Follicular lymphoma (FL) is a major type of indolent B-cell lymphoma and remains an incurable disease [Citation1]. The introduction of anti-CD20 monoclonal antibodies has improved the clinical outcomes of patients with FL. However, survival outcomes remain heterogeneous, with a subset of patients with FL showing an aggressive clinical course.
Prognostic markers for patients with FL have been reported, including progression of disease within 24 months of front-line treatment (POD24) [Citation2], complete response at 30 months (CR30) [Citation3], and positron emission tomography complete response (PET-CR) [Citation4]. POD24 is a widely accepted and validated significant poor prognostic marker [Citation5]. In contrast, patients with FL with CR30 have shown similar survival to a sex- and age-matched general population [Citation6]. The secondary analysis of the GALLIUM trial revealed that response assessment at the end of therapy using 18F-fluorodeoxyglucose (FDG)-PET/computed tomography (CT) was a better predictor of FL survival than CT [Citation7]. Recently, PET-CR has been evaluated as a surrogate endpoint [Citation4].
Histologic transformation (HT) is strongly associated with mortality in patients with FL [Citation1,Citation8,Citation9]. However, the prognostic impact of HT in patients with early progression of FL remains to be fully elucidated due to the low rate of biopsy for HT diagnosis at the time of disease progression. According to previous studies [Citation8–10], biopsy specimens are obtained from only 20–40% of patients at the time of disease progression of FL, suggesting that a small proportion of patients is diagnosed with HT. Therefore, we aimed to retrospectively analyze 50 patients who received chemoimmunotherapy, among whom 82% of patients underwent biopsy at the time of disease progression.
In order to evaluate the clinical impact of HT on survival in patients with early progression of FL, we performed a single-center, retrospective study of patients who were initially diagnosed with FL (grades I, II, or IIIa) according to the World Health Organization’s classification and treated with first-line chemoimmunotherapy at Kumamoto University Hospital between March 2010 and December 2020. Patients with grade IIIb FL and composite lymphoma at initial diagnosis were excluded. Definition of HT was based on biopsy involving both an increased number of large cells and loss of follicular structure. Progression from grade I–II to III was not included in HT. We evaluated prognostic factors and treatment outcomes using a cohort of relapsed patients with FL. Clinical data for each patient were extracted from medical records. This study was approved by the Institutional Review Board of Kumamoto University Hospital (No. 2226) and was conducted in accordance with the principles of the Declaration of Helsinki.
Patients with FL were administered first-line chemoimmunotherapy based on the physician’s discretion. All patients received anti-CD20 monoclonal antibodies (rituximab or obinutuzumab) combined with chemotherapy. The chemotherapy regimen was selected from cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), or bendamustine. POD24 [Citation2], CR30 [Citation3], and PET-CR [4] were previously defined as disease progression within 24 months of first-line chemoimmunotherapy, CR at 30 months after induction treatment initiation, and CR via FDG-PET/CT 30 d before and 90 d after induction treatment termination, respectively. A radiologist re-reviewed and assessed all patients who had received FDG-PET/CT. The response assessment was evaluated according to the Lugano classification [Citation11] and the Revised International Working Group criteria for malignant lymphoma [Citation12]. Categorical variables were compared using Fisher’s exact test. Continuous variables were compared using the Mann–Whitney U test. The overall survival (OS) and progression-free survival (PFS) probabilities were calculated using the Kaplan–Meier method, and the groups were compared using the log-rank test. OS was defined as the duration from initial diagnosis to death from any cause or the date of the last follow-up. Statistical significance was defined as a two-sided p value of < 0.05. Statistical analyses were performed using the EZR software package version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [Citation13].
