Abstract
Myelodysplastic syndromes (MDS) are a heterogeneous group of diseases without a care standard and show variability in treatment outcomes. This Spanish, observational, prospective study ERASME (CEL-SMD-2012-01) assessed the evolution of newly diagnosed and treatment-naïve high-risk MDS patients (according to IPPS-R). 204 patients were included: median age 73.0 years, 54.4% males, 69.6% 0-1 ECOG, and 94.6% with comorbidities. Active treatment was the most common strategy (52.0%) vs. stem cell transplantation (25.5%) and supportive care/watchful-waiting (22.5%). Overall (median) event-free survival was 7.9 months (9.1, 8.3, and 5.3); progression-free survival: 10.1 months (12.9, 12.8, and 4.3); and overall survival: 13.8 months (15.4, 14.9; 8.4), respectively, with significant differences among groups. Adverse events (AEs) of ≥3 grade were reported in 72.6% of patients; serious AEs reported in 60.6%. 33.1% of patients died due to AEs. Three patients developed second primary malignant neoplasms (median: 8.2 months). Our study showed better outcomes in patients receiving active therapy early after diagnosis.
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of hematologic neoplasms characterized by ineffective hematopoiesis and an increased risk of progression to acute myeloid leukemia (AML) [Citation1–3].
MDS are common in older adults (average age 70 years) [Citation1,Citation4], with predominance in men (2:1) [Citation3]. Furthermore, advanced age and common aging-related comorbidities make therapeutic decisions difficult [Citation5].
The complex molecular and genetic pathogenesis of MDS entails remarkably heterogeneous clinical outcomes, with life expectancy ranging from a few months to several years. Therefore, evaluation becomes paramount for establishing an accurate prognosis and offering the best treatment option to these patients. The International Prognostic Scoring System (IPSS) [Citation6] and the Revised International Prognostic Scoring System (IPSS-R) [Citation7] are the most common prognostic scores employed and divide MDS patients into four and five groups of risk, respectively [Citation5]. However, in clinical practice, MDS patients are usually divided into two group: lower-risk and higher-risk.
On the one hand, lower risk MDS patients (LR-MDS; IPSS low or intermediate-1 risk and IPSS-R ≤ 3.5 points) [Citation5] have an overall survival (OS) >30 months [Citation8], and the gold standard is the conservative approach with erythropoiesis-stimulating agents (ESAs) and blood transfusions [Citation5,Citation9]. On the other hand [Citation5], the higher-risk MDS patients (HR-MDS; IPSS high or intermediate-2 risk and IPSS-R > 3.5 points) show an OS <30 months [Citation8] and an increased risk of progression to AML. Therefore, intensive treatment with allogeneic hematopoietic stem cell transplantation (HSCT) is desirable [Citation5,Citation9]. HSCT should be the first strategy considered in fit or ≤70 years patients with HR-MDS, according to the guidelines of the Spanish Group of Myelodysplastic Syndromes (Grupo Español de Síndromes Mielodisplásicos or GESMD by its Spanish acronym) [Citation5] and the guidelines of the main international societies (i.e. European Society for Medical Oncology) [Citation10]. Despite this, there is no time limit for HSCT, and depends on the center’s policy and the identification of a donor. Unfit and/or older HR-MDS patients are considered for treatment with hypomethylating agents or new agents under investigation [Citation11–14].
Despite the current MDS treatment landscape, which includes new options, there is no standard of care for the majority of patients (non-eligible for approved therapies or in relapse settings) [Citation15]. Real-world data could help determine predictive response factors or the approaches with the best outcomes. However, there are limited real-world data on treatment strategies available for HR MDS/chronic myelomonocytic leukemia patients [Citation16].
The ERASME study aimed to assess, in real-world practice, the outcome of newly diagnosed patients with MDS (low-risk or high-risk) or chronic myelomonocytic leukemia (CMML) based on the initial therapeutic strategy adopted by the physician. Here, we present data on HR-MDS patients.
Methods
Study design
The ERASME study (CEL-SMD-2012-01) was an observational, post-authorization, prospective study conducted in 53 Spanish hospitals. The aim was to assess the clinical outcome of newly diagnosed patients with MDS or CMML (intention to treat [ITT] analysis) according to the start of the active treatment. The minimum follow-up period lasted 36 months from patient inclusion in the study.
