adjusted Rd-R vs continuous Rd for elderly, intermediate-fit patients with newly diagnosed multiple myeloma. Blood. 2021;137(22):3027–3036.
- Alexanian R, Dimopoulos MA, Delasalle K, et al. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80(4):887–890.
- Dimopoulos MA, Oriol A, Nahi H, POLLUX Investigators, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331.
- Wandler AM, Huang BJ, Craig JW, et al. Loss of glucocorticoid receptor expression mediates in vivo dexamethasone resistance in T-cell acute lymphoblastic leukemia. Leukemia. 2020;34(8):2025–2037.
- Yang Y, Li F, Saha MN, et al. miR-137 and miR-197 induce apoptosis and suppress tumorigenicity by targeting MCL-1 in multiple myeloma. Clin Cancer Res. 2015;21(10):2399–2411.
- Wang X, Li C, Ju S, et al. Myeloma cell adhesion to bone marrow stromal cells confers drug resistance by microRNA-21 up-regulation. Leuk Lymphoma. 2011;52(10):1991–1998.
- Gazitt Y, Fey V, Thomas C, et al. Bcl-2 overexpression is associated with resistance to dexamethasone, but not melphalan, in multiple myeloma cells. Int J Oncol. 1998;13(2):397–405.
- Chen Y, Li R, Zhu Y, et al. Dihydroartemisinin induces growth arrest and overcomes dexamethasone resistance in multiple myeloma. Front Oncol. 2020;10(767), https://doi.org/10.3389/fonc.2020.00767
- Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol. 2010;28(30):4630–4634.
- Tamura H, Ishibashi M, Yamashita T, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27(2):464–472.
- Nojima M, Maruyama R, Yasui H, et al. Genomic screening for genes silenced by DNA methylation revealed an association between RASD1 inactivation and dexamethasone resistance in multiple myeloma. Clin Cancer Res. 2009;15(13):4356–4364.
- Keats JJ, Fonseca R, Chesi M, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12(2):131–144.
- Bassan F, Peter F, Houbre B, et al. Adherence to oral antineoplastic agents by cancer patients: definition and literature review. Eur J Cancer Care. 2014;23(1):22–35.
- Sweiss K, Wirth SM, Sharp L, et al. Collaborative Physician-Pharmacist-Managed multiple myeloma clinic improves guideline adherence and prevents treatment delays. J Oncol Pract. 2018;14(11):e674–e682.
- Bashir Q, Acosta M. Comparative safety, bioavailability, and pharmacokinetics of oral dexamethasone, 4-mg and 20-mg tablets, in healthy volunteers under fasting and fed conditions: a randomized open-label, 3-way crossover study. Clin Lymphoma Myeloma Leuk. 2020;20(11):768–773.