Abstract
The six-year ECHELON-1 update showed a survival advantage for frontline (1 L) A + AVD (brentuximab vedotin, doxorubicin, vinblastine, dacarbazine) vs ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) for stage III/IV classic Hodgkin lymphoma (cHL). As clinical trials have limited ability to track patients for extended periods, we developed an oncology simulation model using ECHELON-1 data to estimate population-based cHL outcomes in the US over 10 years (through 2031). The model included a scenario without (64.5% ABVD, 35.5% PET-adapted ABVD utilization) and scenarios with 1 L A + AVD (27%–80%k utilization). At 27%–80% A + AVD utilization, the model estimated 13.6%–31.7% fewer deaths, 2.4%–6.3% more patients ≥5 years progression free, 9.4%–24.4% fewer stem cell transplants (SCTs), and 7.8%–22.5% fewer second cancers over 10 years. These results suggest that the improved outcomes observed in the ECHELON-1 update with A + AVD vs ABVD may translate to more patients alive and fewer with primary relapsed/refractory cHL, SCTs, and second cancers.
Introduction
Hodgkin lymphoma (HL) is a rare B-cell malignancy [Citation1] that accounts for approximately 10% of newly diagnosed lymphoma cases in the United States [Citation2–4]. Approximately 95% of patients diagnosed with HL have classic HL (cHL) [Citation1], defined by the presence of Reed–Sternberg cells, which express high levels of CD30, making the disease susceptible to CD30-targeting therapies [Citation1,Citation2,Citation5,Citation6].
The standard-of-care frontline (1 L) therapy for patients diagnosed with stage III/IV cHL for nearly 30 years has been combination chemotherapy with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) [Citation7]. However, approximately 30% of patients with stage III/IV cHL experience a relapse or become refractory to 1 L ABVD [Citation7]. Both an escalated dosing regimen of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) and PET-adapted strategies are alternative options [Citation8–15]. While escalated BEACOPP improves disease control compared with ABVD, it is rarely used in the United States due to its high rate of adverse effects [Citation8,Citation9,Citation12,Citation13,Citation15]. In an effort to maintain disease control while minimizing toxicity, studies investigating PET-adapted strategies to remove bleomycin showed improved tolerability in patients eligible for de-escalation [Citation10–15]. However, neither escalated BEACOPP nor PET-adapted strategies has improved overall survival (OS), the most important benchmark in clinical oncology, compared with ABVD [Citation9,Citation12,Citation13,Citation15–19].
Brentuximab vedotin in combination with doxorubicin, vinblastine, and dacarbazine (A + AVD) is also a treatment option for newly diagnosed stage III/IV cHL. The ECHELON-1 trial, which compared A + AVD and ABVD, is the first trial to demonstrate a survival advantage of any regimen over ABVD in patients with previously untreated stage III/IV cHL [Citation8]. With a median follow-up of approximately six years, A + AVD improved OS (93.9% vs 89.4%, hazard ratio [HR]: 0.59; 95% CI: 0.40–0.88; p = .009) and progression-free survival (PFS; 82.3% vs 74.5%; HR: 0.68 [95% CI: 0.53–0.86]; nominal p = .002) compared with ABVD. Additionally, patients treated with A + AVD compared with ABVD received fewer subsequent therapies (20.4% vs 23.8%) including autologous stem-cell transplantations (SCTs; 6.6% vs 9.0%) and allogeneic SCTs (0.6% vs 1.8%) and had fewer second cancers (3.5% vs 4.9%).
The effect of the ECHELON-1 results on the cHL population in the United States over the next 10 years is unknown. Therefore, to estimate the impact of treatment scenarios without and with 1 L A + AVD on the population of US patients with previously untreated stage III/IV cHL, we developed an oncology simulation model (OSM) that incorporated data from the six-year ECHELON-1 update.
