Abstract
This retrospective study using the nationwide de-identified Flatiron Health electronic health record-derived database was designed to evaluate clinical outcomes among patients with chronic lymphocytic leukemia (CLL) who previously received both a covalent Bruton’s tyrosine kinase inhibitor (cBTKi) and B-cell lymphoma 2 inhibitor (BCL2i) in a real-world setting. Outcomes for the immediate next line of therapy following the latter of the cBTKi or BCL2i treatment included: real-world response rate of 34.4% (using methods most consistent with clinical trials); median duration of real-world response of 13.3 months; and median real-world progression-free survival of 9.2 months. Median overall survival was 25.5 months from the start of the immediate next line of therapy. There remains a need for more effective therapies after cBTKi and BCL2i therapy for patients with CLL.
Introduction
Covalent Bruton’s tyrosine kinase inhibitor (cBTKi) therapy has changed the treatment landscape for patients diagnosed with small cell lymphoma (SLL)/chronic lymphocytic leukaemia (CLL); hereafter, simply referred to as CLL [Citation1]. Historically, chemoimmunotherapy regimens were used as the primary upfront treatment [Citation2]. However, in 2014, the first cBTKi, ibrutinib, was approved by the U.S. Food and Drug Administration (FDA) for use among patients after at least one prior line of therapy, based on the improved survival outcomes compared to ofatumumab observed in the RESONATE trial [Citation3]. This approval was expanded to the first-line setting by 2016, following the results of RESONATE-2, which showed significantly improved tumor response, progression-free survival, overall survival, and reductions in hematologic toxicities compared with chlorambucil [Citation4]. Since then, the use of cBTKi therapy has increased; there are currently three approved cBTKi agents for patients with CLL in the U.S. [Citation5–7]. The B-cell lymphoma 2 (BCL2) inhibitor, venetoclax, is approved as single-agent therapy in the US in the second or later line setting, but data from the MURANO trial also demonstrate its efficacy in combination with rituximab [Citation8,Citation9]. cBTKi and BCL2i therapies are the currently preferred treatment options in national guidelines for patients with CLL in all lines of therapy [Citation10].
Unfortunately, once both cBTKi and BCL2i agents have been exhausted, there is a lack of treatment options that continue to control disease in routine clinical practice among those exposed to both agents [Citation11–14]. Other regimens that are included in treatment guidelines include phosphoinositide 3-kinase inhibitors (PI3Ki), chemotherapy, and anti-CD20 agents, none of which are widely used [Citation11,Citation12], likely in part due to the lack of data for their efficacy after cBTKi and BCL2i failure, and for PI3Ki agents in particular, a toxicity profile that limits use for many patients [Citation13,Citation14]. While off-label treatments such as chimeric antigen receptor (CAR)-T therapy and hematopoietic cell transplantation are effective treatments for selected patients with CLL, they are limited in use not only by the complex healthcare resources needed to deliver the therapy but also by the risk of severe life-threatening toxicities; as a result, careful patient selection is needed [Citation15].
While the evidence is emerging regarding real-world outcomes after cBTKi and BCL2i therapy [Citation12,Citation16], there remains a lack of clinical outcome data (e.g. tumor response and progression) to clarify the treatment options and results those patients can expect once these agents have failed. This study was therefore designed to provide more clinically relevant data including tumor response and progression-free survival and to quantify clinical outcomes expected in a large cohort of patients in a real-world setting after exposure to cBTKi and BCL2i therapy.
Methods
Study design and data source
This retrospective observational study utilized the nationwide de-identified patient data from the Flatiron Health electronic health record (EHR)-derived database [Citation17]. During the study period, the de-identified data originated from approximately 280 cancer clinics in the U.S. (∼800 sites of care).