The ethics committee at each participating center approved the study protocol. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients provided written informed consent.
Patients
The ERASME study included patients between 2013–2019 who were ≥18 years old with a recent (<3 months) diagnosis of MDS or CMML, according to WHO 2008 [Citation17], and who were treatment-naïve. Patients were excluded if they were participating in an interventional clinical trial (inclusion of patients participating in other observational studies was allowed) and if the patients themselves did not consent to participate in this study.
The inclusion of patients was stratified into: 1) Group 1 (LR-MDS), consisting of MDS patients with low or intermediate-1 risk, according to IPSS (5); 2) Group 2 (HR-MDS), consisting of MDS patients with intermediate-2 or high risk, according to IPSS; and 3) Group 3 (CMML), CMML patients belonging to any risk group, according to the CMML Prognostic Scoring System (CPSS) [Citation18].
To ensure correct sample stratification, patients were included consecutively, and a centralized register of the patients included in each group was maintained in real-time.
Our prospective analysis focused on patients with high HR-MDS or CMML.
Study treatment
At diagnosis, the physician decided whether the patient should be considered as a candidate for transplantation. Patients received treatment according to the protocols of the participating centers. Treatment options were classified into 1) active therapy in transplant-ineligible patients (azacitidine, immunomodulatory drugs, or chemotherapy, at the discretion of the physician); 2) HSCT (with/without prior treatment) in transplant-eligible patients (treatments administered prior to transplantation were considered to be active treatments in a transplantation strategy); and 3) supportive care (blood transfusions, chelation therapy, erythropoiesis-stimulating agents, granulocyte colony-stimulating factors, thrombopoietin analogues) or watchful-waiting (WW) strategy.
Study procedures
All evaluations were performed following the protocols of the participating centers. The following evaluations were included in the study: complete medical history, ECOG performance status (PS), Cumulative Illnes Rating Scale-Geriatric (CIRS-G).
Scale-Geriatric (CIRS-G), MDS-specific comorbidity index (MDS-CI), hematology tests, and tumor response (assessed in accordance with the 2006 IMWG criteria) [Citation19].
According to standard clinical practice, data were collected every 3 months or whenever an event occurred for patients on active treatment; while data were collected every 6 months or whenever an event occurred for patients on support treatment.
Adverse events (AEs) and concomitant medication were collected throughout the study. AEs were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTC-AE), version 4.0.
Sample size
It was planned to include approximately 138 patients with HR-MDS, among which it had been estimated that approximately 50% would be eligible for active treatment.
Statistical analysis
The primary endpoint was event-free survival (EFS), defined as the time elapsed between the baseline visit and the appearance of one of the following events: change in the initial clinical decision of the therapeutic plan (adoption of a new therapeutic strategy), adverse effects, disease progression, or death. Therefore, EFS will be calculated from the baseline visit or start of treatment until the occurrence of the first event in the successive visits. Secondary endpoints included the following: time to progression (TTP), time to AML, progression-free survival (PFS), and overall survival (OS). All these time-to-event variates were calculated using the Kaplan-Meier method, the log-rank test was used for statistical comparison, and Cox proportional-hazards model was used for multivariate analyses and to obtain hazard ratios (HR) with 95% confidence intervals (CI) (modifying covariates to EFS: gender, ECOG, therapeutic strategy, and hemoglobin values; modifying covariates to PFS and OS: gender, therapeutic strategy, and hemoglobin and platelets values).
To balance differences in baseline patient characteristics and thus reduce possible bias, a propensity-score matching analysis was conducted and subsequently used as an adjustment variable in the Cox analysis. Profensity-score was estimated for each patient using a logistic regression model using the same variables included in the Cox model.
Follow-up data on the patients who died were censored at the date of their death, but data on surviving patients were not censored. The progress of analytical parameters was assessed by means of statistical tests for repeated measures. Safety was evaluated according to the frequency, incidence, and severity of the AEs.
The different study groups were considered as intention to treat (ITT), and specifically, those patients candidates for transplant were analyzed in the transplant group even if they did not receive the transplant
Data analyses were performed using the SAS® statistical package for Windows (v. 9.4, SAS Institute Inc., Cary, the USA).
