Abstract
The optimal salvage chemotherapy regimen (SC) for relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) prior to autologous stem cell transplant remains unclear. Moreover, although chimeric antigen receptor T cell (CAR-T) therapies were recently approved for primary refractory DLBCL, head-to-head comparisons are lacking. We searched MEDLINE, EMBASE and CENTRAL to July 2022, for randomized trials that enrolled adult patients with R/R DLBCL and performed network meta-analyses (NMA) to assess the efficacy of SC and CAR-T therapies. NMA of SC (6 trials, 7 regimens, n = 1831) indicated that rituximab with gemcitabine, dexamethasone, cisplatin (R-GDP) improved OS and PFS over compared regimens. NMA of 3 CAR-T trials (n = 865) indicated that both axi-cel and liso-cel improved PFS over standard of care, with no difference in OS. Our results indicate that R-GDP may be preferred for R/R DLBCL over other SC compared. Longer follow-up is required for ongoing comparative survival analysis as data from CAR-T trials matures.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma, accounting for 30–40% of newly diagnosed cases worldwide [Citation1]. Despite improved outcomes since the addition of rituximab to chemotherapy [Citation2–4], 10–15% of DLBCL patients will have primary refractory disease and 20–30% will still relapse after first remission [Citation5–7]. For these patients, the standard of care (SOC) is salvage chemotherapy (SC) followed by autologous stem cell transplantation (ASCT) for those who are transplant eligible with chemotherapy sensitive disease [Citation5]. A number of salvage chemotherapy regimens have been evaluated, including rituximab, dexamethasone, cytarabine, cisplatin (R-DHAP) [Citation8], ifosfamide, carboplatin, etoposide (ICE) [Citation9], rituximab plus ICE (R-ICE) [Citation10], etoposide, cytarabine, cisplatin, methylprednisolone (ESHAP), and gemcitabine, dexamethasone, cisplatin (GDP) [Citation11]. However, no salvage regimen has been shown to be clearly superior over another and the optimal regimen remains uncertain [Citation12,Citation13].
Furthermore, approximately 60% of patients relapse following ASCT [Citation5,Citation14], and patients with refractory disease have even worse outcomes, with a median survival of 6 months and only 20% of patients alive at 2 years, as shown in SCHOLAR-1 [Citation15]. In recent years, chimeric antigen receptor T-cell (CAR-T) therapy emerged as an effective novel immunotherapy for those with primary refractory disease, chemoresistant disease, and those who relapse following ASCT. Although the superiority of CAR-T therapies (axicabtagene ciloleucel [axi-cel], tisagenlecleucel [tisa-cel], and lisocabtagene maraleucel [liso-cel]) over SC followed by ASCT in R/R DLBCL in the second line setting was recently evaluated in randomized clinical trials (RCTs) [Citation16–18], head-to-head comparisons of CAR-T products are lacking.
Accordingly, we conducted a systematic review and network meta-analysis (NMA) of RCTs for a comparative analysis of available treatments to shed light on the preferred SC and CAR-T regimen for R/R DLBCL.
Materials and methods
Protocol and registration
We registered our review with PROSPERO (CRD42022307996) and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [Citation19] extension statement for reporting systematic reviews incorporating NMAs.
Data sources and search strategies
Pubmed (MEDLINE), EMBASE databases, and Cochrane Central Register of Controlled Trials were searched from January 1, 2006 (as frontline use of rituximab for DLBCL was approved by the FDA in February of 2006) to July 7, 2022. The search strategy is summarized in the Supplementary Methods. For each database, we used keywords to identify the appropriate controlled vocabulary terms (e.g. MeSH headings). We searched for additional articles by scanning the reference list of all relevant reviews as well as the included trials.
Study eligibility
Eligible patients were aged ≥18 years with histologically confirmed DLBCL who relapsed after or were refractory to at least one prior regimen containing anthracycline and rituximab. The inclusion criteria were as follows: RCTs evaluating the effectiveness and safety of a therapeutic regimen over a control intervention; outcome being overall survival (OS) and/or progression-free survival (PFS); if the study included mixed population of lymphoma subtypes, that at least 70% of patients enrolled had DLBCL or transformed lymphoma, or if outcomes were reported separately for DLBCL/transformed lymphoma when less than 70% of enrolled patient. Exclusion criteria were as follows: 10 or fewer patients in each arm; trials of non-pharmacologic therapies; post hoc analyses; conference abstracts.
Study selection
The web-based tool Rayyan (https://www.rayyan.ai) was used to screen titles and abstracts eligible for full-text review by two pairs of reviewers (MA and IYG, MA and IL), which was carried out independently and in duplicate. Full texts of the remaining articles were reviewed to identify studies that met the inclusion criteria. Disagreements were resolved through consensus and consulting with clinical experts.
Data abstraction
The same pairs of reviewers extracted the following data independently and in duplicate: study characteristics (e.g. the first author, year of publication, study setting and funding source), participant and trial characteristics (e.g. sample size, mean age of participants, sex, clinical condition, stage of the disease, prior interventions, line of therapy, number of relapses, time to relapse after diagnosis, characteristics of interventions and comparators, outcomes of interest, and adverse events (AEs). Data from the longest follow-up duration were extracted if studies reported different follow-up lengths. Study authors were contacted for missing or insufficient information.