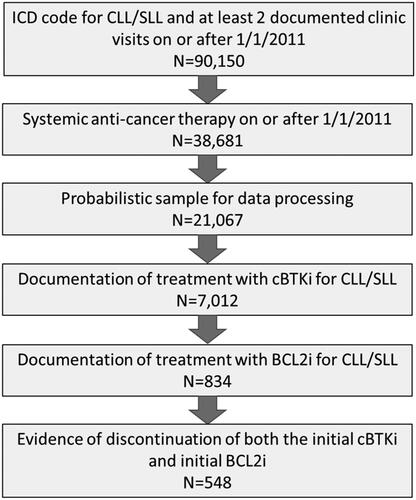
Patients included in the study had been diagnosed with CLL (International Classification of Disease [ICD]-9: 204.1x or ICD-10: C91.1x, C83.0x) with at least two clinic visits in the database showing evidence of receiving systemic therapy on or after 1 January 2011. Patients meeting these criteria were then included in a probabilistic sample for unstructured data processing. To be included in this sample, patients were further required to have been treated specifically for CLL with at least one cBTKi and at least one BCL2i and have discontinued both agents (dual exposed cohort). Once eligibility was confirmed, tumor response, tumor progression, and reasons for treatment discontinuation related to the cBTKi, BLC2i, and subsequent therapies were extracted from the medical record. Additional clinical and demographic variables, including overall survival, were obtained from the electronic health records of eligible patients. Patients were excluded from the study if the medical record was not available, or if the patient was still receiving the initial cBTKi or the initial BCL2i at the time of medical record review.
Subgroups
Subgroups included the timing of clinical outcomes assessment over the course of therapy. These included: (A) patients who initiated first-line therapy with a cBTKi and received BCL2i therapy in the second or third lines of treatment; (B) those who initiated the cBTKi in the second line of therapy, followed by BCL2i in the third or fourth lines; and (C) those who did not receive a cBTKi until the third line or later and a BCL2i in the fourth line of therapy or later.
Clinical outcomes
Clinical outcomes included real-world response (rwR), real-world duration of response (rwDOR), real-world progression-free survival (rwPFS), overall survival (OS), and reasons for treatment discontinuation. Clinical outcomes were reported for the immediate next line of therapy following the cBTKi or BCL2i line of therapy (whichever was later), for patients who received additional therapy.
Real-world response was identified as evidence suggesting tumor response or loss of response from unstructured documents in the medical record after the start of a line of therapy through, but not including the start of the subsequent line of therapy. rwR was evaluated for the immediate next line of therapy after discontinuation of both the initial cBTKi and initial BCL2i lines. Patients without evidence of either response or progression during the line of therapy were included in the denominator for the calculation of tumor response rates. There are known and well-documented differences in how the response is assessed in the real world versus by International Workshop on CLL (iwCLL) criteria, and as such measure different concepts than those evaluated in a real-world setting [Citation18]. Unstructured documentation for rwR or loss of response was therefore categorized by source document type.
Tumor response as evidenced by pathology, radiographic imaging/scans, or laboratory data was considered to be consistent with evidence that would be required in most clinical trial settings and evaluated as the primary measure of the response for this study. rwR rates that included all methods of assessment were also evaluated, which further included data from a physical examination (e.g. physician notes suggesting changes in the size of lymph nodes, liver, spleen, and/or skin), constitutional symptoms (e.g. weight loss, fatigue, fevers, or night sweats), or clinical assessment only (clinician notes with no references as to the source of the evidence).
rwDOR was evaluated using Kaplan–Meier method from the time of first documented rwR (by methods typically used in clinical trials and by all methods of evaluation of response in general practice, respectively) until the date of loss of response, tumor progression, initiation of a subsequent line of therapy, or death, whichever occurred first. Patients without an event were censored from the analysis at the last infusion/administration of the drug in the line of therapy.
Real-world progression (rwP) was evaluated using Kaplan–Meier method, from the start of the line of therapy until the date of progression, loss of response, start of the next line of therapy, or death, whichever occurred first. rwP was identified from unstructured documents in the medical record after the start of a line of therapy through, but not including the start of the subsequent line of therapy. Unstructured documentation for rwP was similarly categorized by source document type, with evidence limited to pathology reports, radiographic imaging, or laboratory findings as the primary measure of progression, but further evaluated using all evidence in the patient record, such as constitutional symptoms, physical exam evidence, or clinical assessment only. Patients without a documented progression event were censored at the last infusion/drug administration observed within the line of therapy.
