 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Real-world US healthcare resource utilization (HRU) and costs during first salvage therapy for relapsed/refractory (R/R) acute myeloid leukemia (AML) are described using IBM MarketScan® data (1/1/2007–6/30/2020). Treatments included high- (HIC) and low-intensity chemotherapy (LIC) alone, and gilteritinib, other FLT3 tyrosine kinase inhibitors (TKIs), and venetoclax with or without chemotherapy. Patients were diagnosed with R/R AML at ≥18 years of age between 1/1/2017–12/31/2019. Patient monthly all-cause HRU and costs were analyzed using a fixed-effects model. Data from 399 patients were analyzed (HIC, n = 104; LIC, n = 133; gilteritinib, n = 14; other FLT3 TKIs, n = 68; venetoclax, n = 80). Inpatient HRU was generally highest with HIC, whereas outpatient HRU was generally highest with LIC and venetoclax. Total all-cause incremental monthly costs appeared to be highest with HIC ($171,982) and similar for LIC ($60,512), gilteritinib ($47,218), other FLT3 TKIs ($43,218), and venetoclax ($77,566). Results highlight HRU and cost differences for R/R AML during first salvage therapy.
Introduction
Acute myeloid leukemia (AML) accounts for one-third of all leukemia cases in the United States (US), with increasing incidence reported [Citation1,Citation2]. In 2022, AML was estimated to affect 20,050 new patients and lead to 11,540 deaths in the US [Citation2]. Patients diagnosed with relapsed or refractory (R/R) AML generally have a poor prognosis [Citation3,Citation4].
The treatment landscape for patients with R/R AML has evolved over the last decade. Treatments now consist of traditional cytotoxic chemotherapies (i.e. high-intensity chemotherapy [HIC] and low-intensity chemotherapy [LIC] including hypomethylating agents) and therapies targeting specific pathways (i.e. venetoclax and FLT3 inhibitors) that can be given alone or in some cases with LIC [Citation5–8]. In patients diagnosed with AML, next-generation sequencing panels are now routinely employed and are guideline-recommended for the most common gene mutations found in AML, including FLT3, TP53, NPM1, RUNX1, ASXL1, IDH1/2, c-KIT and CEBPA [Citation5,Citation8,Citation9]. Treatments can be individualized based on the presence or absence of specific mutations. These individualized therapies may be administered orally, thereby decreasing the time patients spend in the hospital for infusions or monitoring and may have improved safety profiles [Citation6,Citation10–12].
A major driver of cost for patients with R/R AML is inpatient hospital care, including the cost of hospital-based chemotherapy administration that often requires continuous monitoring and treatment of serious adverse effects [Citation6]. Less is known about outpatient healthcare resource utilization (HRU) and costs for R/R AML, although there is often a shift in cost burden from the inpatient to outpatient setting in patients with R/R AML treated with novel therapies that are self-administered [Citation6].
As treatment options continue to evolve for patients with R/R AML, therapies targeting specific mutations are beginning to show promising survival benefits compared with traditional chemotherapies [Citation6,Citation8,Citation13]. A better understanding of the real-world HRU and costs associated with various treatment regimens, which as of 2022 was not available for the US, is hence needed to provide clinicians, decision-makers, and patients with the information required to make informed decisions. Therefore, we aimed to describe the HRU and costs in patients with R/R AML treated with HIC, LIC, gilteritinib, other FLT3 tyrosine kinase inhibitor (TKI) therapies, or venetoclax as first salvage therapy in the US.
Material and methods
Data source and study design
A retrospective cohort analysis was conducted using IBM MarketScan® data from 1 January 2007 to 30 June 2020 to determine HRU and costs of commonly used first salvage therapies for R/R AML. The database contains medical and prescription drug data, including HRU and costs from approximately 40 million deidentified patients annually who are covered by employer-sponsored private health insurance in the US [Citation14].
Patients were indexed on first evidence of R/R AML and followed until the earlier of the first salvage therapy end date or database cutoff date (30 June 2020). All-cause HRU and costs during treatment were estimated for five mutually exclusive regimens commonly used as first salvage therapy for R/R AML, including FLT3-mutation–positive (FLT3+) R/R AML: HIC alone, LIC alone, gilteritinib, other FLT3 TKIs (midostaurin and sorafenib), and venetoclax. Gilteritinib, other FLT3 TKIs, and venetoclax treatments could be administered as a single agent or in combination with chemotherapy. Treatment end date was defined as the earlier of 30 days after the last administration, the day before new treatment initiation, or the day before initiation of hematopoietic stem cell transplantation (HSCT)-related therapy.
