Abstract
The prognostic significance of RAS mutations in AML is poorly understood. In this ambispective cohort study of 239 newly-diagnosed AML patients at the University of Maryland, we assessed the median overall survival (mOS) and median event-free survival (mEFS) in RAS wild-type (WT) AML (n = 196), KRAS-mutated AML (n = 11), NRAS-mutated AML (n = 25), and KRAS/NRAS-mutated AML (n = 7). We used propensity score to adjust outcomes. NRAS-mutated AML had a similar response rate to first-line treatment and mOS compared to RAS-WT AML (57 vs. 54%, p = 0.8, 22.7 vs. 14.6 months, p = 0.7). The mOS of KRAS-mutated AML was shorter compared to RAS-WT AML (p = 0.049) and NRAS-mutated AML (p = 0.02). KRAS-mutated AML treated with anthracycline-based first-line regimens had a lower relative mortality compared to treatment with hypomethylating agents with venetoclax (HR <0.01, p = 0.04) and without venetoclax (HR <0.01, p = 0.04). This study demonstrates that KRAS but not NRAS mutations are associated with worse outcomes in AML.
What is the new aspect of your work? The clinical significance of RAS mutations remains poorly defined and prior studies have yielded conflicting results. We used causal inferential methods, propensity score modeling, to determine the impact of KRAS and NRAS mutation on survival in newly diagnosed AML patients, independent of other risk factors. Moreover, we analyzed the outcomes of KRAS and NRAS-mutated AML patients receiving first-line therapy with hypomethylating agents and other non-anthracycline-based regimens. We provided a detailed description of RAS-mutated AML, including co-occurring mutations and cytogenetic abnormalities.
What is the central finding of your work? KRAS mutations but not NRAS mutations in AML are directly linked to worse outcomes even after controlling for differences in AML type, co-occurring cytogenetic changes, treatment regimens, and comorbidities. KRAS-mutated AML has a higher relative mortality when treated with a hypomethylating agent-based first-line induction regimen compared to treatment with an anthracycline-based regimen.
What is (or could be) the specific clinical relevance of your work? Our findings can help refine our genetic profiles of AML, improve prognostic models, and better stratify treatment regimens.
NOVELTY STATEMENT
Introduction
Acute myeloid leukemia (AML) is a heterogeneous hematologic malignancy arising from clonal expansion of malignant myeloid precursors cells with poor outcomes, especially in older patients. Cytogenetic analysis at the time of diagnosis is the most valuable factor in determining prognosis. Using this baseline cytogenetic profile, AML can be stratified into three risk categories: favorable, intermediate, and adverse [Citation1]. These risk categories are used not only to determine prognosis but also to guide treatment decisions. However, current risk stratification models incorporate only a select few mutations, such as FLT3-ITD, RUNX1, ASXL1, TP53, and others [Citation1]. Further research into other genetic alterations would allow for refined genetic profiles of AML, improved prognostic models, and better stratification of treatment regimens.
Mutations of the RAS proto-oncogenes, NRAS and KRAS, are frequently observed in AML. NRAS mutations have been identified in 11% of patients and KRAS mutations in 5% of patients with AML [Citation2]. The RAS genes encode a membrane-localized G protein that is inactive when bound to GDP and is activated by binding to GTP. Gain-of-function mutations cause the RAS-GTPase protein to be locked into the active GTP-bound states. This results in constitutive activation of the mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase (PI3K) pathways, promoting cell proliferation and survival [Citation3,Citation4].
The clinical significance of RAS mutations remains poorly understood, and prior studies have yielded inconsistent results. In two studies, the presence of a RAS mutation was a significant predictor of longer survival when adjusted for age [Citation5,Citation6]. In other studies, RAS mutations had no independent effect on prognosis, or KRAS mutations were associated with worse outcomes, while NRAS mutations had no effect on outcomes [Citation2,Citation4]. Moreover, there is limited data on the impact of RAS mutations on the treatment response of AML to different regimens. Previous research has shown that the presence of a RAS mutation is associated with a greater benefit from high-dose cytarabine (HiDAC) consolidation therapy, including a lower incidence of relapse and prolonged survival. The proposed mechanism for this improved response to HiDAC is based on the direct effects of RAS mutations on the cell cycle. In vitro studies have shown that AML cells often arrest in S-phase when exposed to cytarabine. However, cells with RAS mutations are less likely to undergo S-phase arrest in response to cytarabine resulting in apoptotic death [Citation7,Citation8]. Prior research has not individually evaluated the prognostic impact of NRAS and KRAS mutations in patients receiving hypomethylating agent-based regimens. In this context, we performed a propensity score-adjusted ambispective cohort study to determine the impact of NRAS and KRAS mutations on outcomes of patients with newly diagnosed AML.