Materials and methods
Oncology simulation model overview
Patients enrolled in clinical trials are generally followed for a limited time period. To overcome this limitation, an OSM can be used to extrapolate clinical trial outcomes over longer time horizons. Specifically, an OSM can be used to estimate the effect of clinical trial outcomes on the prevalent population including how changes in treatment utilization based on clinical trial results may affect population-level outcomes. Additional details on the OSM framework were previously published [Citation20], including validation against published real-world progression rates for other oncological diseases [Citation21]. The robust framework of an OSM builds upon existing health-economic modeling practices and can incorporate multiple treatment pathways, time-varied efficacy rates, and continuous incidence and prevalence estimates across the modeled treatment pathways. The model simulates the real-world by allowing population composition, incidence rates, treatment efficacy, treatment patterns, and treatment availability to vary with time. Using the previously published OSM framework [Citation20], an OSM was developed to estimate the impact of the ECHELON-1 trial results on the cHL population in the United States over a 10-years period (through 2031).
cHL OSM structure and computational approach
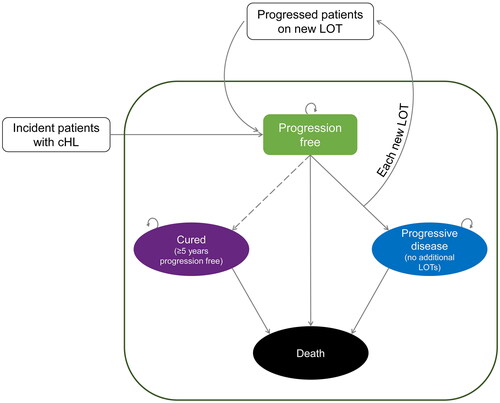
The cHL OSM is a continuous dynamic Markov model developed in Microsoft Excel. The model simulates the real-world cHL US population by incorporating during each one-month treatment cycle (1) continuous incident cohorts and (2) progression across all known lines of therapy to develop an accurate simulation of the cohort composition during later lines of therapy. Each cohort enters the model based on disease progression at diagnosis. For each line of therapy, cohorts enter the progression-free state and are assigned a treatment based on the treatment pattern distributions at the beginning of that cycle. Treatment pattern distributions can change with each cycle reflecting available treatments and current standard of care.
Transitions through the model are based on PFS and OS curves derived from published PFS and OS values and survival point estimates, assuming a constant hazard function. During each one-month treatment cycle, cohorts can remain in the progression-free state or transition to the progressive disease, death, or cured (defined as ≥5 years progression-free) states (). Patients progression-free on their current treatment remain in the progression-free state on their current treatment based on the PFS curve for that treatment. Patients who progress on a treatment and then initiate a new treatment are re-classified as progression-free once the new treatment begins. Patients with progressive disease and no further treatment or those who opt out of treatment transition to the progressive disease state. Patients who die transition to the death state based on underlying population (i.e. age, gender, region) or disease (i.e. OS curve of treatment) mortality. Patients who are cured transition to the cured state following a continuous number of progression-free cycles.
Model inputs
Epidemiology
The annual incidence of cHL was derived using the reported incidence of HL in 2019 from the Surveillance, Epidemiology, and End Results database and assuming 95% of all HL cases are cHL [Citation1] and that 42% of patients newly diagnosed with cHL have stage III/IV disease [Citation2].
Treatment patterns
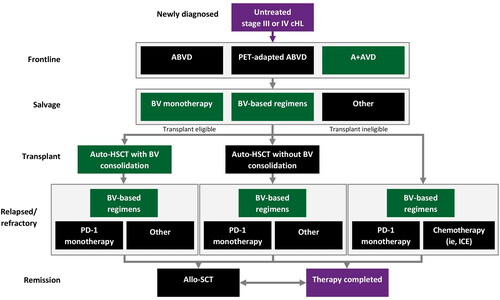
The modeled treatment pathway was developed based on consensus guidelines [Citation16] and input from clinical experts familiar with recommended treatments for stage III/IV cHL (). Patients newly diagnosed with cHL were assumed to be treated with 1 L ABVD, PET-adapted ABVD, or A + AVD in the model; escalated BEACOPP was not included due to its limited use in the United States outside of select academic centers.
Consolidation with autologous SCT was assumed to occur at a single time point following salvage therapy for transplant-eligible patients. Other treatment regimens employed following autologous SCT or failure of 1 L therapy included: brentuximab vedotin monotherapy, brentuximab vedotin in combination (e.g. brentuximab vedotin + nivolumab, brentuximab vedotin + bendamustine), autologous SCT with or without brentuximab vedotin consolidation, PD-1 inhibitor (e.g. nivolumab, pembrolizumab) monotherapy, ICE (ifosfamide, carboplatin, etoposide) combination therapy, allogeneic SCT, or ‘other,’ defined as the average PFS and OS for PD-1 inhibitor monotherapy and chemotherapy (ICE used as a proxy).