OS was evaluated using Kaplan–Meier method as time from the start date of the immediate next line of therapy after discontinuation of the initial cBTKi or initial BCL2i (whichever was later) until death, censoring patients without a death event at the last observation in the database.
Results
A total of 548 patients met the eligibility criteria for dual exposure to cBTKi and BCL2i and were included in this analysis (). Overall, the mean age of the study population was 70.9 years (standard deviation [SD] = 9.0), most patients were White (n = 420, 76.6%), and the majority were male (n = 360, 65.7%). () The most common reasons for discontinuation of the initial cBTKi included toxicity (n = 206, 37.6%) or disease progression/insufficient response (n = 190, 34.7%). Seventeen patients (3.1%) discontinued for both toxicity and progression reasons. Reasons for cBTKi discontinuation were missing for 93 patients (17.0%). The intent of initial BCL2i therapy as fixed duration was noted for 82 patients (15.0%). The most common reasons for discontinuation of the initial BCL2i included toxicity (n = 90, 16.4%), progression or insufficient response (n = 74, 13.5%), or both toxicity and progression (n = 3, 0.5%); however, this only represents a small subset of the study cohort. Reasons for discontinuation of the BCL2i were missing for 49.6% of the cohort (n = 272).
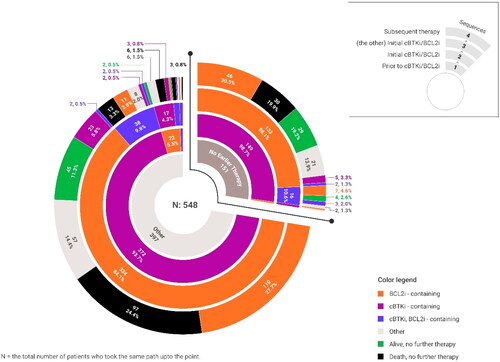
Most patients received the initial cBTKi prior to the initial BCL2i (n = 521, 95.1%); 24 patients (4.4%) received the BCL2i prior to cBTKi therapy, and 3 (0.5%) patients received both agents in the same line of therapy ( and ). The lines of therapy in which the cBTKi and BCL2i received varied, with 136 (24.8%) beginning systemic treatment with a cBTKi-based regimen, 113 (20.6%) began treatment with chemotherapy and/or anti-CD20 therapy followed by cBTKi treatment in the second line, and 127 (23.2%) began cBTKi therapy in the third line or later set, all followed by BCL2i in a subsequent line. Few patients used either additional cBTKi agents (n = 43, 13.6%) or PI3Ki agents (n = 22, 6.9%) as part of the treatment regimen in the immediate next line of therapy.
Table 2. Sequence and timing of cBTKi and BCL2i therapy.
A total of 317 (57.8%) patients with dual exposure had evidence of subsequent therapy. Supplementary Table 1 contains further details of the most common agents (not mutually exclusive) used in the immediate next line of therapy, which included anti-CD20 (n = 223, 70.3%) and/or additional BCL2i (n = 187, 59.0%). Reasons for discontinuation of the immediate next line of therapy were missing for 248 (78.2%) of those receiving additional therapy and are therefore not reported. The general treatment patterns observed in this study are shown in .
Overall, demographic characteristics were similar between those who received at least one additional line of therapy and those who did not, however, there were significant differences between groups for several clinical factors (). There were significant differences in evidence of transformation events that occurred after both cBTKi and BCL2i discontinuation between patients who received a subsequent line of therapy versus those who did not (p = 0.01). There were also statistically significant differences in IgHV status (p = 0.02), year of discontinuation of the initial cBTKi or BCL2i therapy (p = 0.006), and ECOG performance status (p < 0.001) between patients who received additional therapy after dual exposure versus those who did not .
Table 1. Characteristics of patients who discontinued cBTKi and BCL2i therapy.
Table 3. Demographic and clinical characteristics of subgroups evaluated in the study.