Methods
Study design
This was a single-center ambispective cohort study conducted at the University of Maryland Greenebaum Comprehensive Cancer Center (UMGCCC), as previously described [Citation9–11]. The study was reviewed and approved by the University of Maryland Baltimore Institutional Review Board. The aim was to compare overall survival (OS), event-free survival (EFS), complete remission (CR), and complete remission with incomplete hematologic recovery (CRi) rates in patients with AML with and without NRAS and/or KRAS mutations. EFS was defined from time of treatment initiation to induction failure, relapse, or death. OS was defined from time of AML diagnosis to time of death. The 2022 European LeukemiaNet (ELN) criteria were used to define treatment responses. [Citation1] Both CR and CRi were included in composite CR (CRc).
Data source
Data was collected on all consecutive newly diagnosed AML patients treated as inpatients or outpatients at UMGCCC. No AML patients were excluded from the study. The study period was between 2013 and 2020. We utilized both Care Everywhere, a feature of Epic electronic health record, and CRISP to minimize missing data. CRISP is a state-designated Health Information Exchange for Maryland and the District of Columbia, which facilitates the sharing of health information from doctors’ offices, clinical laboratories, radiology centers, hospitals, and other healthcare organizations online to extract relevant data from other clinical sites [Citation12]. All data were stored in the REDCap electronic data capture tool. Independent investigators checked data accuracy [Citation13].
Variables and comparison groups
The following data was extracted: age at diagnosis, gender, ethnicity, performance status, comorbidities, AML type (de novo AML, myelodysplasia-related (MDS) AML, myeloproliferative-related (MPN) AML, therapy-related AML), karyotype, and treatments administered. First-line treatment regimens were categorized as anthracycline-based and non-anthracycline-based regimens. Non-anthracycline-based regimens included treatment with hypomethylating agents (HMAs) with venetoclax, HMAs without venetoclax, and treatment with other agents. Data were also collected on FLT3-ITD, FLT-TKD, IDH1/2, ASXL1, RUNX1, and TP53 mutational status. RAS mutational status was determined using myeloid panel next-generation sequencing. The study only included patients who had myeloid panel results at the time of diagnosis. We compared patients with wild-type (WT) KRAS/NRAS (WT AML), WT NRAS and mutated KRAS (KRAS-mutated AML), mutated NRAS and WT KRAS (NRAS-mutated AML), and mutated KRAS and NRAS AML (KRAS/NRAS-mutated AML).
Propensity score estimation
Propensity score weighting was used to adjust for baseline characteristics, as previously described [Citation9–11]. The baseline characteristics used in the propensity score model included: age, ethnicity, gender, performance status, comorbidities, AML type, Eastern Cooperative Oncology Group (ECOG) performance status, first-line treatment administered, cytogenetic risk group, and mutational status of FLT3-ITD, ASXL1, RUNX1, and TP53. We obtained the average treatment effect (ATE) or the average treatment effect on the treated (ATT), as permitted. To estimate weights, we used multinomial regression or generalized boosted modeling. The following criteria were used to choose the estimand (ATE vs. ATT) and weighting methods: (1) achieving the lowest standardized biases differences, (2) smallest coefficients of variations, and (3) largest estimated sample size. The standardized bias score of <0.25 was used as a cutoff [Citation14]. Both love plots and balance tables were used to confirm adequate balancing after weighting. To account for within-person homogeneity, we used robust variance estimator [Citation15].
Statistical analysis
We used descriptive statistics to compare baseline characteristics of patients with WT KRAS/NRAS, isolated NRAS mutation, isolated KRAS mutation, and dual KRAS/NRAS mutations. Continuous variables were presented as averages with standard deviation (SD) and medians with interquartile ranges (IQR) and compared using t-test or ANOVA test. Categorical variables were presented as absolute numbers with percentages and compared using Pearson chi-square or Fisher’s exact tests. OS and EFS were compared using Gehan Breslow-Wilcoxon rank and log-rank tests. Moreover, weighted Cox proportional hazards models were used to assess OS and EFS in the comparison groups. Hazard ratios (HR) were presented with corresponding 95% confidence intervals (CI). We used regression diagnostics to assess model assumptions. All statistical tests were two-sided, and p-values <0.05 were considered statistically significant. The R statistical package ‘WeighIt’ was used for weighting [Citation16]. We used R-statistical software (version 4.1.1) for statistical analyses.