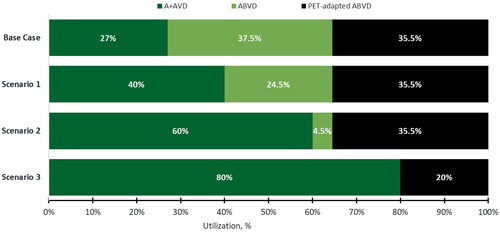
The reference scenario included 0% 1 L A + AVD utilization; in this scenario, 64.5% of patients received 1 L ABVD, and 35.5% of patients received PET-adapted ABVD. In the base case, a reference point for A + AVD utilization, 27% of patients received 1 L A + AVD based on real-world treatment patterns, 37.5% received ABVD, and 35.5% received PET-adapted ABVD (). In scenario analyses, 1 L A + AVD utilization was varied from 40% to 80%, as recommended by clinical experts with corresponding decreases in ABVD and PET-adapted ABVD utilization.
Clinical inputs
Clinical inputs for PFS and OS for all lines of therapy were informed by real-world treatment utilization and treatment-specific clinical trial data obtained from the published literature [Citation8,Citation15,Citation22–32] (). Inputs for A + AVD and ABVD for OS (93.9% [91.6%–95.5%] and 89.4% [86.6%–91.7%]; HR, 0.59; 95% CI: 0.40–0.88; p = .009), PFS (82.3% [95% CI: 79.1%–85.0%] and 74.5% [70.8%–77.7%]; HR: 0.68 [0.53–0.86]), SCTs (7.3% vs 10.8%), and second cancers (3.5% vs 4.9%) were obtained from the six-year ECHELON-1 update [Citation8]. Efficacy inputs for PET-adapted ABVD were informed by Stephens et al. [Citation15] and the rate of second cancer with 1 L PET-adapted ABVD utilization was informed by Johnson et al. [Citation12].
Table 1. Efficacy inputs for treatment of cHL.
Key model assumptions
This cHL OSM was built using several key underlying assumptions:
All patients with previously untreated stage III/IV cHL receive 1L treatment with ABVD, PET-adapted ABVD, or A + AVD
Patients with progressive disease treated with a subsequent therapy follow the PFS and OS curves for that subsequent therapy
Patients with progressive disease who receive no subsequent therapy stay on the OS curve of the prior regimen
The transplant eligibility rate is the same for all salvage therapies
The risk of progression and second cancers is assumed to be constant (ie, constant hazards).
Some treatment options were streamlined to obtain a more parsimonious model using weighted average hazard functions derived from PFS and OS estimates for available therapies.
Results
Reference case: no 1 L a + AVD utilization
With 0% 1 L A + AVD utilization, the model estimates that 20,200 patients treated 1 L for stage III/IV cHL are alive and progression-free in 2031 including 3654 newly diagnosed and 16,547 previously diagnosed patients (). Cumulatively over 10 years, without 1 L A + AVD, the model predicts that 2650 patients treated 1 L for stage III/IV cHL would die, 25,810 would achieve ≥5 years’ PFS, 3200 would receive an SCT, and 1418 would develop a second cancer ().
Figure 4. Patient flow in 2031 for stage III/IV cHL: reference case with 0% A + AVD and base case with 27% A + AVD utilization.
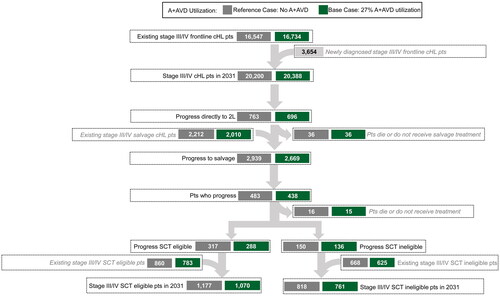
Table 2. No. of 1 L patients with stage III/IV cHL progression-free and deaths, SCTs, and second cancers avoided over 10 years with varying 1 L A + AVD utilization compared with the reference scenario without A + AVD.