Clinical outcomes
The rwR rate as evaluated by pathology, radiographic imaging/scans, or laboratory data was 34.4% for the immediate next line of therapy following the discontinuation of both the initial cBTKi and BCL2i. When including all available methods of assessment in the patient record, rwR was 52.1% for the immediate next line of therapy.
Table 4. Real-world tumor response (rwR) for the immediate next line of therapya.
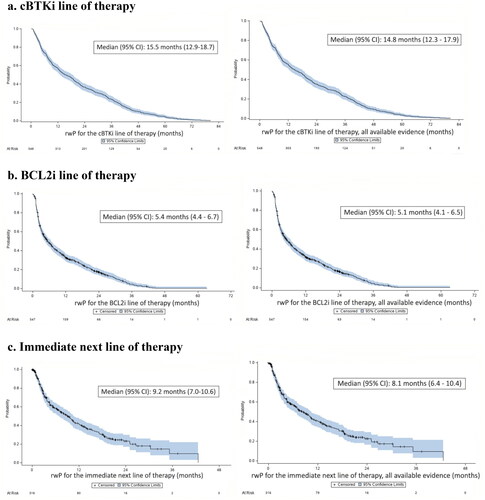
Duration of rwR limited to pathology, imaging or laboratory report data was a median of 13.3 months (95% CI: 10.6–19.5) for the immediate next line of therapy, and is consistent when all methods of assessment were included. Details of the characteristics of subgroups and tumor response data are provided in and .
Median time to rwP or death for the immediate next line of therapy was 9.2 months (95% CI: 7.0–10.6) when limited to evidence from imaging/scan, pathology and laboratory records; findings were consistent when all methods of assessment were evaluated (). Median time to rwP or death for the immediate next line of therapy for the predefined subgroups was 12.6 months (95% CI: 8.6–19.1) first-line cBTKi subgroup), 7.9 months (95% CI: 3.7–13.1) second-line cBTKi subgroup), and 6.7 months (95% CI: 4.3–10.1; third-line or later cBTKi subgroup) using imaging/scan, pathology and laboratory data.
Figure 3. Time to real-world progression (rwP) or death (outcomes based on imaging, pathology, or laboratory data on left; all available evidence/methods of assessmenta on right). (a). cBTKi line of therapy. (b). BCL2i line of therapy. (c). Immediate next line of therapyb. aAll available evidence included all methods of assessment as observed in the patient record: imaging, pathology, laboratory report, constitutional symptoms, physical exam, or clinical assessment only. bOne patient was not evaluable in as the progression event occurred prior to the start of the immediate next line of therapy.
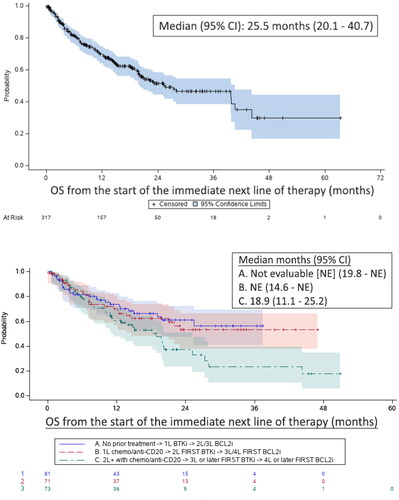
As shown in , overall survival from the start of the immediate next line of therapy was 25.5 months (95% CI: 20.1–40.7). Median OS was not estimable for the first- or second-line cBTKi subgroups but was 18.9 months (95% CI: 11.1–25.2) for those in the third- or later-line cBTKi subgroup.
Figure 4. Overall survival (OS) from the start of the immediate next line of therapya (all patients above; subgroups by treatment historyb below). aThe immediate next line of therapy following the discontinuation of the initial cBTKi or initial BCL2i, whichever was later: bSubgroups included: (A) patients who initiated first-line therapy with a cBTKi and received BCL2i therapy in the second or third lines of treatment; (B) those who initiated the cBTKi in the second line of therapy, followed by BCL2i in the third or fourth lines; and (C) those who did not receive either a cBTKi until the third line or later and a BCL2i at the fourth line of therapy or later.