Study outcomes
The primary outcome was OS and the secondary outcome was PFS. OS was defined as the time from randomization to death, and PFS was defined as the time from randomization to disease progression or death from any cause, whichever occurred first. Additional secondary outcomes were most frequent AEs categorized into 5 categories: hematologic, gastrointestinal (GI), infectious, treatment-related death and other.
Data synthesis and statistical analysis
We calculated log (ln) of hazard ratio (HR) (lnHR), the variance of lnHR (var(lnHR)), and standard error of lnHR (se(lnHR)) directly from unadjusted HR and 95% confidence intervals (CIs), as the measure of effect estimate for quantitative synthesis. If trials did not report HR and 95% CI but provided sufficient data on OS and PFS, the log HRs and variances were estimated from the total number of events and p-value from the log-rank test using the formulas introduced by Tierney et al. [Citation20]. If trials reported insufficient data, lnHR and var(lnHR) were estimated from Kaplan–Meier (KM) curves [Citation20]. For AEs, we calculated an odds ratio (OR) with 95% CI for each study for an overall estimation. HR and OR < 1 indicates a reduction in the risk of the outcome.
NMAs of outcomes were conducted separately for salvage chemotherapy and CAR-T trials, for outcomes reported by at least 3 trials. We first generated graphs for the study outcomes to identify disconnected comparisons and excluded trials with nodes disconnected from the rest of the network. We then conducted NMA to synthesize the available evidence from the entire network by combining direct and indirect estimates for each comparison into a single summary treatment effect. A Frequentist fixed-effect model was used throughout for comparative analysis [Citation21]. Although our networks were connected and all nodes had a pathway to other nodes, our networks do not have closed loops which are required to test for consistency.
Transitivity was assumed a priori. Given the nature of the networks (tree-shaped networks) built in this study and our inability to assess the inconsistency assumptions using statistical methods, we assessed the transitivity assumption in the included studies and took these into consideration when evaluating the certainty of evidence. To this end, we compared the distribution of effect modifiers across different comparisons and rated down the certainty of evidence in the comparisons where imbalanced distributions may affect the plausibility of transitivity assumption.
We applied probability rankings to report the rank order of compared therapies. However, given the limitations of this approach [Citation22], simplifying the interpretation of the network results only based on the probabilities may be misleading, particularly when comparisons are not well connected to each other [Citation22]. As such, we also estimated the ranking probability and interpreted the relative effect of the therapies by using the surface under the cumulative ranking curve (SUCRA) and rankograms for displaying rank probabilities. The larger the SUCRA value, the higher the rank of the corresponding treatment among the networks, whereby a SUCRA of 1 indicates the most effective and 0 indicates the least effective intervention [Citation23]. P-scores were measured to indicate the extent of certainty that a treatment is better than another treatment, averaged over all competing treatments. All analyses were performed using R version 4.1.2 (2021-11-01) using the ‘netmeta’ package [Citation24].
Data quality assessment
Two reviewers independently assessed the risk of bias using a modified version of the Cochrane risk of bias instrument [Citation25]. This instrument assesses the following responses including ‘definitely yes’ or ‘probably yes’ (considered as low risk of bias), or ‘definitely no’ or ‘probably no’ (considered as high risk of bias) to the following components: random sequence generation; allocation concealment; blinding of patients, caregivers, outcome assessors, outcome adjudicators and data analysts; and incomplete outcome data with ≥ 20% missing data assessed as high risk of bias.
We applied the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach to assess the certainty of evidence on a component-by-component basis according to the following domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias [Citation26,Citation27]. This approach specifies four levels of the certainty for a body of evidence for a given outcome: high certainty indicating very confident that the true effect lies close to that of the estimate of the effect; moderate certainty indicating moderately confident in the effect estimate; low certainty indicating limited confidence in the effect estimate; very low certainty indicating we have very little confidence in the effect estimate [Citation26]), and we applied this approach to each network and indirect effect estimates [Citation27,Citation28].
Results
Search results and study characteristics
The search strategies identified 5871 unique citations, for which the titles and abstracts were screened for inclusion. The full texts of 121 articles were retrieved, of which 22 trials [Citation8,Citation11,Citation16,Citation17,Citation29–46], including one trial in Chinese language [Citation46], met inclusion criteria (), comprising of 4503 enrolled patients from North America, Europe, Middle East, and China.
Figure 1. PRISMA Flowchart illustrating the selection of studies included in our analysis. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses
All included trials were two-arm trials, and 15 trials were multicenter trials. The median of the mean age of included patients was 59 years (interquartile range [IQR], 55 to 66 years), and all trials included participants with Ann Arbor stage I-IV. Five trials [Citation31,Citation32,Citation36,Citation38,Citation43] enrolled patients ineligible for curative treatment due to demographics such as age and comorbidity burden. Eight trials enrolled only DLBCL patients while the remainder enrolled patients with different non-Hodgkin lymphoma, whereby DLBCL and transformed lymphoma comprised of >70% of the cohort (Supplement Table S1). There were 13 studies not eligible for quantitative synthesis and included for qualitative synthesis only (details presented in Supplement Tables S2 and S3).