Results
The database close was on 24th may 2019.
Patients characteristics and distribution
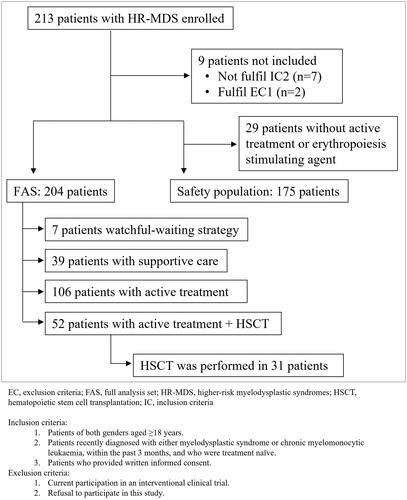
A total of 213 patients with HR-MDS were enrolled, 204 patients from 53 centers were included in the full analysis set (FAS), and 175 patients were included in the safety population (). The center that recruited the most patients included 36 patients and 36 centers enrolled >2 patients.
The most frequent therapeutic strategy was active treatment (52.0%), followed by intention to HSCT (25.5%), supportive care (19.1%), and WW strategy (3.4%) ().
Characteristics of HR-MDS patients are shown in . The age (median) of patients in the HSCT group was lower than that of patients on active treatment (59.0 vs. 75.0 years old, p < 0.0001). In turn, the age of patients on active treatment was lower than those under supportive care/WW strategy (75.0 vs. 80.0 years old, p = 0.0010). The risk IPSS categories were similar across treatment groups (). Approximately half of all patients had IPSS intermediate-2-risk (50.9% for active treatment, 55.8% for HSCT, and 50.0% for supportive care/WW strategy), whereas the other half had IPSS high-risk (48.1%, 42.3%, and 50.0%, respectively). According to IPSS-R, 40.2% of HR-MDS patients were in the poor or very poor category of cytogenetic risk ().
Table 1. Patient baseline demographics and disease characteristics.
Treatment
Preferred treatment strategies were considered based on the IPSS risk classification (87.7%), age (78.4%), symptomatology (54.4%), and comorbidities (52.5%).
Patients on active therapy received mainly azacitidine (88.7%). Chemotherapy drugs were used by 9.4% of patients (7 + 3 induction in most cases, with a median [interquartile range, IQR] of 1.0 [1.0–2.0] cycles), and immunomodulators (lenalidomide) were used by 0.9% (n = 2) of patients.
Azacitidine was mostly administered (91.1% [n = 82] of patients) at a median (IQR) dose of 75.0 (74.0–75.0) mg/m2 per day for 7 days, every 28 days. Eight patients (8.9%) received it for 5 days, every 28 days. The median (IQR) number of cycles was 5.0 (3.0–10.0). The dose was changed once, twice, and three times in 29, 13, and 4 patients, respectively.
In the HSCT group (n = 52), only 3 (5.8%) patients received a direct transplant. Forty-nine (94.2%) patients received active treatment before HSCT. The most common treatment administered before HSCT was azacitidine (55.8%), followed by chemotherapy drugs (34.6%). At the end of this study, 31 (59.6%) patients had received HSCT from those who were eligible to receive it. Seventeen patients (32.7%) died before HSCT, the median (IQR) day from the date of diagnosis being 169 (94.0–306.0), and 4 (7.7%) were waiting for it at the time of the last follow-up.
In the supportive care group, the main supportive therapies were red blood cell transfusion (82.1%), ESAs (33.3%), and platelet transfusion (30.8%). Eleven patients (23.9%) under supportive care/WW strategy switched to active treatment after a median (IQR) period of 120.0 (70.0–210.0) days.
Effectiveness
Death was the main reason for discontinuing the study (71.1%, n = 145). Loss of follow-up was reported in 29 (14.2%) patients, and 26 (12.7%) patients ended it according to the protocol. The median (IQR) follow-up time for survivors was 18.7 (3.7–36.7) months.