Institutional review board approval was not required for this study as the deidentified data analyzed do not meet the criteria for human subject research as defined by 45 CFR 46.102.
Operational definitions
A series of algorithms were constructed to allow for the identification and categorization of R/R disease and treatment episodes to the extent possible as refractory status and certain treatments (HIC, LIC) are not readily identifiable in claims data. In these algorithms, published literature [Citation15] and factors such as diagnosis codes, care setting, length of inpatient stay, duration of outpatient chemotherapy treatment cycles, observed chemotherapy regimens, AML-related medications and procedures, and treatment switching and sequencing were utilized.
Outpatient and inpatient regimens
Outpatient chemotherapy regimens were identified using administrative records of specific chemotherapy agents (Supplemental Table 1) based on either 21- or 28-day cycles. Inpatient chemotherapy regimens were identified using AML diagnosis codes and codes for specific chemotherapies (Supplemental Tables 2, 3).
Induction and consolidation chemotherapy
Induction chemotherapy was defined as chemotherapy administered 7 days before to 14 days after the first AML diagnosis or the onset of AML relapse. Consolidation therapy with HIC was defined as HIC administered within 180 days of the end of HIC induction therapy, and consolidation therapy with LIC was defined as LIC administered post-induction therapy.
High-intensity and low-intensity chemotherapy
HIC was defined as the administration of cytarabine in combination with daunorubicin, doxorubicin, etoposide, idarubicin, mitoxantrone, cladribine, or clofarabine in either an inpatient or outpatient setting.
LIC was defined as any chemotherapy regimen not meeting HIC criteria and containing at least one of the following therapies: azacitidine, cytarabine, decitabine, enasidenib, gemtuzumab, glasdegib, and ivosidenib.
As dose was not available in the data, distinctions between HIC and LIC that were based on dose were not possible to distinguish.
Relapsed or refractory AML
Relapse was identified as either the first ICD diagnosis code for AML relapse, or the initiation of a new systemic therapy following remission or completion of consolidation therapy without subsequent HSCT, or after a time-lapse of more than 100 days post-HSCT.
Refractory AML in patients treated with HIC was identified by HIC induction therapy lasting more than 42 days without complications. Refractory AML in patients treated with LIC was identified by either a switch in chemotherapy regimen after three LIC cycles without subsequent HSCT, or initiation of FLT3 TKI therapy that was not administered as HSCT maintenance therapy.
Study population
Included patients had at least one AML diagnosis in the inpatient setting or at least two AML diagnoses within 30 days in the outpatient setting. Additionally, patients were aged 18 years or older at first AML diagnosis and had continuous health plan enrollment for ≥180 days prior to first AML diagnosis and through the start of first salvage therapy. Patients had evidence of R/R AML between 1 January 2017 and 31 December 2019 and received HIC, LIC, gilteritinib, other FLT3 TKIs, or venetoclax as first salvage therapy. Evidence of R/R AML was determined by diagnosis code and use of a treatment-based algorithm [Citation15].
Patients were excluded if they had acute promyelocytic leukemia, coverage under a capitated payment plan during the observation period, or concurrent administration of any FLT3 TKI with venetoclax.
Statistical analysis
Descriptive statistics were used to characterize baseline patient demographic and clinical characteristics. HRU and costs (US dollars) during the treatment period were descriptively analyzed by care setting and were adjusted for inflation (year 2019). To account for varying follow-up periods, per patient per month (PPPM) HRU and costs were descriptively summarized.
A fixed-effects model was used to assess the incremental cost associated with HIC, LIC, gilteritinib, midostaurin, sorafenib, and venetoclax. Patient-level analytical months were constructed as 30-day intervals for all observed data from the earlier of first AML diagnosis and 3 years prior to R/R onset. The multivariate regression analysis used the resultant patient-month level data to assess incremental costs associated with each treatment. Each patient was assigned a fixed class effect to account for covariates that did not vary over time or varied at a steady pace (e.g. sex and age). The results from these regression models were the estimated monthly incremental costs associated with each treatment.