Risk of bias
All included trials were at high risk of bias for at least one domain: 9 (40%) were at high risk of bias for all domains except for one domain, all 22 (100%) had adequate randomization sequence generation, 15 (68%) had adequate allocation concealment, 3 (14%) blinded patients, 1 (4%) blinded health care providers, 1 (4%) blinded data collectors, and 5 (23%) blinded outcome assessors. Fourteen trials (64%) reported ≥20% missing outcome data (Supplement Table S4).
Network meta-analysis
The NMA was undertaken through constructing several individual networks, as there was not sufficient overlap between included trials to produce a single coherent network for each outcome. Moreover, each comparison was informed by one trial, and each outcome was analyzed based on the available data. There were 9 trials eligible for quantitative NMA synthesis, with 6 trials included in the SC NMA and 3 trials included in the CAR-T NMA ().
Table 1. Summary of characteristics of trials included in network meta-analysis.
NMA of salvage chemotherapy trials
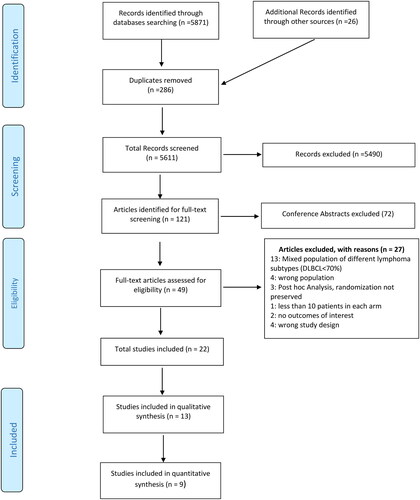
NMA of SC was conducted on 6 studies, comprising 1831 participants, comparing 7 treatments [Citation8,Citation11,Citation29,Citation34,Citation42,Citation46]. For the primary outcome of OS, Supplementary Figure S1 shows network plot of eligible comparisons. Compared with R-GDP, DHAP (HR 1.93, 95% CI 1.03-3.63), ESHAP (HR 2.98, 95% CI 1.40-6.35), ofatumumab with DHAP (O-DHAP) (HR 2.82, 95% CI 1.30-6.12) and R-DHAP (HR 2.54, 95% CI 1.22- 5.29) were all associated with worse OS, albeit with low certainty (Supplemental Tables S5A and Table S6). Compared with GDP, R-GDP was associated with improved OS (HR 0.53, 95% CI 0.29-0.97) ( and Supplemental Table S5A) supported by low certainty of evidence (Supplemental Table S6). In terms of SUCRA rankings, R-GDP ranked highest (SUCRA value 0.92), while ESHAP ranked lowest (SUCRA values 0.15) (Supplementary Figure S2 and ).
Figure 2. Network meta-analysis of eligible salvage chemotherapy trials for primary outcome of overall survival (OS). the comparative effectiveness of regimens and corresponding hazard ratio (HR) and 95% confidence interval (CI) are shown in panel A, and the estimated surface under the cumulative ranking (SUCRA) values based on 10,000 simulations are shown in panel B.
Abbreviations: GDP (gemcitabine, dexamethasone, cisplatin), R-GDP, (GDP + rituximab), ICE (ifosfamide, mesna, carboplatin, etoposide), R-ICE (ICE + rituximab), CAR-T (chimeric antigen receptor T-cell therapy), DHAP (dexamethasone, cytarabine, cisplatin), R-DHAP (rituximab + DHAP), O-DHAP (DHAP + ofatumumab), ESHAP (etoposide, cytarabine, cisplatin, methylprednisolone).
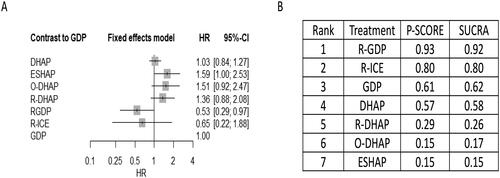
Supplementary Figure S3 shows network plot of eligible comparisons for PFS in salvage chemotherapy trials. When compared to GDP, R-GDP was associated with better PFS (HR 0.56, 95% CI 0.33-0.93), while R-ICE was associated with worse PFS (HR 1.76, 95% CI 1.09-2.85) () supported by low and moderate certainty of evidence respectively. Compared with R-GDP, ESHAP (HR 2.75, 95% CI 1.42-5.36), O-DHAP (HR 2.28, 95% CI 1.15- 4.53) and R-DHAP (HR 2.55, 95% CI 1.34- 4.87) were all associated with a significantly decreased PFS, supported by moderate to low certainty of evidence (Supplemental Tables S5B and S7). Low certainty of evidence indicated that compared with R-ICE, DHAP (HR 0.57, 95% CI, 0.37-0.89) and R-GDP (HR 0.32, 95% CI 0.16- 0.64) significantly improved PFS (Supplemental Tables S5B and S7). R-GDP and ESHAP ranked highest and lowest among the treatments with respect to PFS (SUCRA values 0.99 and 0.10, respectively) (Supplement Figure S4 and ).
Figure 3. Network meta-analysis of eligible salvage chemotherapy trials for primary outcome of progression-free survival (PFS). the comparative effectiveness of regimens and corresponding hazard ratio (HR) and 95% confidence interval (CI) are shown in panel A, and the estimated surface under the cumulative ranking (SUCRA) values based on 10,000 simulations are shown in panel B.