Treatment effectiveness, including OS, DFS, and PFS results, are summarized in . In the active treatment group, the main responses (>10% of patients) were complete response (CR, 20.8%), stable disease (SD, 17.9%), and progressive disease (PD, 15.1%). However, in a large proportion of patients under supportive care/WW strategy (67.4%), the best overall response was not assessed ().
Table 2. Main effectiveness results.
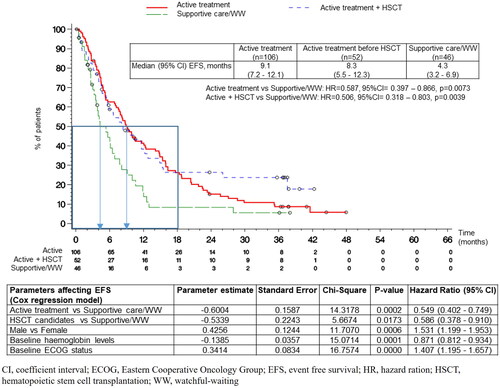
The median (95% CI) EFS was 7.9 (6.1-9.5) months, with significant differences observed among treatment groups (log-rank p = 0.0069) (). The longest EFS was reported in the patients on active treatment, followed by HSCT patients, and then by patients under supportive care/WW strategy (9.1 [7.2–12.1], 8.3 [5.5–12.3], and 4.3 [3.2–6.9] months, respectively) (, ). The median (95% CI) EFS for patients under supportive care/WW strategy and who switched to active treatment was 4.0 (2.2–6.9) months, showing significant differences when compared to patients who had been allocated to the active treatment group from the beginning (log-rank p = 0.0002). The COX regression model adjusted by propensity-score and baseline covariates supported the univariate results: the therapeutic strategy affected the EFS (p = 0.0007; active treatment vs. supportive care/WW strategy, HR [95% CI], 0.55 [0.40–0.75], p = 0.0002; HSCT candidates vs. supportive/WW strategy, HR [95% CI], 0.59 [0.38–0.91], p = 0.0173).
The baseline covariates affecting the EFS were gender (male vs. female; p = 0.0006; HR [95% CI], 1.53 [1.20–1.95]), ECOG PS (p < 0.0001; HR [95% CI], 1.41 [1.20–1.66]) and hemoglobin levels (p = 0.0001; HR [95% CI], 0.87 [0.81–0.93]).
Thirty-eight (18.6%) patients showed progress to AML at a median of 5.6 months. The treatment group with the highest progress to AML was the group with active treatment (27.4%), followed by the supportive care/WW strategy (13.0%), and HSCT (5.8%), the time (median) corresponding to this progress was 9, 3.7, and 4 months, respectively (). The COX regression model did not identify any covariate that modified the progress to AML.
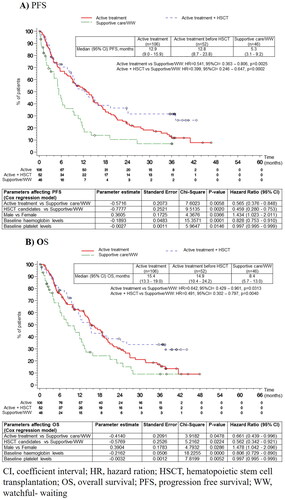
The median PFS was 10.1 (95% CI: 8.7–12.8) months for the whole HR-MDS population, with poor PFS being observed in the supportive care/WW strategy group (). Significant differences were identified between treatment groups (p = 0.0004) ( and ). The COX regression model supported the univariate results: the therapeutic strategy affected PFS (active treatment vs. supportive care/WW strategy, HR [95% CI], 0.57 [0.38-0.85], p = 0.0058; HSCT candidates vs. supportive care/WW strategy, HR [95% CI], 0.46 [0.28–0.75], p = 0.0020). The COX regression model identified gender (male vs. female; p = 0.0366; HR [95% CI], 1.43 [1.02–2.01]), hemoglobin levels (p = 0.0001; HR [95% CI], 0.83 [0.75–0.91]) and platelet levels (p = 0.0146; HR [95% CI], 0.99 [0.99–1.00]) as modifying covariates.
The median OS was 13.8 (11.8–15.9) months for the whole HR-MDS population, with poor OS observed in the supportive care/WW strategy group. There were also significant differences between treatment groups (p = 0.0119) ( and ).