In the fixed-effects model, events that occurred both before and after the first date of R/R AML as well as first salvage therapy or first line of treatment (LOT1) and second line of treatment (LOT2) were modeled. The model was expressed as
where Ci,t is the cost of a patient i in month t; αi is an individual fixed effect for each patient that captures their baseline monthly costs without AML, reflecting age, sex, comorbidities, and other patient-specific factors that impact healthcare costs and were constant throughout the study period; and µi,t is the error term. All other covariates were measured in days. Specifically, Ai,t reflects the number of days in month t that fall in the 30-day window before initial AML diagnosis, while Ri,t reflects the number of days in month t that fall in the 30-day window before R/R AML onset. The coefficients of these two variables estimate the potential increases in daily cost due to symptoms and diagnostic testing prior to initial AML diagnosis and R/R AML. Treatment variables TRTk,i,t reflect the number of days in month t the patient was exposed to HIC, LIC, gilteritinib, other FLT3 TKIs, venetoclax, or HCST, with targeted therapies further modeled by disease stage (i.e. non-R/R AML, R/R LOT1, and R/R LOT2) if there were at least three non-zero observations. Consequently, the following treatment covariates were included in the model:
gilteritinib (R/R LOT1, R/R LOT2)
other FLT3 TKI (non-R/R AML, R/R LOT1, R/R LOT2)
venetoclax (non-R/R AML, R/R LOT1, R/R LOT2)
HIC only
LIC only
HSCT during the month
The coefficients of treatment variables TRTk,i,t estimate average daily incremental costs while the patients were exposed to respective treatment. Variable NoTRT_AMLi,t reflects the number of days patient i was not exposed to any modeled treatment in month t while the disease had not progressed to R/R. Variable NoTRT_RRi,t reflects the number of days patient i was not exposed to any modeled treatment in month t after the disease has progressed to R/R. The coefficients of these two variables estimate average daily incremental cost in the absence of modeled treatment during the disease phases before and after R/R, respectively. All estimates of daily costs were converted to 30-day monthly costs.
Results
Patient characteristics
A total of 399 patients were included in the analysis (Supplemental Figure 1). Most baseline characteristics were similar across treatment groups (), although the mean (standard deviation [SD]) age for the HIC group appeared younger (50.9 [11.3] years) and the venetoclax group appeared older (61.2 [13.8] years) compared with a mean age ranging from 50.9–53.6 years across the other treatment groups. Additionally, the percentage of females was lower in the HIC and gilteritinib groups compared with the other treatment groups. Overall, 38.1% of patients received HIC at any time prior to their first R/R AML diagnosis; most patients treated with gilteritinib (64.3%) and other FLT3 TKIs (55.9%) had prior HIC treatment. Concomitant chemotherapy (HIC or LIC) was received by six (42.9%) patients receiving gilteritinib, 33 (48.5%) receiving other FLT3 TKIs, and 77 (96.3%) receiving venetoclax. Two (14.3%) patients received concomitant azacitidine or decitabine while receiving gilteritinib; 52 (65.0%) patients received concomitant azacitidine or decitabine, and 12 (15.0%) patients received concomitant cytarabine while receiving venetoclax. Approximately 20% of patients had a previous HSCT.
Table 1. Baseline characteristics.
Inpatient HRU
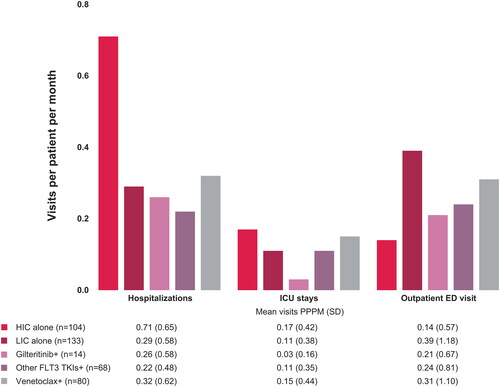
Among patients receiving HIC for first salvage therapy after diagnosis of R/R AML, almost all patients were hospitalized (n = 100, 96%); across other treatment groups, the percentages of patients requiring hospitalization during treatment were comparable and all appeared lower than HIC, ranging from 57% of patients (n = 39) in the other FLT3 TKI group to 71% of patients (n = 10) in the gilteritinib group (). Mean hospital length of stay ranged from 9.6 days during gilteritinib treatment (SD = 7.80) to 38.6 days during HIC (SD = 19.83).