Abbreviations: GDP (gemcitabine, dexamethasone, cisplatin), R-GDP, (GDP + rituximab), ICE (ifosfamide, mesna, carboplatin, etoposide), R-ICE (ICE + rituximab), CAR-T (chimeric antigen receptor T-cell therapy), DHAP (dexamethasone, cytarabine, cisplatin), R-DHAP (rituximab + DHAP), O-DHAP (DHAP + ofatumumab), ESHAP (etoposide, cytarabine, cisplatin, methylprednisolone).
In terms of AEs, infection was the only frequent AE reported in 3 SC trials (n = 749) eligible for NMA [Citation11, Citation29, Citation46], with no significant differences between trials in the NMA (Supplemental Table S8, Supplemental Figures S8 and S9).
NMA of CAR-T trials
NMA was conducted for 3 CAR-T trials comprising of 865 participants [Citation16,Citation17,Citation44]. For the primary outcome of OS, Supplement Figure 5 shows network plot of eligible comparisons. We found no significant difference in OS between the CAR-T arm and the SOC arm (SC followed by ASCT) (). Low certainty of evidence indicated that axi-cel had significantly better OS compared to tisa-cel (HR 0.59, 95% CI 0.35-0.98), and liso-cel significantly improved OS over tisa-cel (HR 0.41, 95% CI 0.19-0.90) (Supplementary Tables S9A and S10). Amongst the CAR-T therapy OS rankings, liso-cel ranked highest (SUCRA value 0.92) (Supplement Figure S6 and ).
Figure 4. Network meta-analysis of eligible chimeric antigen receptor T-cell (CAR-T) trials for primary outcome of overall survival (OS). the comparative effectiveness of regimens and corresponding hazard ratio (HR) and 95% confidence interval (CI) are shown in panel A, and the estimated surface under the cumulative ranking (SUCRA) values based on 10,000 simulations are shown in panel B.
Abbreviations: SC (salvage chemotherapy followed by ASCT), axi-cel (axicabtagene ciloleucel), liso-cel (lisocabtagene maraleucel), tisa-cel (tisagenlecleucel).
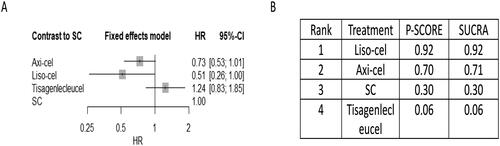
Figure 5. Network meta-analysis of eligible chimeric antigen receptor T-cell (CAR-T) trials for primary outcome of progression-free survival (PFS). the comparative effectiveness of regimens and corresponding hazard ratio (HR) and 95% confidence interval (CI) are shown in panel A, and the estimated surface under the cumulative ranking (SUCRA) values based on 10,000 simulations are shown in panel B.
Abbreviations: SC (salvage chemotherapy followed by ASCT), axi-cel (axicabtagene ciloleucel), liso-cel (lisocabtagene maraleucel), tisa-cel (tisagenlecleucel).
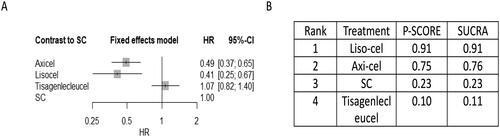
For the secondary outcome of PFS, Supplement Figure S5 shows network of eligible comparisons. axi-cel (HR 0.49, 95% CI, 0.37-0.65) and liso-cel (HR 0.41, 95% CI 0.25-0.67) significantly improved PFS when compared to SOC, supported by moderate to low certainty of evidence (, Supplemental Tables S9B and S11). Compared to tisa-cel, axi-cel (HR 0.46, 95% CI 0.31-0.67) and liso-cel (HR 0.38, 95% CI 0.25-0.67) had improved PFS, supported by low certainty of evidence (Supplemental Tables S9B and S11). For CAR-T therapy PFS rankings, liso-cel (SUCRA value 0.91) ranked highest (Supplement Figure S7 and ).
In terms of AEs, tisa-cel and axi-cel had significantly higher probability of cytokine release syndrome (CRS), when compared to SOC (tisa-cel OR 17.66, 95% CI 1.01-308.59; axi-cel OR 24.30, 95% CI 1.42-415.75), supported by low certainty of evidence (Supplemental Figure S10, Supplemental Table S11). Similarly, compared to SOC, axi-cel (OR 44.87, 95% CI 6.07-331.50) had significantly higher probability of neurologic toxicity while liso-cel and tisa-cel did not, supported by low certainty of evidence (Supplemental Figure S12, Supplementary Table S12). Of the CAR-T trials, liso-cel had the highest SUCRA value for CRS, followed by tisa-cel and axi-cel, while tisa-cel had the highest SUCRA value for neurotoxicity, followed by liso-cel and axi-cel, indicating that axi-cel had the highest risk for CRS and neurotoxicity (Supplementary Figures S11 and S13, respectively).