Contrary to what has been observed with EFS, early active treatment did not lead to favorable OS, compared to patients under supportive care/WW strategy who switched to active treatment (median [95% CI]: 15.4 [13.3–19.0] vs. 15.9 [7.3-24.9], respectively; log-rank p = 0.5047). The COX regression model supported the univariate results: the therapeutic strategy affected OS (active treatment vs. supportive care/WW strategy, HR [95% CI] 0.66 [0.44–1.00], p = 0.0478; HSCT candidates vs. supportive care/WW strategy, HR [95% CI] 0.56 [0.34–0.92], p = 0.0224). The COX regression model identified gender (male vs. female; p = 0.0286; HR [95% CI], 1.48 [1.04–2.10]), hemoglobin levels (p < 0.0001; HR [95% CI], 0.81 [0.73–0.89]) and platelets levels (p = 0.0052; HR [95% CI], 0.99 [0.99–1.00]) as modifying covariates.
Safety
summarizes the incidence of AEs during the study, both in the whole safety population and by the strategy selected. A total of 1,173 AEs were reported, with 90.3% patients suffering ≥1 AE and 72.6% suffering any AE of grade ≥3 AE. At least one serious AE (SAE) was reported in 60.6% of patients, and 33.1% died due to an AE ().
Table 3. Summary of adverse events in the safety population.
The most frequently reported AEs were asthenia (26.9%), febrile neutropenia (22.9%), pyrexia (22.3%), thrombocytopenia (18.9%), anemia (17.7%), and neutropenia (17.1%) ().
The comparison made at each visit with regard to the previous one showed that 53.4% (n = 109) patients experienced a worsening comorbidity, 51.5% (n = 105) developed new symptomatology, and 42.2% (n = 86) developed a new comorbidity. The treatment group with the lowest percentage of patients with these events (comorbidity worsening, new symptomatology, and new comorbidity, respectively) was the HSCT group (28.8%, 42.3%, and 30.8%), compared with the group including active treatment patients (60.4%, 53.8%, and 48.1%,) and supportive care/WW strategy patients (65.2%, 56.5%, and 41.3%).
The main AEs affecting comorbidities (>10% of patients) were anemia (n = 92, 45.1%), infections (n = 68, 33.3%), bruises/bleeding (n = 38, 18.6%), and pneumopathies (n = 28, 13.7%).
By the end, only 3 (1.5%) patients, one in each treatment group, had developed second primary malignant neoplasms at a median (IQR) of 8.2 (1.1–15.2) months.
Discussion
This real-world study showed that HR-MDS patients were managed mainly with active treatment (mostly azacitidine). The treatment decision was based on risk classification according to age, symptomatology, and comorbidities, following the main guidelines for MDS patient management [Citation5,Citation10].
This study also found that those HR-MDS patients selected to receive active therapy or a transplant had a better EFS and OS than those who received supportive therapy/WW strategy. However, the outcome for transplanted patients is not better than the overall outcome, probably due to the short follow-up period (recent publications have suggested that transplant benefits start after two years from the procedure [Citation20]) or to the small sample size (n = 31), which probably makes it more difficult to find differences. It should also be noted that only 60% of the patients initially considered candidates for transplant received it; this probably explains the absence of benefit compared to active treatment without transplantation. Two recent studies have used biological randomization to compare the role of transplantation in higher-risk MDS patients; patients who have a donor underwent allogeneic stem cell transplantation, whereas those without a donor received the best available therapy (mainly hypomethylating agents) [Citation20,Citation21]. The studies showed higher overall survival for those patients with a donor highlighting the benefit of transplantation in higher-risk MDS patients.
In this study, which compared an early active treatment with supportive care/WW strategy, the early active treatment showed longer EFS (9.1 vs. 4.3 months), and similar findings were observed for PFS (12.9 vs. 5.3 months, respectively) and OS (15.4 vs. 8.4 months, respectively).