Table 2. Hospitalization healthcare resource utilization.
Hospitalization visits PPPM appeared highest during HIC and similar across other groups (). Intensive care unit stays PPPM appeared highest during HIC (mean hospitalizations with ICU PPPM, 0.17, SD = 0.42) and venetoclax treatment (mean = 0.15, SD = 0.44) and the lowest during gilteritinib treatment (mean = 0.03, SD = 0.16).
Figure 1. HRU PPPM in the inpatient and emergency department setting for patients with R/R AML receiving first salvage therapy. Abbreviations: +: with or without HIC or LIC; AML: acute myeloid leukemia; ED: emergency department; HIC: high-intensity chemotherapy; HRU: healthcare resource utilization; ICU: intensive care unit; LIC: low-intensity chemotherapy; PPPM: per patient per month; R/R: relapsed or refractory; SD: standard deviation; TKI: tyrosine kinase inhibitor.
Outpatient HRU
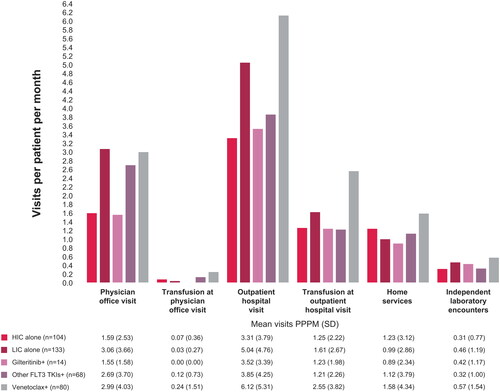
In the outpatient setting, results suggest that physician office visits and outpatient hospital visits PPPM were higher in the LIC, other FLT3 TKI, and venetoclax groups and lower in the HIC and gilteritinib groups (). Transfusion-related physician office and outpatient hospital visits PPPM appeared to be higher in the venetoclax group than in the other groups.
Figure 2. HRU PPPM in the outpatient setting for patients with R/R AML receiving first salvage therapy. Abbreviations: +: with or without HIC or LIC; AML: acute myeloid leukemia; HIC: high-intensity chemotherapy; HRU: healthcare resource utilization; LIC: low-intensity chemotherapy; PPPM: per patient per month; R/R: relapsed or refractory; SD: standard deviation; TKI: tyrosine kinase inhibitor.
Incremental hospitalization, outpatient, and prescription costs
Across all treatment groups, patients receiving HIC appeared to have the highest overall monthly incremental healthcare costs (). Incremental costs were lower for the remaining treatments, ranging from a mean (standard error [SE]) of $43,218 (2795) for patients treated with other FLT3 TKIs to a mean (SE) of $77,566 (3531) for patients treated with venetoclax, with patients treated with gilteritinib having mean (SE) incremental monthly costs of $47,218 (5523). For patients treated with HIC, the primary cost driver was inpatient costs with a mean (SE) monthly incremental cost of $167,460 (2841). Costs for patients treated with LIC, venetoclax, gilteritinib, or other FLT3 TKIs were distributed across inpatient, outpatient, and pharmacy costs. Incremental monthly prescription costs for gilteritinib appeared higher than for other FLT3 TKIs and for venetoclax. Conversely, inpatient and outpatient costs appeared lower for gilteritinib and other FLT3 TKIs than venetoclax ().
Table 3. Incremental monthly healthcare costs.
Discussion
Results from our analyses, which provide an overview of HRU and costs during treatment of R/R AML in first salvage therapy in the US, show considerable variation in HRU and costs during treatment across evaluated treatments and settings. Not surprisingly, inpatient HRU was generally the highest with HIC. In the outpatient setting, outpatient HRU was generally the highest with LIC and venetoclax, and prescription costs were the highest with gilteritinib.
Analysis of total all-cause costs during treatment showed that costs were highest with HIC, potentially due to long hospital stays, and were similar among other treatments evaluated. Among the other therapies evaluated, prescription drug costs for gilteritinib were the highest; however, all healthcare costs were similar for gilteritinib and other FLT3 TKIs and somewhat higher for venetoclax, suggesting that the high prescription drug costs reported for gilteritinib are potentially offset by the lower HRU seen in both the inpatient and outpatient settings.