Discussion
Prior studies have suggested poor outcomes among patients with CLL following cBTKi and BCL2i therapies but were limited by the lack of clinical outcomes that are typically measured in clinical trials (such as tumor response and progression) [Citation11,Citation12]. Based on our findings from these prior database studies, the current study was designed to further this work by abstracting clinical evidence of tumor response and progression from the patient record to directly evaluate these clinical outcomes. The outcomes observed in this study continue to support the need for more effective therapies after cBTKi and BCL2i therapy as evidenced by the low response rates and time to progression observed in this setting.
It is important to note that tumor response and progression as measured in the real world are not directly comparable to clinical trial outcomes in hematology. Therefore, comparisons between these data and that observed in clinical trials among patients with CLL should be made with caution, considering the differences in the source of evidence and timing of assessments used to determine response and progression. This is particularly evident when evaluating all forms of evidence in the patient record versus those considered more consistent with IWCLL approaches. The current study attempted to address the differences in response assessment between the real world and in clinical trials that use IWCLL by evaluating both response and outcomes based on the source of evidence in the patient record. By limiting the evidence to imaging, laboratory reports and pathology scans, the results may be slightly more consistent with trial data, but this has not been validated. It is clear that the totality of real-world evidence in the patient record (including notes unsubstantiated by clinical data) is not consistent with the more limited data, suggesting that decision-making for care based on real-world outcomes may occur quite differently than what may occur in a trial. When all evidence was considered, there were a greater number of responses identified, but it is very likely that these may not have reached the definition of partial or complete response as defined by IWCLL criteria. However, the duration of the response remained consistent regardless of the source of evidence used. With higher rates of response observed in all the real-world data, it is possible that decisions for subsequent care take place later in the course of treatment that may occur in a trial setting. The differences in real-world practices from trial-based settings have yet to be fully evaluated, but the evidence from this study suggests the differences may be substantial.
While this study overcome many of the limitations of health record data by supplementing with a thorough chart review, missing data remain. The rate of missing data could have impacted the outcomes observed in this study, Reasons for discontinuation of the BCL2i and for the immediate next therapy had high rates of missingness in particular, therefore, these data cannot be considered reliable for interpretation. The assumption for this study was made that data were missing at random, however, there could have been systematic reasons for missing data that could have affected some of the study findings. The rate of unknown prior therapies (line Ø) ranged from approximately 20-30% across study cohorts. This made impossible the clear definition of the exact line of therapy that these patients received. Due to this limitation, patients with evidence of line Ø were excluded from the subgroup analyses that relied on ‘line of therapy’ definitions. Also, missing data related to tumor response may not have been missing at random. The lack of response data may have reflected stable disease or truly missing evidence for a progression or response. Therefore, there are also limitations to the interpretation of tumor response in the real-world setting, given the rates of missing data observed with this outcome as patients without a response were by default included in the denominator for the rate of response estimates.
Despite the limitations of data obtained in a real-world setting, the rates of tumor response (when limited to imaging, laboratory results, and pathology) are very similar to those observed in other trials of patients with CLL. This study observed a 34.4% response rate in the immediate next line of therapy of a blended treatment group after exposure to both a cBTKi and BCL2i. This is similar to that observed in the post cBTKi/post BCL2i setting in another retrospective multicenter study, which showed an overall response rate of 31.8% of chemoimmunotherapy (n = 23), 40.9% for PI3Ki therapy (n = 24), 85.7% for CAR T-cell therapy (n = 9), and 40.0% for venetoclax re-treatment (number of patients not reported); however, sample sizes were extremely low and data are limited to those presented in the study abstract, limiting the ability to interpret the findings [Citation14]. The evaluation of outcomes by specific subgroups of treatments was beyond the scope of the current descriptive study but would be a valuable addition to the body of literature in a future study with larger sample sizes and appropriate adjustments for comparative interpretation. It is imperative that studies utilizing real-world data be conducted in compliance with best practices for the evaluation of specific treatment outcomes in real-world data to ensure the accuracy of conclusions in a non-randomized setting [Citation19–21].