Results
Cohort characteristics
A database of 467 AML patients treated at UMGCCC was analyzed and 239 patients with pretreatment next-generation sequencing were included in our cohort. The median age of the cohort was 65.2 years [IQR 56.2–74.1], and 41% were female. Median follow-up was 30.0 months (CI: 19.1–46.9). 196 patients (82%) had RAS WT AML, 11 patients (4.6%) had KRAS-mutated AML, 25 patients (10.5%) had NRAS-mutated AML, and 7 patients (2.9%) had KRAS/NRAS-mutated AML. The median follow-up was 28.4 months (CI: 18.3–50.3) for RAS WT AML and 34.4 months (CI: 21.1–39.9) for RAS-mutated AML. 41% of patients received an anthracycline-based first-line treatment, 54% received a non-anthracycline-based regimen, and 4% received no first-line treatment. shows the unadjusted baseline characteristics of patients based on RAS mutation status. Covariate balance before and after propensity score weighting for all comparisons is shown in Supplemental Figures 1–3.
Table 1. Unadjusted baseline characteristics of patients with RAS-mutated and wild-type AML.
When comparing the frequency of other cytogenetic abnormalities between RAS mutation types, there was a statistically significant difference in the prevalence of inv(16)(p13.1;q22) or t(16;16)(p13.1;q22); CBFB-MYH11 (WT 1%, KRAS-mutated 9%, NRAS-mutated 20%, KRAS/NRAS-mutated 29%, p < 0.01), t(9;11)(p21.3;q23.3); MLLT3-KMT2A (WT 1%, KRAS-mutated 18%, NRAS-mutated 4%, KRAS/NRAS-mutated 14%, p < 0.01), t(6;9)(p23;q34.1); DEK-NUP214 (WT 0%, KRAS-mutated 0%, NRAS-mutated 4%, KRAS/NRAS-mutated 0%, p = 0.04), monosomal karyotype (WT 1%, KRAS-mutated 18%, NRAS-mutated 0%, KRAS/NRAS-mutated 0%, p < 0.01), and −5 or del(5q) (WT 17%, KRAS-mutated 0%, NRAS-mutated 0%, KRAS/NRAS-mutated 29%, p = 0.04). The prevalence of cytogenetic abnormalities in all patients in this study is shown in Supplemental Table 1.
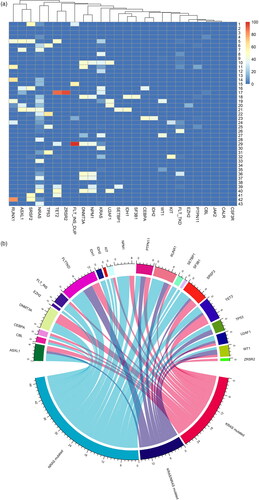
The most common co-occurring mutations in patients with RAS-mutated AML were FLT3-TKD (26%), ASXL1 (21%), and RUNX1 (21%). Mutation prevalence did not differ significantly when comparing RAS-mutated and RAS WT AML. A heat map of co-occurring mutations in RAS-mutated AML is shown in and a chord diagram of co-occurring mutations in RAS-mutated and WT AML is shown in . 11 different KRAS mutation variants and 14 different NRAS mutation variants were detected. The most common KRAS mutation variant was c.38g > a; p.Gly13Asp (28%) and the most common NRAS mutation variant was c.35g > a; p.Gly12Asp (31%). A list of detected NRAS and KRAS mutation variants for NRAS-mutated, KRAS-mutated, and KRAS/NRAS-mutated AML is included in Supplemental Table 2.
Response rate
Unadjusted composite response rate (CRc) (CR + CRi) was 40.0% for KRAS-mutated AML compared to 53.7% for RAS WT AML, 56.0% for NRAS-mutated AML, and 66.6% for KRAS/NRAS-mutated AML (p = 0.75, Supplemental Table 3). There was not a statistically significant difference in adjusted or unadjusted CRc when comparing KRAS-mutated, NRAS-mutated, and RAS WT AML (Supplemental Table 4).