Targeting specific mutations in AML, including those in the R/R setting, is an important next step in optimizing precision medicine [Citation16,Citation17]. The routine use of molecular assays to uncover recurrent or evolving mutations that may be targeted in R/R AML may facilitate a shift away from traditional cytotoxic therapies to the use of novel therapies selected based on somatic mutations [Citation18]. In patients with AML, agents are available to treat FLT3 and IDH mutations, the former of which may confer a worse prognosis than wild type [Citation19,Citation20]. In clinical practice, patients with FLT3+ R/R AML may be treated with several different FLT3- targeted therapies including the first-generation FLT3 inhibitors sorafenib and midostaurin and the second-generation FLT3 inhibitor gilteritinib [Citation21]. As of 2022, only gilteritinib showed a significant improvement in overall survival (9.3 vs 5.6 months; p < 0.001) and one-year survival (37.1% vs 16.7%) in patients with FLT3+ R/R AML compared with standard chemotherapy [Citation13].
Although this analysis included data on first salvage therapy for R/R AML administered from January 2017 through June 2020, few patients were treated with gilteritinib because it was only approved by the US Food and Drug Administration for FLT3+ R/R AML in November 2018. This study provides an early indicator that using an approved targeted agent may change the nature of HRU during treatment.
Limitations
The planned study utilized healthcare insurance data in order to estimate HRU and costs during first salvage treatment for R/R AML. Important limitations exist when using administrative data for research because the data were generated for the purpose of reimbursing healthcare providers for care.
Administrative records may not always accurately reflect a patient’s true medical condition. Specific to this study, algorithms were used to identify first salvage treatment episodes, R/R disease status, and differentiate between HIC and LIC treatment. However, distinctions between HIC and LIC that were based on dose were not possible. Additionally, the MarketScan database does not contain a substantial itemization of medications received during an inpatient hospital stay; hence, patients who stayed in the hospital for an extended period for LIC induction and received subsequent LIC treatments in an inpatient setting may be misclassified as receiving HIC. Finally, within claims, the intention of therapy (treatment vs maintenance) cannot be determined. We assumed that most patients receiving gilteritinib were receiving therapy for R/R disease versus post-chemotherapy maintenance therapy given the indication for gilteritinib during the study period.
We excluded patients who received concurrent administration of any FLT3 TKI with venetoclax. At the time this study was performed (i.e. prior to 2020), few patients treated with an FLT3 TKI received treatment in combination with venetoclax and the classification of these patients was not straightforward. Therefore, we opted to exclude patients receiving FLT3 TKI and venetoclax combination therapy. Only two patients were removed from the study due to this exclusion criterion.
FLT3 status is not directly identifiable in administrative claims data. Consequently, heterogeneity with regard to FLT3+ status is likely much greater in patients receiving HIC, LIC, or venetoclax than those receiving gilteritinib or other FLT3 TKI treatments. The extent to which underlying FLT3 status influences associations between treatment and HRU and costs is unknown. Literature has found that FLT3 mutations are associated with poor disease prognosis, including a greater risk of R/R disease and death [Citation19,Citation20].
This study attempted to measure HRU and costs incurred during treatment in light of the recent development of FLT3 treatments for R/R AML. In addition to the descriptive analysis of on-treatment HRU and costs, all-cause costs were modeled in a fixed-effects model to estimate the mean incremental cost of each treatment. Direct HRU and healthcare costs were reported PPPM to account for censoring. This study did not attempt to estimate the lifetime costs of treatment, indirect costs, or the economic value of potential improved quality or duration of life.
Conclusions
Overall, HRU and costs during first salvage R/R AML treatment varied across evaluated therapies. All-cause total costs were highest for HIC but similar for LIC, gilteritinib, other FLT3 TKIs, and venetoclax in this commercially insured US patient population. As more real-world data become available for recently approved therapies, particularly targeted therapies such as FLT3 inhibitors, further analyses are warranted to determine the effect of these therapies on HRU and costs alongside potential societal benefits including quality of life and survival.