Conclusion
This chart review-based retrospective real-world study contributes to the growing body of evidence regarding the lack of effective treatment options for patients with CLL after receiving cBTKi and BCL2i-based therapy. There is a need for more effective therapies after cBTKi and BCL2i therapy for patients with CLL.
Author contributions
All authors were involved in the conceptualization of the study concept and interpretation of study results. LMH and JMP were responsible for designing the study, LMH and MK developed the statistical analysis plan. LMH wrote the draft manuscript. TS, DH, and MK were responsible for all data analysis. All authors contributed substantially to the final manuscript and have approved the final version for submission.
Supplemental Material
Download MS Word (14.3 KB)Disclosure statement
LMH and MK are employees of Eli Lilly and Company. JMP, RAW, PBA, and HK are employees of Loxo@Lilly. TS and DH are employees of Syneos Health, which receives funding from Eli Lilly and Company for analytical support of observational studies.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Gordon MJ, Danilov AV. The evolving role of Bruton’s tyrosine kinase inhibitors in chronic lymphocytic leukemia. Ther Adv Hematol. 2021;12:204062072198958.
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174.
- Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437.
- FDA [Internet]. Drugs@FDA: FDA-Approved Drugs; Calquence 2019. Available from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=210259.
- FDA [Internet]. Drugs@FDA: FDA-Approved Drugs; Imbruvica 2018. Available from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=210563.
- FDA [Internet]. Drugs@FDA: FDA-Approved Drugs; Brukinsa 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/213217s007lbl.pdf.
- FDA [Internet]. Drugs@FDA: FDA-Approved Drugs; Venclexa 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf.
- Seymour JF, Kipps TJ, Eichhorst B, et al. MURANO trial establishes feasibility of time-limited venetoclax-rituximab (VenR) combination therapy in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Blood. 2018;132(Supplement 1):184–184.
- NCCN [Internet]. NCCN clinical practice guidelines in oncology: chronic lymphocytic leukemia/small lymphocytic leukemia, Version 2. 2023. 2023, www.nccn.org, cited on February 17, 2023.
- Eyre T, Hess LM, Sugihara T, et al. Outcomes following treatment with a covalent BTKi and BCL2 inhibitor among patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): a real-world study of a large US database. HemaSphere. 2022;6:551–552. https://journals.lww.com/hemasphere/Fulltext/2022/06003/P653__OUTCOMES_FOLLOWING_TREATMENT_WITH_A_COVALENT.551.aspx
- Mato AR, Hess LM, Chen Y, et al. Outcomes for patients with chronic lymphocytic leukemia (CLL) previously treated with both a covalent BTK and BCL2 inhibitor in the United States: a Real-World database study. Clin Lymphoma Myeloma Leuk. 2023; 23(1):57–67.
- Mato AR, Roeker LE, Eyre TA, et al. Efficacy of therapies following venetoclax discontinuation in CLL: focus on B-cell receptor signal transduction inhibitors and cellular therapies. Blood. 2019;134(Supplement_1):502–502.
- Thompson MC, Roeker LE, Coombs CC, et al. Addressing a new challenge in chronic lymphocytic leukemia: outcomes of therapies after exposure to both a covalent bruton’s tyrosine kinase inhibitor and venetoclax. Blood. 2021;138(Supplement 1):2628–2628.
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69.
- Lew TE, Lin VS, Cliff ER, et al. Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood Advances. 2021;5(20):4054–4058.
- Ma X, Long L, Moon S, et al. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron health, SEER, and NPCR. MedRxiv 2020; doi: https://doi.org/10.1101/2020.03.16.20037143.
- Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. J Am SocHematol. 2018;131(25):2745–2760.
- Berger ML, Sox H, Willke RJ, et al. Good practices for real‐world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR‐ISPE special task force on real‐world evidence in health care decision making. Value Health. 2017;20(8):1003–1008.
- Johnson ML, Crown W, Martin BC, et al. Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR good research practices for retrospective database analysis task force report—part III. Value Health. 2009;12(8):1062–1073.
- Berger ML, Mamdani M, Atkins D, et al. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR good research practices for retrospective database analysis task force report—part I. Value Health. 2009;12(8):1044–1052.