Unadjusted survival outcomes
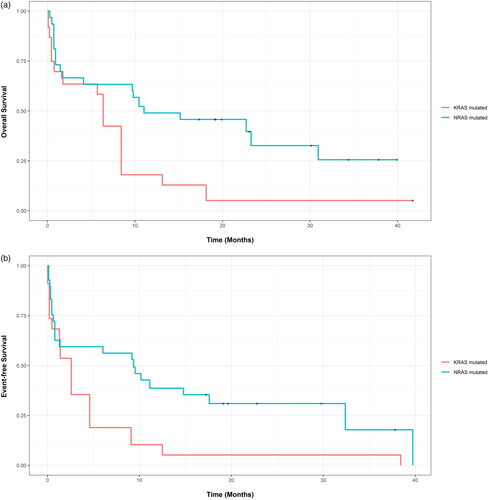
KRAS-mutated AML had the shortest unadjusted mOS of 5.7 months (CI: 0.8-Not calculable (NC)) and unadjusted mEFS of 2.0 months (CI: 0.5-NC) compared to a mOS of 14.6 months (CI: 10.2-17.6, p = 0.02) and mEFS of 5.7 months (CI: 4.2-8.1, p = 0.09) for RAS WT AML, mOS of 15.2 months (CI: 9.7-NC, p = 0.04) and mEFS of 9.5 months (CI: 1.3-NC, p = 0.09) for NRAS-mutated AML, and mOS of 8.9 months (CI: 0.3-NC, p = 0.10) and mEFS of 5.5 months (CI: 2.1-NC, p = 0.10) for KRAS/NRAS-mutated AML (, Supplemental Tables 5 and 6). On unweighted univariable Cox proportional hazards regression, KRAS-mutated AML had higher relative mortality (HR 2.10, CI: 1.10-4.40, p = 0.02) compared to RAS WT AML. Relative mortality did not differ significantly between NRAS-mutated AML and RAS WT AML (HR 0.94, CI: 0.56-1.58. p = 0.81) or between KRAS/NRAS-mutated AML and RAS WT AML (HR 0.88, CI: 0.32-2.37, p = 0.79). Similarly, KRAS-mutated AML had a trend toward higher relative mortality or relapse compared to WT AML, but this was not statistically different (HR 1.72, CI: 0.91-3.27, p = 0.10) (Supplemental Table 7). At time of analysis, 66%, 91%, 64% and 57% of patients died in RAS WT, KRAS-mutated, NRAS-mutated and KRAS/NRAS-mutated AML groups, subsequently.
Adjusted survival outcomes
When using propensity score weighted outcomes, KRAS-mutated AML had a shorter adjusted mOS of 5.7 months (CI: 0.8–NC) and adjusted mEFS of 2.0 months (CI: 0.5–NC) compared to an adjusted mOS of 15.1 months (CI: 6.0–30.3, p = 0.049) and adjusted mEFS of 5.1 months (CI: 4.2–12.1, p = 0.080) for WT AML. KRAS-mutated AML also had a shorter adjusted mOS of 6.4 months (CI: 0.8–18.1) and adjusted mEFS of 2.6 months (CI: 0.2–9.1) when compared to an adjusted mOS of 11.0 months (CI: 1.6–NC, p = 0.015) and adjusted mEFS of 9.4 months (CI: 0.8–NC, p = 0.026) for NRAS-mutated AML. There was not a statistically significant difference in adjusted mOS and adjusted mEFS when comparing NRAS-mutated AML and WT AML. NRAS-mutated AML had an adjusted mOS of 22.8 months (CI: 9.8–NC) and an adjusted mEFS of 9.2 months (CI: 0.6–32.4) when compared to an adjusted mOS of 14.6 months (CI: 10.2–17.6, p = 0.660) and adjusted mEFS of 5.7 months (CI: 4.2–8.7, p = 0.920) for WT AML (Supplemental Table 6). There was not a statistically significant difference in adjusted OS and EFS at years 1 and 2 when comparing KRAS-mutated, NRAS-mutated, and WT AML (Supplemental Table 8). On weighted univariable Cox proportional hazards regression, NRAS-mutated AML had a significantly lower relative mortality (HR 0.44, CI: 0.21–0.91, p = 0.03), and relative mortality or relapse (HR 0.46, CI: 0.22–0.96, p = 0.04) compared to KRAS-mutated AML (Supplemental Table 9). and Supplemental Figures 5 and 6 show propensity score-adjusted OS and EFS when comparing KRAS-mutated, NRAS-mutated, and WT AML.