Regarding the OS data, the values of the results are higher than those recorded in the register of the Spanish Group of Myelodysplastic Syndromes (GESMD), reported in 2005 by Bernal et al. [Citation22], which showed a value as low as 13.4 months of OS in patients receiving azacitidine. This value is similar to the one reported with conventional treatment (12.2 months). However, the median OS observed in the HR-MDS patients of this study who received active treatment (15.4 months) is in line with recent records and with real-life studies on patients treated with active treatment (with OS ranging between 12 and 20 months) [Citation22–26].
Comparing the OS of this study (real-world data) with the OS reported in a previous phase 3, multicentre, open-label trial with azacitidine (AZA-001) (24.5 months) [Citation27], the OS value showed in this study is lower, as observed in subsequent studies with azacitidine [Citation28]. This may be attributable to differences in the design of the studies, and it reflects differences between the patients selected for clinical trials (according to relatively stringent inclusion criteria) and those treated in the real world (in which fewer patients are selected). For instance, this study included more patients with unfavorable features than the AZA-001 trial did, such as the poor/very poor cytogenetic risk categories (37% vs. 28%) and advanced age (75 vs. 69 years) [Citation27].
Although we did not observe a favorable OS with early active treatment compared to switching to active treatment from the supportive care/WW strategy, a better prognosis for survival would be expected if treatment were administered as early as possible, as with transplantation [Citation29].
Overall, a small number of HR-MDS patients progressed to AML. The percentage of patients showing progression to AML in the active treatment group (27.4%) was similar to that observed in the records of the GESMD for patients on azacitidine (26%) [Citation22]. The median AML progression time was shorter than the AML progression time reported in the AZA-001 trial (9.0 vs. 17.8 months) [Citation27]. This difference may be explained by the heterogeneity in the patients’ characteristics mentioned above.
Moreover, it was found that the following covariates affected EFS, PFS, and OS: gender, hemoglobin levels, and treatment. Furthermore, the ECOG PS affected EFS, and platelet levels affected PFS and OS. Thus, it is important to pay more attention to these parameters with respect to newly diagnosed HR-MDS patients.
Lastly, regarding the best haematological response, the percentage of patients on active treatment with a CR (21%) in this study was similar to that observed in other records, and real-life studies on patients treated with azacitidine (ranging between 12% and 17%) [Citation23–25], and in the AZA-001 trial (17%) [Citation27].
Limitations
One of the limitations of this study is its observational design (Spanish real-world practice), which involves clear decision-making on the treatment to be given, which may lead to a selection bias. Additionally, it entails either a high number of missing values for certain parameters or some missing data (e.g. quality of life), as well as difficulty in extrapolating data on populations from other locations due to the heterogeneity of the clinical practice and patient populations.
When the study was initiated, NGS techniques were unavailable in most centers. Therefore, no molecular data are available, and it is not possible to know whether there were high-risk mutations that could have influenced treatment decision-making.
Finally, the fact that some patients under supportive care/WW strategy switched to active treatment could have biased the results of EFS, PFS, AML progression time, and OS of the former group of patients.
General conclusion
This study highlights the importance of initiating active treatment in newly diagnosed intermediate-2/high risk MDS patients. This treatment group showed results with higher EFS, PFS, and OS values than those related to patients under another therapeutic strategy within the same risk group. Ultimately, selecting an active treatment for HR-MDS patients had a protective effect against the risk of events, disease progression, and death.
Author contributions
D.V. conceived and designed the study, and contributed to the quality control and interpretation of the data, data collection, and drafting and revision of the manuscript. R.L. and M.R. conceived and designed the study, and contributed to the interpretation of the data and revision of the manuscript. The remaining authors participated in the data collection and manuscript review. All authors have read and agreed to the final published version of the manuscript.