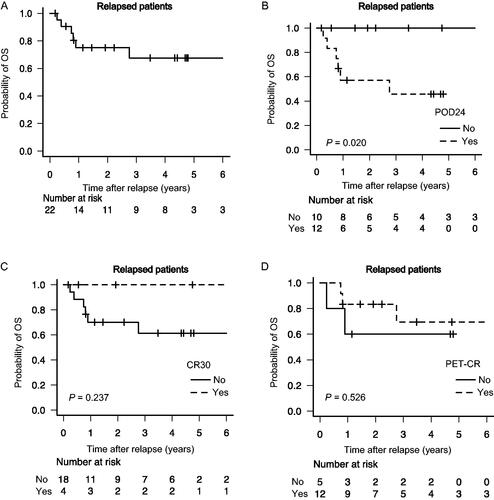
During the study period, 50 patients were initially diagnosed with FL and treated at the Kumamoto University Hospital (Table S1). Most patients (92%) were staged via FDG-PET/CT (Table S1). OS/PFS curves for the whole cohort were shown in Figure S1(A/B). In total, 22 patients (44%) had a relapse after receiving chemoimmunotherapy, and most (82%) underwent biopsy at the time of disease progression (Figure S2). Consequently, seven relapsed patients were diagnosed with HT. Table S2 lists the clinical characteristics of the 22 relapsed patients with FL at the time of disease progression. Their median age at the time of disease progression was 63 years (range, 46–80 years), and 68.2% of patients were over 60 years of age. Most patients (82%) were treated with CHOP-based regimens as induction therapy. Of the 22 relapsed patients, 54.5% (n = 12) had a relapse within 24 months after induction therapies, and most patients (82%) did not reach CR30. Among 17 patients who received PET/CT around the induction treatment termination, 12 achieved PET-CR.
With a median follow-up of 70.7 months (range, 9.7–142.2 months), the 4-year OS probability after disease progression was 67.6% (95% confidence interval [CI], 40.5–84.3%) in 22 relapsed patients with FL (). Among them, the 4-year OS probability after disease progression was 45.7% and 100.0% in patients with POD24 (n = 12) and without POD24 (n = 10), respectively (p = 0.0204, ). Similarly, patients with CR30 (n = 4) had a greater 4-year OS after disease progression than those without CR30 (n = 18; 100 vs. 61.3%; p = 0.237, ). Among patients who received PET/CT (n = 17), the 4-year OS following disease progression did not differ between patients who achieved PET-CR and those who did not (69.4 vs. 60.0%; p = 0.526, ).
Figure 1. Outcomes in patients with early FL progression. OS probability in relapsed patients with FL in the whole cohort (A); comparison of OS in relapsed patients with POD24 vs. without POD24 (B), in relapsed patients with CR30 vs. without CR30 (C), and in relapsed patients with PET-CR vs. without PET-CR (D). FL: follicular lymphoma; OS: overall survival; POD24: progression of disease within 24 months; CR30: complete response at 30 months; PET-CR: positron emission tomography complete response.
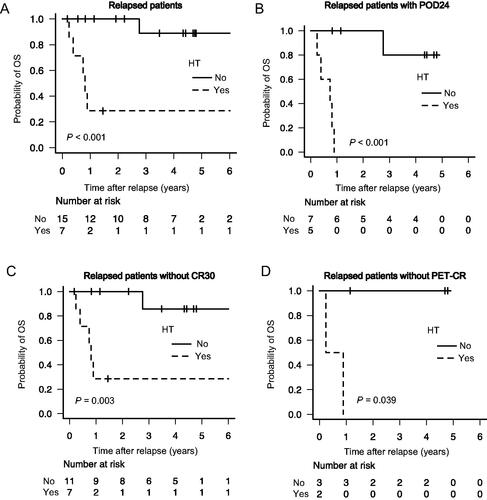
The 4-year OS probabilities in patients with (n = 7) and without HT (n = 15) were 28.6% (95% CI, 4.1–61.2%) and 88.9% (95% CI, 43.4–98.4%), respectively (p < 0.001, ). Focusing on patients with POD24, non-CR30, and non-PET-CR, no significant differences were observed between FL and HT in their clinical characteristics (Table S3). Notably, among patients with POD24 (n = 12), those with HT (n = 5) had a worse prognosis than those without HT (n = 7) (4-year OS: 0.0 vs. 80.0%; p < 0.001, ). Further, of the 18 patients without CR30, 4-year OS probabilities in patients with HT (n = 7) and without HT (n = 11) were 28.6 and 85.7%, respectively (p = 0.0033, ). Among non-PET-CR patients, the 4-year OS probabilities in patients with HT (n = 2) and without HT (n = 3) were 0.0 and 100.0%, respectively (p = 0.0389, ).