Outcomes stratified by first-line regimen
CRc for KRAS-mutated AML was 80.0% (4 of 5 patients) for patients receiving anthracycline-based first-line therapies compared to 0.0% (0 of 5 patients) for patients receiving non-anthracycline-based regimens (p = 0.08). CRc for NRAS-mutated AML was 66.6% (8 of 12 patients) for patients receiving anthracycline-based regimens compared to 33.3% (2 of 6 patients) for patients receiving HMAs without venetoclax, 50.0% (3 of 6 patients) for HMAs with venetoclax, and 100.0% (1 of 1 patients) for other non-anthracycline-based regimens (p = 0.44, Supplemental Table 10). There was not a statistically significant difference in mOS when comparing the first-line treatment regimens for KRAS-mutated (p = 0.20) and NRAS-mutated AML (p = 0.08). mOS was 13.0 months for KRAS-mutated and 30.5 months for NRAS-mutated AML treated with anthracycline-based regimens. For AML treated with non-anthracycline-based regimens, unadjusted mOS was 0.5 months for KRAS-mutated and 5.4 months for NRAS-mutated AML treated with HMAs without venetoclax, 1.5 months for KRAS-mutated and 9.2 months for NRAS-mutated AML treated with HMAs plus venetoclax, and 5.2 months for KRAS-mutated and 23.0 months for NRAS-mutated AML treated with other non-anthracycline-based agents (Supplemental Figure 7, Supplemental Table 11). On multivariable Cox proportional hazards regression KRAS-mutated AML had higher relative mortality when treated with HMAs without venetoclax (HR >20, p = 0.04) and HMAs plus venetoclax (HR >20, p = 0.04) when compared to treatment with anthracycline-based regimens (Supplemental Table 12). There was not a statistically significant difference in relative mortality for NRAS-mutated AML treated with HMAs without venetoclax (HR 3.23, CI: 0.89–11.80, p = 0.08) and HMAs plus venetoclax (HR 0.96, CI: 0.19–4.76, p = 0.96) when compared to treatment with anthracycline-based regimens (Supplemental Table 12).
Discussion
Detection and analysis of cytogenetic changes have greatly enhanced the prognostication of AML and are now used to guide treatment decisions [Citation1]. However, many mutations and mutation subtypes remain poorly understood.
Previous research on the prognostic significance of RAS mutations has shown inconsistent results. In one study, the presence of a RAS mutation (NRAS and/or KRAS) did not affect patient outcomes but was a significant predictor of improved survival when adjusted for age (n = 99, p = 0.03) [Citation5]. In another study, KRAS and NRAS mutations had no independent effect on prognosis, while in a third study KRAS mutations were associated with worse survival [Citation2,Citation4]. Bowen et al. found NRAS and KRAS mutations did not affect prognosis (n = 1106). However, all patients included in the study were younger than 60 years old at the time of diagnosis [Citation2]. Ball et al. performed a subsequent study of 232 patients with a higher median age and found that NRAS mutations did not impact survival but that KRAS mutations were associated with shorter mOS and mEFS [Citation4]. In another study by Rivera et al, RAS mutated-AML (n = 273) was associated with 38% three-year OS compared to 28% in RAS WT AML (n = 1137) (p < 0.05). However, the analysis included both NRAS (n = 195) and KRAS (n = 78) mutated subtypes aggregated.
Our cohort of 239 patients had a median age of 65.2 years and our results were consistent with those of Ball et al. We found that NRAS mutations did not have a statistically significant impact on outcomes. However, KRAS-mutated AML was associated with a lower mOS compared to RAS WT AML and NRAS-mutated AML. Ball et al. attributed the worse prognosis of KRAS-mutated AML to the higher proportion of patients with therapy-related AML, secondary AML, KMT2A translocations, and prior treatment with hypomethylating agents [Citation4]. Unlike in prior studies, we used propensity score analysis and Cox proportional hazards regression to adjust outcomes for baseline confounding. As in the unadjusted data, KRAS-mutated AML had a lower adjusted mOS compared to RAS WT AML and NRAS-mutated AML. In contrast to the findings of Ball et al. this suggests that KRAS mutations in AML are directly linked to worse outcomes even after controlling for differences in AML type, co-occurring cytogenetic changes, treatment regimens, and comorbidities. The exact mechanism by which KRAS mutations result in worse survival is not known. It has been hypothesized that KRAS mutations may confer resistance to induction therapy in AML, but further research is required [Citation3].