Acknowledgements
The authors wish to thank all the participating Investigators and hospitals of the ERASME Study: Hospital Virgen de la Victoria, Hospital Puerta del Mar, Hospital de Antequera, Hospital Nuestra Señora De Valme, Hospital Virgen de la Macarena, Hospital Punta de Europa, Hospital Costa del Sol, Hospital Universitario Reina Sofía de Córdoba, Hospital Universitario Torrecardenas, Hospital Universitario Miguel Servet, Hospital Clínico Universitario Lozano Blesa, leoHospital Universitario Central de Asturias, Hospital Universitario Son Llàtzer, Hospital Can Misses, Hospital Universitario Insular de Gran Canaria, Hospital Universitario de Gran Canaria Doctor Negrín, Hospital Universitario Marqués de Valdecilla, Complejo Hospitalario La Mancha Centro, Hospital General Nuestra Señora del Prado, Hospital Universitario Río Hortega de Valladolid, Hospital Clínico Universitario de Valladolid, Hospital Santa Bárbara, Hospital General de Segovia, Hospital Clínico Universitario de Salamanca, Hospital Universitario de León, Hospital Duran y Reynals, Hospital Universitari Vall d‘ Hebron, Hospital de la Santa Creu i Sant Pau, Hospital Universitari Dr. Josep Trueta de Girona, Hospital Universitario Germans Trias i Pujol, Complejo Hospitalario Universitario de Santiago de Compostela, Complejo Hospitalario de Pontevedra, Hospital Universitario de La Ribera, Hospital Universitario Lucus Augusti, Complejo Hospitalario de Ourense, Complejo Hospitalario de Navarra, Hospital Universitario Clínico San Carlos, Hospital Universitario Ramón y Cajal, Hospital Universitario Infanta Leonor, Hospital Universitario de Getafe, Hospital Universitario Fundación Jiménez Díaz, Hospital Universitario Fundación Alcorcón, Hospital General Universitario Santa Lucía, Hospital Clínico Universitario Virgen de la Arrixaca-IMIB, Hospital Universitario Donostia, Hospital Txagorritxu, Hospital Arnau de Vilanova, Hospital Universitario Doctor Peset, Hospital Clínico Universitario de Valencia, Hospital Universitario y Politécnico La Fe, Hospital del Mar, Mutua de Terrassa, Hospital Clínic de Barcelona. Writing assistance was supported by Celgene S.L.U., a Bristol-Myers Squibb Company, and provided by Irene Mansilla, MSc, from TFS Develop.
Disclosure statement
DV has received research support from Celgene, a Bristol-Meyers Squibb Company, has received speaker honorariums and has served on advisory boards for Celgene, Amgen, Novartis, Jazz Pharmaceuticals, Janssen, Sanofi and Pfizer, has received speaker honorariums from MSD and Astellas, and has served on advisory boards for Sobi. M.J.M has received research support from Celgene. MT has received speaker honorariums and has served on advisory boards for Celgene, Pfizer, Novartis; has received speaker honorariums from Janssen, MSD, Daiichi Sanyo, Servier; and has served on advisory boards for Roche and Astellas. M.D.C has received research support, speaker honorariums, and has served on consultancy and advisory boards for Celgene, Novartis, Takeda. F.R. has received research support and traveling fees; and has served on consultancy and advisory boards for Celgene; has received traveling fees and served on consultancies for Novartis, Amgen, Abbvie, Takeda; and received traveling fees from Roche, Rovi, Janssen, MSD and Saiichi Sankyo. E.S.F has received research support and has served on advisory boards for Agios; and he has served on advisory boards for Novartis. H.P., R.C., T.B.C., A.J., S.B., J.C., I.O., R.R., C.P., J.B. and C.M.N. declare they have no conflict of interest. R.L. and M.R.are employees of Celgene, a Bristol-Meyers Squibb Company.
Additional information
Funding
References
- Grupo Español de Síndromes Mielodisplásicos (GESMD), Sociedad Española de Hematología y Hemoterapia (SEHH). Guías españolas de diagnóstico y tratamiento de los síndromes mielodisplásicos y la leucemia mielomonocítica crónica. Haematologica. 2012;97:5–46.
- Rahmé R, Adès L. An update on treatment of higher risk myelodysplastic syndromes. Expert Rev Hematol. 2019;12(1):61–70.
- Rodríguez JH, Acosta I. Actualización en síndromes mielodisplásicos (SMD). Rev. Méd. Rosario. 2011;77:24–41.
- Komrokji RS, Bennett JM. Evolving classifications of the myelodysplastic syndromes. Curr Opin Hematol. 2007;14(2):98–105.
- Grupo Español de Síndromes Mielodisplásicos (GESMD). Guías españolas para el diagnóstico y tratamiento de los SMD y la LMMC. 2020. http://www.gesmd.es/wp-content/uploads/2021/10/GuiaSMDLMMC2020.pdf
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088.