Figure 2. Impact of HT on survival in patients with early FL progression. Comparison of OS in relapsed patients with HT vs. without HT (A), in POD24 patients with HT vs. without HT (B), in non-CR30 patients with HT vs. without HT (C), and in non-PET-CR patients with HT vs. without HT (D). HT: histologic transformation; OS: overall survival; POD24: progression of disease within 24 months; CR30: complete response at 30 months; PET-CR: positron emission tomography complete response.
Of the 50 patients with FL, six patients died. All six patients had a relapse within 24 months. Among them, five patients died of lymphoma, 80.0% (n = 4) of whom had developed HT.
Herein, we demonstrated that HT has a robust poor prognostic impact on survival in patients with early FL progression. Surrogate markers might predict FL prognosis; however, histologic confirmation at the time of disease progression is important to clarify the accurate prognosis and treatment strategy for relapsed patients with FL, even at an early stage of disease progression.
Stratifying a subset of patients with FL with poor survival is an unmet clinical need. Early FL disease progression is a major predictor for poor prognosis in patients with FL [Citation2,Citation3,Citation5]. HT is also strongly associated with mortality in patients with FL [Citation8,Citation9]. However, the accurate prognostic impact of HT on survival remains unknown in patients with early progression of FL. This could be because biopsy specimens at the time of disease progression were obtained in only 20–40% of patients with FL [Citation8–10], potentially resulting in a small number of patients being diagnosed with HT. In contrast, 82% of relapsed patients with FL were diagnosed via biopsy in our study. This is a major strength of our study. Using this patient cohort, we discovered that HT has a robust poor prognostic value in patients with FL with POD24, CR30, and PET-CR. Histologic confirmation is important even in patients with early FL progression because treatment strategies for HT, such as hematopoietic stem cell transplantation, could be more intensive than those for FL [Citation8].
The exploratory analysis of the GALLIUM trial demonstrated that 16 out of 30 patients with POD24 and HT died during study follow-up [Citation14], suggesting that HT is strongly associated with mortality in patients with FL with POD24. Interestingly, a recent retrospective study from the GELTAMO group discovered that OS in patients with POD24 without HT was comparable to that in patients without POD24 [Citation15]. Furthermore, the MSKCC group reported that PET-based staging potentially excluded early-onset HT, resulting in prolonged OS in non-transformed patients with POD24 after standard treatment [Citation16]. However, the biopsy rate at the time of disease progression is unknown in these studies. Notably, our study which analyzed a cohort of 82% biopsy-confirmed patients, highlighted that the prognosis of POD24 with HT was inferior to that of POD24 without HT. Moreover, even in the 92% PET/CT-based staged patient cohort, HT was crucial for early death, especially within one year, in patients with early progression.
This study has several limitations. First, the number of patients who developed HT was small. Second, the chemoimmunotherapy regimens were diverse and determined at the physician’s discretion because of the study’s retrospective nature. Therefore, future investigation using a larger number of patients is required to confirm our findings.
In conclusion, our results are based on a cohort with most biopsy-confirmed patients, indicating that HT requires special attention, even in patients with early progression.
Ethics approval statement
This study was approved by the Institutional Review Board of Kumamoto University Hospital (No. 2226) and was conducted in accordance with the principles of the Declaration of Helsinki.
Author contributions
Takafumi Shichijo: Conceptualization (lead); data curation (equal); formal analysis (lead); investigation (lead); project administration (equal); visualization (lead); writing – original draft (lead); writing – review and editing (equal). Hiro Tatetsu: Conceptualization (equal); data curation (lead); funding acquisition (lead); investigation (equal); project administration (lead); resources (lead); writing – review and editing (equal); supervision (lead). Kisato Nosaka: Resources (equal); writing – review and editing (equal); supervision (equal). Yusuke Higuchi: Resources (equal); writing – review and editing (supporting). Yoshitaka Kikukawa: Resources (equal); writing – review and editing (supporting). Kosuke Toyoda: Writing – review and editing (equal). Shinya Shiraishi: Data curation (equal); resources (equal); investigation (equal). Jun-ichirou Yasunaga: Resources (equal); writing – review and editing (equal); supervision (equal). Masao Matsuoka: Writing – review and editing (equal); supervision (equal).
Supplemental Material
Download Zip (332.2 KB)Acknowledgments
The authors thank the medical, nursing, data-processing, laboratory, and clinical staff members at the participating center for their important contributions to this study and their dedicated patient care.