When compared to SOC, axi-cel and tisa-cel had significantly lower odds of febrile neutropenia (axi-cel OR 0.06, 95% CI 0.02-0.18; tisa-cel OR 0.45, 95% CI 0.25–0.80), supported by moderate certainty of evidence. Furthermore, axi-cel had the lowest odds of febrile neutropenia compared to liso-cel and tisa-cel, supported by low certainty of evidence (Supplementary Figure S14, Supplement Table S13). Axi-cel had the highest SUCRA value (1.0) indicating the lowest odds of febrile neutropenia (Supplementary Figure S15). NMA of other hematologic AEs anemia, thrombocytopenia, and leukopenia are shown in Supplement Table S13 and Supplementary Figures S16–S21. For ranking of hematologic AEs, tisa-cel had the highest probability of anemia (SUCRA value 0.99) and leukopenia (SUCRA 0.9), axi-cel had the highest probability of thrombocytopenia (SUCRA 1.0), and SOC had the lowest probability of neutropenia (SUCRA 1.0).
Moderate certainty of evidence showed that compared with SOC, tisa-cel (OR 0.19, 95% CI 0.04–0.87) significantly reduced the odds of nausea (Supplementary Figure S22 and Supplementary Table S14). Tisa-cel ranked highest among SUCRA values (0.87) indicating the least toxicity among evaluated treatments (Supplementary Figure S23).
Our NMA revealed no significant association between CAR-T therapy and the risk of diarrhea, vomiting, dyspnea or fatigue (Supplemental Table S14, Supplemental Figures S24–S31).
Discussion
This systematic review and network meta-analysis was carried out to compare the efficacy and safety of curative intent treatment approaches for relapsed or refractory DLBCL including various salvage regimens (GDP, R-GDP, DHAP, O-DHAP, R-DHAP, ESHAP, R-ICE) and CAR-T therapies (axi-cel, liso-cel, tisa-cel). This analysis included 1831 DLBCL patients from six salvage chemotherapy trials and 865 patients from three CAR-T trials. The findings of this study indicated that R-GDP may be associated with improved OS and PFS when compared to other evaluated regimens. For CAR-T therapies, both axi-cel and liso-cel may have improvement in PFS over SOC, while no difference was observed for OS.
Although there are prior studies report on systematic reviews and/or meta-analyses for treatment of/R DLBCL [Citation47–49], they are limited by the following: 1) non-comprehensive search strategy [Citation49]; 2) lack of statistical pooling of effect estimates [Citation47,Citation48]; 3) limited evaluation of the certainty of evidence [Citation49]; 4) outdated literature search (up to 2016) [Citation47,Citation49]. As such, our study provides an up-to-date systematic review and NMA for direct and indirect comparisons to elucidate the optimal salvage regimen for patients with R/R DLBCL based on outcomes OS and PFS.
Salvage regimens are designed with the goal of achieving high remission rates prior to ASCT with a favorable toxicity profile and minimal impairment of peripheral blood stem cell mobilization. The results of this study indicated that R-GDP ranked highest for primary outcomes OS and PFS when compared to other salvage regimens through direct and indirect evidence. Accordingly, our data support the use of R-GDP as the preferred regimen, particularly given its favorable safety profile, improved patient-reported quality of life, fewer hospitalizations and outpatient delivery [Citation11]. The role of rituximab in salvage therapy in the rituximab era has been called into question and its’ potential benefit in combination with chemotherapy required additional evaluation. For instance, in the HOVON-44 study, the addition of rituximab improved PFS in patients who were not exposed to rituximab in first-line treatment [Citation42]; though response rates were significantly lower among those that received frontline rituximab. While there is uncertainty regarding the merit of re-treatment with rituximab in the current era, our NMA supports combined use of rituximab with SC. This is also concordant with the results of a systematic review of 4 RCTs including 409 patients indicating that rituximab salvage therapy may be effective in improving outcomes for patients with R/R DLBCL [Citation50]. The role of ofatumumab as another anti-CD20 antibody was evaluated in the ORCHARRD study, which did not show improved outcomes over rituximab [Citation34].
For almost three decades, the SOC for transplant-eligible patient has been platinum-based SC followed by ASCT. Although our results suggest that R-GDP may be the favored salvage regimen for R/R DLBCL, it did not increase the proportion of patients proceeding to ASCT beyond 50% [Citation11]. As such, second-line SC followed by ASCT is being challenged by the development of novel, non-chemotherapy-based treatment strategies. Autologous CD19-directed CAR-T therapy became the curative treatment strategy for patients with DLBCL in the third-line setting; its use in the second-line setting was the next step in clinical development [Citation16–18,Citation44,Citation51–54]. For the first time since the PARMA trial [Citation55], three ground-breaking CAR-T trials set out to challenge the current SOC for DLBCL patients who relapse within 12 months of first-line therapy: ZUMA-7 (axi-cel, NCT03391466) [Citation16], Belinda (tisa-cel, CT03570892) [Citation44], and Transform (liso-cel, NCT03575351) [Citation17] for patients with R/R DLBCL within 12 months of first-line therapy completion. Based on the favorable PFS and event-free survival (EFS) outcomes over SOC in ZUMA-7 and transform, but not Belinda, both axi-cel and liso-cel were approved by the FDA for its use in the second-line setting.