Supplemental Material
Download MS Word (95.9 KB)Acknowledgments
Medical writing/editorial support was provided by Sarah A Thompson, PharmD, from OPEN Health, Parsippany, NJ, and editorial support by Cheryl Casterline, MA, from Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by the study sponsor.
Disclosure statement
L Muffly reports consultancy fees from Amgen Inc., CTI BioPharm Corp., Kite Pharma, Medexus Pharmaceuticals Inc., Astellas, and Pfizer Inc.; research funding/grants from Jasper Therapeutics, Adaptive Biotechnologies, Kite Pharma, and Bristol Myers Squibb; and honoraria for speaker engagements from Adaptive Biotechnologies and Pfizer Inc. C Young, D Nimke, Q Feng, and BJ Pandya are employees of Astellas Pharma, Inc.
Data availability statement
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com.
For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
- American Cancer Society. Key statistics for acute myeloid leukemia (AML). Accessed October 17, 2022. Available from: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html.
- Anders B, Veltri L, Kanate AS, et al. Outcomes of six-dose high-dose cytarabine as a salvage regimen for patients with relapsed/refractory acute myeloid leukemia. Adv Hematol. 2017;2017:6464972. doi:10.1155/2017/6464972
- Ramos NR, Mo CC, Karp JE, et al. Current approaches in the treatment of relapsed and refractory acute myeloid leukemia. J Clin Med. 2015;4(4):665–695. doi:10.3390/jcm4040665
- National Comprehensive Cancer Network (NCCN). NCCN guidelines acute myeloid leukemia (Version 1.2022). Accessed December 14, 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- Forsythe A, Sandman K. What does the economic burden of acute myeloid leukemia treatment look like for the next decade? An analysis of key findings, challenges and recommendations. J Blood Med. 2021;12:245–255. doi:10.2147/JBM.S279736
- Kennedy VE, Keegan THM, Li Q, et al. Frontline treatment patterns and outcomes among older adults with acute myeloid leukemia: a population-based analysis in the modern era. Cancer. 2022;128(1):139–149. doi:10.1002/cncr.33873
- Leisch M, Jansko B, Zaborsky N, et al. Next generation sequencing in AML-nn the way to becoming a new standard for treatment initiation and/or modulation? Cancers. 2019;11(2):252. doi:10.3390/cancers11020252
- Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. doi:10.1056/NEJMoa1112304
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50
- Lin TL, Levy MY. Acute myeloid leukemia: focus on novel therapeutic strategies. Clin Med Insights Oncol. 2012;6:205–217. doi:10.4137/CMO.S7244
- Talati C, Sweet K. Recently approved therapies in acute myeloid leukemia: a complex treatment landscape. Leuk Res. 2018;73:58–66. doi:10.1016/j.leukres.2018.09.001
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740. doi:10.1056/NEJMoa1902688
- Watson Health. IBM MarketScan research databases for life sciences researchers. Accessed December 10, 2021. Available from: https://www.ibm.com/products/marketscan-research-databases.
- Grinblatt DL, Pandya BJ, Han W, et al. Real-world use of FLT3-TKIs in R/R FLT3-mutated AML in the United States. Blood. 2020;136(Supplement 1):24–25. doi:10.1182/blood-2020-136391
- Jabbour E, Cortes J, Ravandi F, et al. Targeted therapies in hematology and their impact on patient care: chronic and acute myeloid leukemia. Semin Hematol. 2013;50(4):271–283. doi:10.1053/j.seminhematol.2013.09.006
- Nair R, Salinas-Illarena A, Baldauf HM. New strategies to treat AML: novel insights into AML survival pathways and combination therapies. Leukemia. 2021;35(2):299–311. doi:10.1038/s41375-020-01069-1
- Perl AE. The role of targeted therapy in the management of patients with AML. Hematology Am Soc Hematol Educ Program. 2017;2017(1):54–65. doi:10.1182/asheducation-2017.1.54
- Chevallier P, Labopin M, Turlure P, et al. A new leukemia prognostic scoring system for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia. 2011;25(6):939–944. doi:10.1038/leu.2011.25
- Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221. doi:10.1056/NEJMoa1516192
- Smith CC. The growing landscape of FLT3 inhibition in AML. Hematology Am Soc Hematol Educ Program. 2019;2019(1):539–547. doi:10.1182/hematology.2019000058