Base case: 27% a + AVD utilization
With 27% A + AVD utilization, the model estimates that 20,388 patients treated 1 L for stage III/IV cHL are alive and progression free in 2031 () representing an additional 188 patients (0.9%) alive and progression free compared with the reference scenario (i.e. 0% A + AVD utilization; ). Cumulatively over 10 years with 27% 1 L A + AVD utilization for stage III/IV cHL, an additional 13.6% of patients would avoid death, 2.4% more patients would achieve ≥5 years PFS, 9.4% fewer patients would receive an SCT, and 7.8% fewer patients would develop a second cancer compared with the reference scenario (i.e. no A + AVD utilization; ). Annually, for every 100 patients treated with 1 L A + AVD in the base case, an estimated 3.7 additional deaths would be avoided, 6.7 additional patients would be progression free for ≥5 years, 2.9 fewer patients would require SCT, and 1.1 fewer patients would be diagnosed with a second cancer.
Scenario analyses: 40% to 80% a + AVD utilization
With 40%–80% 1 L A + AVD utilization, the model estimates an additional 278 (1.4%) to 517 (2.6%) patients alive and progression free in 2031 compared with the reference scenario (i.e. 0% A + AVD utilization; ). Cumulatively over 10 years with 40%–80% 1 L A + AVD utilization for stage III/IV cHL, an additional 20.0% to 31.7% of patients would avoid death, 3.5% to 6.3% would achieve ≥5 years PFS, 13.8% to 24.4% fewer patients would receive an SCT, and 11.5% to 22.5% fewer patients would develop a second cancer compared with the reference scenario with no A + AVD utilization. Scenario analyses of events avoided per 100 patients treated per year were consistent with base case rates.
Discussion
Disease burden and benefit of a + AVD
This cHL OSM was developed to estimate survival and prevalence outcomes as well as SCT and second cancer rates for patients with newly diagnosed stage III/IV cHL in treatment scenarios without and with 1 L A + AVD in the forthcoming decade (i.e. through 2031). Cumulative results over 10 years and events avoided per 100 patients treated per year for the cHL OSM base case, which assumed 27% 1 L A + AVD utilization, show increases in the estimated prevalent population of patients with cHL in the United States who are alive and progression-free and decreases in the number of patients who die, require an SCT, or develop a second cancer compared to the reference scenario with 0% 1 L A + AVD utilization. Varying 1 L A + AVD utilization from 40% to 80% in scenario analyses further increased the number of deaths avoided and of patients remaining alive and progression-free and further decreased the number of patients needing SCTs or developing a second cancer compared with the reference scenario with 0% A + AVD. As reported, results from scenario analyses of events avoided per 100 patients treated per year were consistent with base case rates as one would not expect these to deviate on a patient(s) per year basis.
Results from ECHELON-1, the first to show an overall survival benefit versus classic ABVD, suggest that A + AVD compared with ABVD has the potential to improve the overall prognosis of patients with stage III/IV cHL [Citation8]. With a median follow-up of approximately six years, the ECHELON-1 trial update confirmed that patients with stage III/IV cHL continue to benefit from treatment with A + AVD beyond five years. Patients treated with A + AVD compared with ABVD had significantly improved OS with a 41% reduction in the risk of death (HR: 0.59; 95% CI: 0.40–0.88; p = .009) and a 32% reduction in the risk of death or progression (HR, 0.68; 95% CI, 0.53–0.86) [Citation8]. As most cHL relapses occur during the first two years of treatment [Citation33], the long-term PFS and OS improvement observed with A + AVD compared with ABVD in ECHELON-1 suggests a higher proportion of patients may achieve long-term remission and live longer when treated 1 L with A + AVD than ABVD [Citation8].