While differences in CRc rates were not statistically significant, KRAS-mutated AML had a trend toward a lower unadjusted CRc rate following induction chemotherapy. Interestingly, unadjusted CRc rates were higher for NRAS-mutated AML and KRAS/NRAS-mutated AML relative to RAS WT AML. When compared to RAS WT AML, NRAS-mutated AML also had a higher adjusted CRc as well as longer unadjusted and adjusted mOS and mEFS. While our study lacks the power to establish a statistically significant association, these results suggest that NRAS mutations may confer an improved prognosis relative to RAS WT AML. It also suggests that the presence of an NRAS mutation in dual KRAS/NRAS-mutated AML confers an improved prognosis relative to AML with an isolated KRAS mutation. The exact mechanism behind this association is unclear. It may be due to the increased prevalence of inv(16)(p13.1;q22) or t(16;16)(p13.1;q22); CBFB-MYH11 or other co-occurring favorable-risk mutations in NRAS-mutated and KRAS/NRAS-mutated AML [Citation1]. Both mechanistic studies and single cell sequencing studies can help unravel this interesting observation.
Interestingly, prior research has shown a greater benefit of high-dose cytarabine (HiDAC) consolidation therapy in patients with RAS-mutated AML. Ahmed et al. found that patients with RAS-mutated AML had longer OS and EFS when treated with HiDAC consolidation regimens compared to lower dose regimens. For RAS WT AML, longer EFS was also observed with HiDAC therapy but to a lesser extent than RAS-mutated AML and there was no difference in OS [Citation7]. Similarly, Neubauer et al. found that both patients with RAS WT and with RAS-mutated AML benefited from HiDAC consolidation but patients with RAS-mutated AML experienced a much greater reduction in rates of relapse [Citation8]. However, it is important to note that these results might be confounded by indication.
We examined the impact of RAS mutations on patients receiving HMA-based induction regimens, a relationship not previously evaluated in prior studies. For both KRAS-mutated and NRAS-mutated AML, there was a trend toward higher CRc and longer OS in patients treated with anthracycline-based regimens. There was also higher relative mortality for KRAS-mutated AML treated with HMA-based regimens when compared to treatment with anthracycline-based regimens. This trend is consistent with the findings of Ahmed et al. and Neubauer et al. that patients with RAS-mutated AML receive greater benefits from cytarabine [Citation7,Citation8]. In addition, Rivera et al. examined the response of RAS-mutated AML to different regimens compared to RAS WT AML. They found that RAS WT AML had higher CRc compared to RAS-mutated AML when treated with HMA with venetoclax (72 vs. 45%, p < 0.05) but not with other regimens (p > 0.05). Compared to our study, we examined the outcomes of each regimen in RAS-mutated AML, stratified by isoform.
Our study has limitations. First, it is an ambispective and single-center study, limiting its generalizability. Second, our sample size is limited. Lastly, we elected to do pair-wise comparisons instead of single statistical testing for all sub-groups. Multiple statistical testing increases the risk of type I error. Pair-wise comparisons improve the weighting process and improve causal associations’ estimation. Given all the above, our study results need to be confirmed using data derived from several institutions. Nevertheless, our study has several strengths; it has very low missing data. Moreover, we analyzed outcomes of AML stratified by each RAS subtype rather than aggregated analysis. We also controlled for confounding and adjusted our outcomes to baseline characteristics. Because age, comorbidities, and first-line therapy administered impact survival, not controlling for these variables leads to biased estimates.