A strength of this study is that we used NMA to compare the effectiveness between the three CAR-T therapies, particularly given the lack of head-to-head comparisons. The results presented indicate that axi-cel and liso-cel may have improved PFS over tisa-cel, and that liso-cel ranked highest for PFS amongst the three CAR-T therapies. This PFS benefit is not associated with a statistically significant OS benefit for liso-cel, but is associated with OS benefit for axi-cel; however, longer follow-up is required for more definitive OS results [Citation16,Citation17,Citation44,Citation56]. Although axi-cel and liso-cel were associated with improved PFS over SOC, both axi-cel and liso-cel were associated with a significantly greater risk of CRS, and axi-cel was associated with greater risk of neurotoxicity. Although any CRS occurred in 49–92% of study participants in the three trials, rates of grade ≥3 CRS was much lower at 1%, 5%, and 6%, for liso-cel, tisa-cel, and axi-cel respectively. Neurotoxicity was noticeably more common for axi-cel, at 21% of patients with grade ≥3 toxicity, compared to 4% for liso-cel, and 2% for tisa-cel. Our NMA indicated that axi-cel was associated with the greatest odds of CRS and neurotoxicity. This may be in part related to the different costimulatory signaling domain, CD28, used by axi-cel, as opposed to 41BB for liso-cel and tisa-cel is 41BB, potentially driven by the greater cytokines elevation associated with greater T cell expansion [Citation57]. In a previously published matching-adjusted indirect comparison of axi-cel vs. liso-cel in the third line or later setting for R/R DLBCL, liso-cel had a more favorable safety profile than axi-cel [Citation58]. Generally, cytopenias were less common in the CAR-T arms compared to SOC as was febrile neutropenia. Our NMA indicated that the risk of febrile neutropenia and thrombocytopenia was indeed reduced compared to SOC.
Nevertheless, an important limitation that may bias the CAR-T comparative results reported here is the variability in design and conduct between l trials, and particualrly the Belinda evaluating tisa-cel. Differences that may have negatively impacted outcomes in Belinda include allowance of bridging chemotherapy which likely biased toward less efficacy from tisa-cel, prolonged time from randomization to CAR-T infusion due to manufacturing considerations (median 52 days compared to 29 days in Zuma-7; not reported for Transform), and enrollment of more patients with high-risk features (active B-cell subtype and double/triple hit status) in Belinda. Although Belinda met the inclusion criteria for our NMA, its inclusion likely introduced significant heterogeneity to the analysis and the potential lower efficacy of tisa-cel beyond study design differences cannot be inferred from these results. Furthermore, the full benefit and limitations of CAR-T will require more mature follow-up. Moreover, despite the success of this treatment modality, there is much to understand and optimize regarding efficacy, safety, and delivery to patients. Further studies regarding outcomes based on disease histology, biology, and clinical subgroups are more important than ever to better stratify how to select therapy for individual patients and identify patients best suited to receive CAR-T over ASCT.
There are several limitations to consider when interpreting the results of this study. An important limitation is the relatively small number of eligible studies for the evaluated therapies, and that 13 eligible studies could not be connected to the network and were included for qualitative analysis only. Moreover, our NMA results were limited by the lack of multiple studies per comparison, precluding comprehensive comparative analysis as we were unable to estimate the between-study variability of effect sizes. Though a random effects model for NMA may have been more generalizable than a fixed effects model, this was not feasible due to the limited number of trial comparisons.
Due to the lack of closed loops in the networks, we were unable to assess how effect modifiers such as molecular subtype, stage, and prognostic index may impact comparisons of this NMA. Although we assumed transitivity a priori based on extracted study characteristics of included studies, clinical and methodological differences are unavoidable between studies in a systematic review. Additional effect modifiers not available from the included studies that may introduce heterogeneity and affect the plausibility of transitivity assumption include histology type, stage, prognostic indices, and history of prior treatments. Given the limited direct evidence comparing evaluated therapies, our NMA results were supported by low to moderate certainty of evidence. Our risk of bias assessment of the included trials may differ from the readership.
As the majority of included studies had high risk of bias, caution should be exercised when interpreting the results of this study as the risk of bias may limit the confidence of estimated treatment effects. Due to the limited number of trials available, sensitivity analysis to assess the impact of risk of bias could not be performed. These limitations highlight the need to improve the quality and rigor of future trials to strengthen the evidence base. Finally, although SUCRA is a commonly used approach in NMA to rank treatments based on probability, it has several limitations including lack of confidence intervals and assuming homogenous treatment effects, and its ranking interpretation should be in conjunction with NMA estimates along with its confidence intervals and certainty of evidence.
Despite these limitations, the benefit of NMA such as ours is aggregating available evidence to estimate more precise estimates of outcomes, particularly given the limited number of trials available and the fact that there is unlikely to be head-to-head comparisons among the compared therapies under study.
Conclusion
Of the compared salvage chemotherapy regimens, our network meta-analysis aggregating direct and indirect evidence showed that rituximab added to GDP was associated with improved OS and PFS over other evaluated regimens. CD19 directed CAR-T therapy with axi-cel and liso-cel improved PFS over SC followed by ASCT with no difference in OS based on published data included for analysis. While our study shed light on potentially preferred therapies, we appreciate that treatment selection is a complex process accounting for both patient outcomes and resource utilization. As such, our study is not meant to identify the most efficacious regimen clinicians should use for all patients but rather to synthesize trial data to help guide treatment decision-making.