Disclosure statement
HT has received honoraria from AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical, Eisai, Novartis, Ono Pharmaceutical, SymBio Pharmaceuticals Limited, and Takeda Pharmaceutical and patents and royalties from Mesoblast. KN has received consultancy fees, research funding, and honoraria from Kyowa Kirin, research funding from Chugai Pharmaceutical, and honoraria from Celgene, Eisai, Meiji Seika Pharma, Janssen Pharmaceutical, Abbvie Inc., and Bristol Myers Squibb. MM has received research funding from Chugai Pharmaceutical and Kyowa Kirin. TS, YH, YK, KT, and J-i.Y have no conflicts of interest to disclose.
Data availability statement
Inquiries for data should be directed to [email protected] or [email protected]. The data will be available for achieving aims in the approved proposal.
Additional information
Funding
References
- Sarkozy C, Maurer MJ, Link BK, et al. Cause of death in follicular lymphoma in the first decade of the rituximab era: a pooled analysis of French and US cohorts. J Clin Oncol. 2019;37(2):144–152. doi: 10.1200/JCO.18.00400.
- Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the national LymphoCare study. J Clin Oncol. 2015;33(23):2516–2522. doi: 10.1200/JCO.2014.59.7534.
- Shi Q, Flowers CR, Hiddemann W, et al. Thirty-month complete response as a surrogate end point in first-line follicular lymphoma therapy: an individual patient-level analysis of multiple randomized trials. J Clin Oncol. 2017;35(5):552–560. doi: 10.1200/JCO.2016.70.8651.
- Dixon JG, Dimier N, Nielsen T, et al. End of induction positron emission tomography complete response (PET-CR) as a surrogate for progression-free survival in previously untreated follicular lymphoma. Br J Haematol. 2022;198(2):333–337. doi: 10.1111/bjh.18217.
- Casulo C, Dixon JG, Le-Rademacher J, et al. Validation of POD24 as a robust early clinical end point of poor survival in FL from 5225 patients on 13 clinical trials. Blood. 2022;139(11):1684–1693. doi: 10.1182/blood.2020010263.
- Magnano L, Alonso-Alvarez S, Alcoceba M, et al. Life expectancy of follicular lymphoma patients in complete response at 30 months is similar to that of the Spanish general population. Br J Haematol. 2019;185(3):480–491. doi: 10.1111/bjh.15805.
- Trotman J, Barrington SF, Belada D, et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (gallium): secondary analysis of a randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1530–1542. doi: 10.1016/S1470-2045(18)30618-1.
- Sarkozy C, Trneny M, Xerri L, et al. Risk factors and outcomes for patients with follicular lymphoma who had histologic transformation after response to first-line immunochemotherapy in the PRIMA trial. J Clin Oncol. 2016;34(22):2575–2582. doi: 10.1200/JCO.2015.65.7163.
- Shichijo T, Maruyama D, Yamauchi N, et al. Transformation scoring system (TSS): a new assessment index for clinical transformation of follicular lymphoma. Cancer Med. 2020;9(23):8864–8874. doi: 10.1002/cam4.3501.
- Federico M, Caballero Barrigón MD, Marcheselli L, et al. Rituximab and the risk of transformation of follicular lymphoma: a retrospective pooled analysis. Lancet Haematol. 2018;5(8):e359-67–e367. doi: 10.1016/S2352-3026(18)30090-5.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244.
- Seymour JF, Marcus R, Davies A, et al. Association of early disease progression and very poor survival in the gallium study in follicular lymphoma: benefit of obinutuzumab in reducing the rate of early progression. Haematologica. 2019;104(6):1202–1208. doi: 10.3324/haematol.2018.209015.
- Muntañola A, Mozas P, Mercadal S, et al. Early progression in follicular lymphoma in the absence of histological transformation or high-risk follicular lymphoma international prognostic index still has a favourable outcome. Br J Haematol. 2023;200(3):306–314. doi: 10.1111/bjh.18522.
- Batlevi CL, Sha F, Alperovich A, et al. Positron-emission tomographye-based staging reduces the prognostic impact of early disease progression in patients with follicular lymphoma. Eur J Cancer. 2020;126:78–90. doi: 10.1016/j.ejca.2019.12.006.