Results from ECHELON-1 and this model suggest a lower rate of SCT in patients treated with A + AVD than ABVD. The economic burden of SCT for patients with hematologic malignancies, including those with cHL, is substantial [Citation34]. In the ECHELON-1 trial, fewer second cancers, including hematologic malignancies, were observed among patients treated with A + AVD (3.5%) than ABVD (4.9%) [Citation8]. While the exact driving cause for this lower rate of second cancers is not fully known, a pooled analysis of data from 4 HL clinical trials across 1227 patients found secondary cancers occurred in 4.0% and 6.4% of patients treated with ABVD and BEACOPP [Citation35]. The risks of downstream second cancers are important to both health care providers and patients. Results from the CONNECT physician survey found that 60% of physicians surveyed stated that a treatment’s efficacy and safety profile had the most essential impact on 1 L treatment decisions for patients with stage III/IV cHL [Citation36]. Similarly, 81% of patients aged <40 years and 46% of those aged ≥40 years who participated in the CONNECT patient survey reported that secondary cancers were a top concern regarding the long-term effects of treatment [Citation37].
Limitations
Several assumptions were incorporated into this model. First, treatment patterns may be heterogeneous in late-stage or progressive disease where multiple treatment options exist and may continue to change as novel therapies enter the market. We streamlined treatment regimen groups and used weighted average hazard functions to represent broader treatment groups; salvage therapies were categorized into only three options. Second, the model only includes autologous SCT at a single time point, following second-line therapy in remission; in practice autologous SCT is utilized beyond second-line therapy and in patients with partial remission. Lastly, we assumed that the risk of disease progression remained constant in the model through use of an exponential function for PFS and OS based on the key model assumption of constant hazards; different assumptions for the risk of progression were not examined.
Conclusion
Results from this OSM suggest improved outcomes for the population of patients newly diagnosed with stage III/IV cHL in the United States treated 1 L with A + AVD versus ABVD. Although results from real-world studies are needed to confirm these modeled results, evidence from OSMs developed for other oncologic disease were similar to published real-world data [Citation20,Citation21]. In settings where 1 L A + AVD is available, these modeled results suggest that the significant improvement in OS and improvement in PFS seen in the ECHELON-1 trial with A + AVD, the first to show an improvement in OS versus classic ABVD, may translate to more deaths avoided, more patients being cured of their disease, fewer patients with primary refractory or relapsed cHL, fewer patients needing additional high-cost therapies including SCT, and fewer second cancers.
Acknowledgments
Medical writing support was provided by Kristin Mickle, MPH, and Beth Lesher, PharmD, BCPS, from OPEN Health, and was funded by the study sponsor.
Disclosure statement
Tycel Phillips is a consultant to AbbVie, ADC, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb, Epizyme, Gilead, Lilly, Pharmacyclics, Regeneron, Seagen, and Xencor, and he is on the boards of Epizyme, Genentech, and Merck. Kristina S. Yu, Nicholas Liu, and Michelle A. Fanale are employees of Seagen and own stock in Seagen. Kristen Migliaccio-Walle and Brian Bloudek are employees of Curta, and are paid consultants to Seagen in connection with this study. John M. Burke has been a member of the speakers bureaus at Seagen and BeiGene, and he is a consultant to AbbVie, Adaptive Biotechnologies, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb, Epizyme, Foresight Diagnostics, Genentech/Roche, Kura, Kymera, Lilly, MorphoSys, Novartis, Nurix, Seagen, TG Therapeutics, Verastem, and X4 Pharmaceuticals.
References
- Piccaluga PP, Agostinelli C, Gazzola A, et al. Pathobiology of Hodgkin lymphoma. Adv Hematol. 2011;2011:1–18.
- National Institutes of Health. Cancer stat facts: Hodgkin lymphoma 2022 [cited 2022 Aug 9]. Available from: https://seer.cancer.gov/statfacts/html/hodg.html
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7–33.
- Deutsch YE, Tadmor T, Podack ER, et al. CD30: an important new target in hematologic malignancies. Leuk Lymphoma. 2011;52(9):1641–1654.
- Kuppers R, Hansmann ML. The Hodgkin and reed/Sternberg cell. Int J Biochem Cell Biol. 2005;37(3):511–517.
- Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478–1484.
- Ansell SM, Radford J, Connors JM, et al. Overall survival with brentuximab vedotin in stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2022;387(4):310–320.
- Borchmann P, Goergen H, Kobe C, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2017;390(10114):2790–2802.
- Casasnovas RO, Bouabdallah R, Brice P, et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): a randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019;20(2):202–215.
- Gallamini A, Tarella C, Viviani S, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: long-term results of the GITIL/FIL HD 0607 trial. J Clin Oncol. 2018;36(5):454–462.
- Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374(25):2419–2429.
- Kreissl S, Goergen H, Buehnen I, et al. PET-guided eBEACOPP treatment of advanced-stage Hodgkin lymphoma (HD18): follow-up analysis of an international, open-label, randomised, phase 3 trial. Lancet Haematol. 2021;8(6):e398–e409.
- Ricardi U, Levis M, Evangelista A, et al. Role of radiotherapy to bulky sites of advanced Hodgkin lymphoma treated with ABVD: final results of FIL HD0801 trial. Blood Adv. 2021;5(21):4504–4514.
- Stephens DM, Li H, Schoder H, et al. Five-year follow-up of SWOG S0816: limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood. 2019;134(15):1238–1246.
- National Comprehensive Cancer Network. Hodgkin lymphoma (Version 1.2022) 2021 [cited 2021 Nov 29]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf
- Allen PB, Gordon LI. Frontline therapy for classical Hodgkin lymphoma by stage and prognostic factors. Clin Med Insights Oncol. 2017;11:117955491773107.
- Ansell SM. Hodgkin lymphoma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol. 2018;93(5):704–715.
- Relecom A, Federico M, Connors JM, et al. Resources-stratified guidelines for classical Hodgkin lymphoma. Int J Environ Res Public Health. 2020;17(5).
- Bloudek B, Wirtz HS, Hepp Z, et al. Oncology simulation model: a comprehensive and innovative approach to estimate and project prevalence and survival in oncology. CLEP. 2022;14:1375–1386.
- Gallicchio L, Devasia TP, Tonorezos E, et al. Estimation of the number of individuals living with metastatic cancer in the United States. J Natl Cancer Inst. 2022;114(11):1476–1483.
- Advani RH, Moskowitz AJ, Bartlett NL, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. 2021;138(6):427–438.
- Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428–1439.
- Casadei B, Argnani L, Morigi A, et al. Effectiveness of chemotherapy after anti-PD-1 blockade failure for relapsed and refractory Hodgkin lymphoma. Cancer Med. 2020;9(21):7830–7836.
- Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562–1566.
- Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144–1153.
- Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131(11):1183–1194.
- Kersten MJ, Driessen J, Zijlstra JM, et al. Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: the phase II HOVON/LLPC transplant BRaVE study. Haematologica. 2021;106(4):1129–1137.
- Kuruvilla J, Ramchandren R, Santoro A, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(4):512–524.
- LaCasce AS, Bociek RG, Sawas A, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018;132(1):40–48.
- Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862.
- Santoro A, Mazza R, Pulsoni A, et al. Five-year results of the BEGEV salvage regimen in relapsed/refractory classical Hodgkin lymphoma. Blood Adv. 2020;4(1):136–140.
- Hapgood G, Zheng Y, Sehn LH, et al. Evaluation of the risk of relapse in classical Hodgkin lymphoma at event-free survival time points and survival comparison with the general population in British Columbia. J Clin Oncol. 2016;34(21):2493–2500.
- Bonafede M, Richhariya A, Cai Q, et al. Real-world economic burden of hematopoietic cell transplantation among a large US commercially insured population with hematologic malignancies. J Med Econ. 2017;20(12):1244–1251.
- André MPE, Carde P, Viviani S, et al. Long-term overall survival and toxicities of ABVD vs BEACOPP in advanced Hodgkin lymphoma: a pooled analysis of four randomized trials. Cancer Med. 2020;9(18):6565–6575.
- Evens AM, Yu KS, Liu N, et al. Classic Hodgkin lymphoma: real-world observations from physicians, patients, and caregivers on the disease and its treatment (CONNECT): physician frontline treatment preferences for stage III or IV classic Hodgkin lymphoma. Pan Pacific Lymphoma Conference; 2022 Jul 18–22; Koloa, HI.
- Flora DR, Parsons SK, Liu N, et al. Classic Hodgkin lymphoma: real-world observations from physicians, patients, and caregivers on the disease and its treatment (CONNECT)—a cross-sectional survey of patients with stage III or IV classic Hodgkin lymphoma compared by age. Pan Pacific Lymphoma Conference; 2022 Jul 18–22; Koloa, HI.