Conclusion
Our ambispective cohort study shows that KRAS-mutated AML is associated with worse OS compared to RAS WT AML and NRAS-mutated AML using both unadjusted and propensity score weighted outcomes to adjust for confounding factors including AML type, co-occurring cytogenetic changes, and initial treatments, among others. Unlike prior studies, our analysis includes patients receiving treatment with HMAs and other non-anthracycline-based regimens stratified by RAS isoform mutational subtype. We found that KRAS-mutated AML has higher relative mortality when treated with an HMA-based induction regimen compared to treatment with an anthracycline-based regimen. Additionally, while our study lacks the power to establish a statistically significant association, our results suggest that the presence of an NRAS mutation may confer an improved prognosis both in the absence and presence of a co-occurring KRAS mutation. Prior research has suggested that the worse prognosis of KRAS-mutated AML is due to KRAS mutation-induced resistance to induction therapies, but further study is needed to fully understand the nuanced impact RAS mutations have on AML prognosis.
Ethical approval
This study used de-identified data and was approved by human protection oversight by the institutional review board.
Author contributions
Study conception and design: MMA, MW, SN. Data collection: EMC, MMA. Analysis and interpretation: MW, MMA, EMC, FA, SN. Draft manuscript preparation: MW, MMA, SN. Statistical analysis: MMA. Critical review of manuscript: EMC, FA, SN. Administrative and technical support: MMA. Supervision: MMA, SN.
Supplemental Material
Download MS Word (2.3 MB)Disclosure statement
No potential conflict of interest was reported by the author(s). Moaath Mustafa Ali MD, MPH and Sandrine Niyongere, MD affirm that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and any discrepancies the study as planned have been explained. The study is not reproducing material from other sources.
Data availability statement
The data that support the findings of this study are available from MMA upon reasonable request.
Additional information
Funding
References
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 ELN recommendations from an international expert panel. Blood. 2022;140(12):1345–1377.
- Bowen DT, Frew ME, Hills R, et al. RAS mutation in acute myeloid leukemia is associated with distinct cytogenetic subgroups but does not influence outcome in patients younger than 60 years. Blood. 2005;106(6):2113–2119.
- Zhou JD, Yao DM, Li XX, et al. KRAS overexpression independent of RAS mutations confers an adverse prognosis in cytogenetically normal acute myeloid leukemia. Oncotarget. 2017;8(39):66087–66097.
- Ball BJ, Hsu M, Devlin SM, et al. The prognosis and durable clearance of RAS mutations in patients with acute myeloid leukemia receiving induction chemotherapy. Am J Hematol. 2021;96(5):E171–E175.
- Neubauer A, Dodge R, George S, et al. Prognostic importance of mutations in the ras proto-oncogenes in de novo acute myeloid leukemia. Blood. 1994;83(6):1603–1611.
- Rivera D, Kim K, Kanagal-Shamanna R, et al. Implications of RAS mutational status in subsets of patients with newly diagnosed acute myeloid leukemia across therapy subtypes. Am J Hematol. 2022 Dec; 97(12):1599–1606.
- Ahmad EI, Gawish HH, Al Azizi NM, et al. The prognostic impact of K-RAS mutations in adult acute myeloid leukemia patients treated with high-dose cytarabine. Onco Targets Ther. 2011;4:115–121.
- Neubauer A, Maharry K, Mrózek K, et al. Patients with acute myeloid leukemia and RAS mutations benefit most from postremission high-dose cytarabine: a cancer and leukemia group B study. J Clin Oncol. 2008;26(28):4603–4609.
- Mustafa Ali MK, Corley EM, Alharthy H, et al. Outcomes of Newly Diagnosed Acute Myeloid Leukemia Patients Treated With Hypomethylating Agents With or Without Venetoclax: A Propensity Score-Adjusted Cohort Study. Front Oncol. 2022;12:858202.
- Corley EM, Mustafa Ali MK, Alharthy H, et al. Impact of IDH1 c.315C > T SNP on Outcomes in Acute Myeloid Leukemia: A Propensity Score-Adjusted Cohort Study. Front Oncol. 2022 Mar 18;12:804961.
- Corley EM, Mustafa Ali MK, Alharthy H, et al. Impact of FLT3-ITD insertion length on outcomes in acute myeloid leukemia: a propensity score-adjusted cohort study. Biology. 2022;11(6):916.
- Epic Systems. 2019. [2019-05-26]. Epic: About https://www.epic.com/about.
- Harris PA, Taylor R, Minor BL, REDCap Consortium, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
- Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15(3):234–249.
- Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–5655.
- Greifer N. WeightIt: weighting for covariate balance in observational studies [cited 2022 Jul 15]. Available from: https://ngreifer.github.io/WeightIt/.