- Voso MT, Fenu S, Latagliata R, et al. Revised international prognostic scoring system (IPSS) predicts survival and leukemic evolution of myelodysplastic syndromes significantly better than IPSS and WHO prognostic scoring system: validation by the gruppo romano mielodisplasie italian regional database. J Clin Oncol. 2013;31(21):2671–2677.
- Valcárcel D, Sanz G, Ortega M, Grupo Español de Síndromes Mielodisplásicos (GESMD), et al. Use of newer prognostic indices for patients with myelodysplastic syndromes in the low and intermediate-1 risk categories: a population-based study. Lancet Haematol. 2015;2(6):e260-266–e266.
- Comont T, Delavigne K, Cougoul P, et al. [Management of myelodysplastic syndromes in 2019: an update]. Rev Med Interne. 2019;40(9):581–589.
- Fenaux P, Haase D, Santini V, ESMO Guidelines Committee. Electronic address: [email protected], et al. Myelodysplastic syndromes: ESMO clinical practice guidelines for diagnosis, treatment and follow-up(†⋆). Ann Oncol. 2021;32(2):142–156.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
- DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216–228.
- Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–1117.
- Platzbecker U, Germing U, Götze KS, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017;18(10):1338–1347.
- Platzbecker U, Kubasch AS, Homer-Bouthiette C, et al. Current challenges and unmet medical needs in myelodysplastic syndromes. Leukemia. 2021;35(8):2182–2198.
- Bell JA, Galaznik A, Huelin R, et al. Systematic literature review of treatment options and clinical outcomes for patients with Higher-Risk myelodysplastic syndromes and chronic myelomonocytic leukemia. Clin Lymphoma Myeloma Leuk. 2018;18(4):e157–e166.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the world health organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951.
- Such E, Cervera J, Costa D, et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica. 2011;96(3):375–383.
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425.
- Kröger N, Sockel K, Wolschke C, et al. Comparison between 5-Azacytidine treatment and allogeneic Stem-Cell transplantation in elderly patients with advanced MDS according to donor availability (VidazaAllo study). J Clin Oncol. 2021;39(30):3318–3327.
- Nakamura R, Saber W, Martens MJ, et al. Biologic assignment trial of Reduced-Intensity hematopoietic cell transplantation based on donor availability in patients 50-75 years of age with advanced myelodysplastic syndrome. J Clin Oncol. 2021;39(30):3328–3339.
- Bernal T, Martínez-Camblor P, Sánchez-García J, Spanish Society of Hematology, et al. Effectiveness of azacitidine in unselected high-risk myelodysplastic syndromes: results from the Spanish registry. Leukemia. 2015;29(9):1875–1881.
- Dinmohamed AG, van Norden Y, Visser O, et al. Effectiveness of azacitidine for the treatment of higher-risk myelodysplastic syndromes in daily practice: results from the dutch population-based PHAROS MDS registry. Leukemia. 2015;29(12):2449–2451.
- Itzykson R, Thépot S, Quesnel B, Groupe Francophone des Myelodysplasies(GFM), et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117(2):403–411.
- Mozessohn L, Cheung MC, Fallahpour S, et al. Azacitidine in the 'real-world’: an evaluation of 1101 higher-risk myelodysplastic syndrome/low blast count acute myeloid leukaemia patients in Ontario, Canada. Br J Haematol. 2018;181(6):803–815.
- Zeidan AM, Stahl M, DeVeaux M, et al. Counseling patients with higher-risk MDS regarding survival with azacitidine therapy: are we using realistic estimates? Blood Cancer J. 2018;8(6):55.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, International Vidaza High-Risk MDS Survival Study Group, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–232.
- Garcia JS, Swords RT, Roboz GJ, et al. A systematic review of higher-risk myelodysplastic syndromes clinical trials to determine the benchmark of azacitidine and explore alternative endpoints for overall survival. Leuk Res. 2021;104:106555.
- Abel GA, Kim HT, Hantel A, et al. Fit older adults with advanced myelodysplastic syndromes: who is most likely to benefit from transplant? Leukemia. 2021;35(4):1166–1175.