Supplemental Material
Download MS Word (440.4 KB)Disclosure statement
The authors report there are no competing interests to declare. All authors have no financial relationships with any organizations that might have an interest in this work and no other relationships or activities that could appear to have influenced this submission.
Data availability statement
The data that support the findings of this study are openly available in referenced studies. The data abstraction form is available on request from the corresponding author.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by world health organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. doi:10.3322/caac.21357
- Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi:10.1056/NEJMoa011795
- Johnston KM, Marra CA, Connors JM, et al. Cost‐effectiveness of the addition of rituximab to CHOP chemotherapy in first‐line treatment for diffuse large B‐cell lymphoma in a population‐based observational cohort in British Columbia. Value Health. 2010;13(6):703–711. doi:10.1111/j.1524-4733.2010.00737.x
- Maurer M, Habermann T, Shi Q, et al. Progression-free survival at 24 months (PFS24) and subsequent outcome for patients with diffuse large B-cell lymphoma (DLBCL) enrolled on randomized clinical trials. Ann Oncol. 2018;29(8):1822–1827. doi:10.1093/annonc/mdy203
- Skrabek P, Assouline S, Christofides A, et al. Emerging therapies for the treatment of relapsed or refractory diffuse large B cell lymphoma. Curr Oncol. 2019;26(4):253–265. doi:10.3747/co.26.5421
- Ladicka M. Relapsed/refractory diffuse large B-cell lymphoma. Onkologia (Bratislava). 2015;10(4):232–235.
- Coiffier B, Sarkozy C. Diffuse large B-cell lymphoma: r -CHOP failure—what to do? Hematology Am Soc Hematol Educ Program. 2016;2016(1):366–378. doi:10.1182/asheducation-2016.1.366
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi:10.1200/JCO.2010.28.1618
- Zelenetz AD, Hamlin P, Kewalramani T, et al. Ifosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2003;14(Suppl 1):i5–10. doi:10.1093/annonc/mdg702
- Kewalramani T, Zelenetz AD, Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103(10):3684–3688. doi:10.1182/blood-2003-11-3911
- Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY. 12. J Clin Oncol. 2014;103(10):3684–3688.
- Kondo E. Autologous hematopoietic stem cell transplantation for diffuse large B-cell lymphoma. J Clin Exp Hematop. 2016;56(2):100–108. doi:10.3960/jslrt.56.100
- Zahid U, Akbar F, Amaraneni A, et al. A review of autologous stem cell transplantation in lymphoma. Curr Hematol Malig Rep. 2017;12(3):217–226. doi:10.1007/s11899-017-0382-1
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:498–505. doi:10.1182/asheducation-2011.1.498
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood J Am Soc Hematol. 2017;130(16):1800–1808. doi:10.1182/blood-2017-03-769620
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. doi:10.1056/NEJMoa2116133
- Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi:10.1016/S0140-6736(22)00662-6
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi:10.1056/NEJMoa1804980
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9. doi:10.1186/2046-4053-4-1
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. doi:10.1186/1745-6215-8-16
- White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Res Synth Methods. 2012;3(2):111–125. doi:10.1002/jrsm.1045
- Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6(1):5. doi:10.1186/s13643-017-0473-z
- Salanti G, Ades A, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi:10.1016/j.jclinepi.2010.03.016
- Balduzzi S, Rücker G, Nikolakopoulou A, et al. Netmeta: an R package for network meta-analysis using frequentist methods. J Stat Soft. 2023;106(2):1–40. doi:10.18637/jss.v106.i02
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(2):d5928. doi:10.1136/bmj.d5928
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi:10.1016/j.jclinepi.2010.07.015
- Brignardello-Petersen R, Bonner A, Alexander PE, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36–44. doi:10.1016/j.jclinepi.2017.10.005
- Salanti G, Higgins JP, Ades A, et al. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. doi:10.1177/0962280207080643
- Aribi M, Mesli N, Remla N, et al. Gemcitabine and treatment of diffuse large B-cell lymphoma in relapsed or refractory elderly patients: a prospective randomized trial in Algeria. J Cancer Res Ther. 2010;6(1):41–46. doi:10.4103/0973-1482.63572
- Assouline SE, Nielsen TH, Yu S, et al. Phase 2 study of panobinostat with or without rituximab in relapsed diffuse large B-cell lymphoma. Blood, J Am Soc Hematol. 2016;128(2):185–194. doi:10.1182/blood-2016-02-699520
- Avilés A, Neri N, Huerta-Guzmán J, et al. ESHAP versus rituximab-ESHAP in frail patients with refractory diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2010;10(2):125–128. doi:10.3816/CLML.2010.n.017
- Czuczman MS, Trněný M, Davies A, et al. A phase 2/3 multicenter, randomized, Open-Label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2017;23(15):4127–4137. doi:10.1158/1078-0432.CCR-16-2818
- Fayad L, Ansell SM, Advani R, et al. Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: a randomized, double-blind, placebo-controlled phase 2b trial. Leuk Lymphoma. 2015;56(9):2569–2578. doi:10.3109/10428194.2015.1007504
- Van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35(5):544–551. doi:10.1200/JCO.2016.69.0198
- Morschhauser F, Flinn IW, Advani R, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol. 2019;6(5):e254–e265. doi:10.1016/S2352-3026(19)30026-2
- Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155–165. doi:10.1200/JCO.19.00172
- Vose JM, Carter S, Burns LJ, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. J Clin Oncol. 2013;31(13):1662–1668. doi:10.1200/JCO.2012.45.9453
- Dang NH, Ogura M, Castaigne S, et al. Randomized, phase 3 trial of inotuzumab ozogamicin plus rituximab versus chemotherapy plus rituximab for relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Br J Haematol. 2018;182(4):583–586. doi:10.1111/bjh.14820
- Pettengell R, Coiffier B, Narayanan G, et al. Pixantrone dimaleate versus other chemotherapeutic agents as a single-agent salvage treatment in patients with relapsed or refractory aggressive non-Hodgkin lymphoma: a phase 3, multicentre, open-label, randomised trial. Lancet Oncol. 2012;13(7):696–706. doi:10.1016/S1470-2045(12)70212-7
- Pettengell R, Długosz‐Danecka M, Andorsky D, et al. Pixantrone plus rituximab versus gemcitabine plus rituximab in patients with relapsed aggressive B‐cell non‐hodgkin lymphoma not eligible for stem cell transplantation: a phase 3, randomized, multicentre trial (PIX306). Br J Haematol. 2020;188(2):240–248. doi:10.1111/bjh.16255
- Shimoni A, Avivi I, Rowe JM, et al. A randomized study comparing yttrium‐90 ibritumomab tiuxetan (zevalin) and high‐dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma. Cancer. 2012;118(19):4706–4714. doi:10.1002/cncr.27418
- Vellenga E, van Putten WL, Van’t Veer MB, et al. Rituximab improves the treatment results of DHAP-VIM-DHAP and ASCT in relapsed/progressive aggressive CD20+ NHL: a prospective randomized HOVON trial. Blood J Am Soc Hematol. 2008;111(2):537–543. doi:10.1182/blood-2007-08-108415
- Yang X-G, Jiang C. Ligustrazine as a salvage agent for patients with relapsed or refractory non-Hodgkin’s lymphoma. Chin Med J (Engl). 2010;123(22):3206–3211.
- Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–639. doi:10.1056/NEJMoa2116596
- Srour SA, Li S, Popat UR, et al. A randomized phase II study of standard‐dose versus high‐dose rituximab with BEAM in autologous stem cell transplantation for relapsed aggressive B‐cell non‐hodgkin lymphomas: long term results. Br J Haematol. 2017;178(4):561–570. doi:10.1111/bjh.14731
- Muhebaier AA, Liu H, Guzailinuer MM, et al. Prognosis and safety of rituximab combined with GDP regime for patients with relapsed diffuse large B-cell lymphoma. Anti-Tumor Pharm. 2018;8(6):0897–0902.
- Galaznik A, Huelin R, Stokes M, et al. Systematic review of therapy used in relapsed or refractory diffuse large B-cell lymphoma and follicular lymphoma. Future Sci OA. 2018;4(7):FSO322. doi:10.4155/fsoa-2018-0049
- Thuresson P-O, Vander Velde N, Gupta P, et al. A systematic review of the clinical efficacy of treatments in relapsed or refractory diffuse large B cell lymphoma. Adv Therapy. 2020 Dec;37(12):4877-4893. doi:10.1007/s12325-020-01507-7
- Wang L, Lam H, Shou Y, et al. Meta-analytical methods for estimating outcomes from overall response rate in patients with relapsed/refractory diffuse large B-cell lymphoma. Oncotarget. 2019;10(35):3285–3293. doi:10.18632/oncotarget.26904
- Ren Y-R, Jin Y-D, Zhang Z-H, et al. Rituximab treatment strategy for patients with diffuse large B-cell lymphoma after first-line therapy: a systematic review and meta-analysis. Chin Med J (Engl). 2015;128(3):378–383. doi:10.4103/0366-6999.150111
- Bao F, Wan W, He T, et al. Autologous CD19-directed chimeric antigen receptor-T cell is an effective and safe treatment to refractory or relapsed diffuse large B-cell lymphoma. Cancer Gene Ther. 2019;26(7-8):248–255. doi:10.1038/s41417-018-0073-7
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi:10.1056/NEJMoa1707447
- Nastoupil LJ, Jain MD, Spiegel JY, et al. Axicabtagene ciloleucel (axi-cel) CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory large B-cell lymphoma: real world experience. Blood. 2018;132(Supplement 1):91–91. doi:10.1182/blood-2018-99-114152
- Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285–295. doi:10.1016/j.ymthe.2016.10.020
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi:10.1056/NEJM199512073332305
- Abramson JS, Garcia J, Abramson JS, et al. Cytokine release syndrome and neurological event costs in lisocabtagene maraleucel–treated patients in the TRANSCEND NHL 001 trial. Blood Adv. 2021;5(6):1695–1705. doi:10.1182/bloodadvances.2020003531
- Westin J, Sehn LH. CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift? Blood. 2022;139(18):2737–2746. doi:10.1182/blood.2022015789
- Maloney DG, Kuruvilla J, Liu FF, et al. Matching-adjusted indirect treatment comparison of liso-cel versus axi-cel in relapsed or refractory large B cell lymphoma. J Hematol Oncol. 2021;14(1):140. doi:10.1186/s13045-021-01144-9
