To the Editor,
VENICE-II was an open-label, single-arm, phase 3b, study (NCT02980731) which assessed health-related quality of life (HRQoL) improvements in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) receiving venetoclax monotherapy, according to the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30), a self-report questionnaire validated for use in oncology studies [Citation1,Citation2] including the CLL16 module. The trial met the primary HRQoL endpoint at week 48 with a mean improvement in EORTC QLQ-C30 global health status (GHS)/QoL of +9.3 points (n = 156, 95% confidence interval (CI) 6.1–12.5; p = .004) [Citation1]. At week 48, there were also clinically meaningful improvements in the role functioning, fatigue, and insomnia domains of EORTC QLQ-C30, and the fatigue and future health domains of the CLL-16 module [Citation1]. Taken together, these data suggest that venetoclax monotherapy has a positive impact on HRQoL. Additionally, at week 48, median duration of response (DoR), time to progression (TTP), progression-free survival (PFS), and overall survival (OS) were not reached, and no new safety signals were reported. Follow-up data are now available up to week 108; this letter aims to provide updated HRQoL results, time-to-event endpoint analyses, and safety information from the VENICE-II trial.
Detailed methods for the trial have been previously published [Citation1]. In brief, the trial enrolled patients with relapsed/refractory CLL across 32 research sites in Latin America, Eastern Europe, and the Asia-Pacific area. Data were collected using the EORTC QLQ-C30 survey; scores were calculated based on the scoring manual and mean change from baseline was summarized descriptively along with interval estimates at each time point. Data for all respondents were included in QoL analyses at each timepoint; no data were censored. Improvement in EORTC QLQ-C30 GHS or functioning domains is indicated by a positive change, while improvement in symptoms or financial difficulties is indicated by a negative change. For EORTC QLQ-CLL16 symptom or problem scores, a negative change represents an improvement. For the primary endpoint, a ≥5-point change in the GHS/QoL scale indicated a clinically meaningful difference. For the remaining domains, a ≥10-point change in mean scores for either scale indicated a clinically meaningful difference [Citation1]. All time-to-event endpoints were analyzed using Kaplan–Meier’s methodology. Safety analyses included only treatment-emergent adverse events (AEs) with onset or worsening after the first dose of venetoclax and ≤30 days after discontinuation. Preferred terms were used according to the Medical Dictionary for Regulatory Activities (MedDRA) and severity was rated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTAE) v4.03.
Patient baseline demographics and primary endpoint data (at week 48) for the VENICE-II trial have been previously published (N = 210) [Citation1]. At the end of the trial (week 108), median (range) of venetoclax duration was 127 (0.1–218.3) weeks.
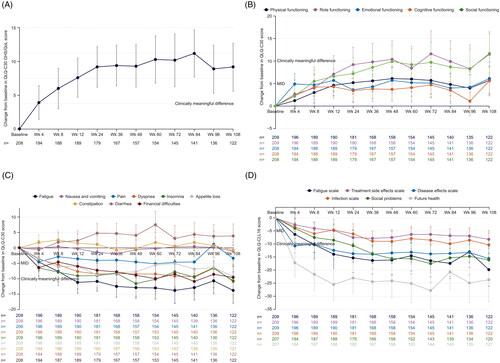
This current, updated analysis identified a mean (95% CI) change in EORTC QLQ-C30 GHS/QoL from baseline to week 108 of 9.2 (n = 122; 5.6–12.7), which was maintained from 9.3 (n = 157; 6.2–12.5) at week 48 (()). Clinically meaningful improvements in EORTC QLQ-C30 functioning subscales were seen at week 108 in role functioning (+11.5) and social functioning (+11.7; ()), and there were no clinically relevant deteriorations from baseline or from week 48. Similarly, clinically meaningful improvements in EORTC QLQ-C30 symptom subscales were seen at week 108 for fatigue (–13.8), dyspnea (–10.9), and insomnia (–10.7; ()), again with no clinically relevant deteriorations from baseline or from week 48. Clinically meaningful improvements were also seen at week 108 in EORTC QLQ-C30 CLL-16 subscales for fatigue (–19.8), disease effects (–15.6), infection (–10.3), social problems (–16.1), and future health (–23.7; ()) with no clinically relevant deteriorations from baseline or from week 48.
Figure 1. Mean change in EORTC QLQ-C30 GHS/QoL (A), functioning (B), and symptoms (C) subscales, and EORTC QLQ-CLL16 subscales (D) from baseline to week 108 in all treated patients. Overall changes from baseline in a patient-reported EORTC QLQ-C30 (A–C) and EORTC QLQ-CLL16 (D) scores until week 108 of venetoclax treatment. (A) ≥5-point change indicated a clinically meaningful difference for the GHS/QoL subscale; error bars are 95% CI. (B, C, D) Clinical relevance of changes in HRQoL was determined based on the MID of values from baseline to each assessment time point. Five- to ten-point considered a little change while a ≥10-point change indicated a clinically meaningful difference. Improvement in functional subscales are indicated by positive change; improvement in symptom subscales are indicated by negative change. BL: baseline; CI: confidence interval; EORTC: European Organization for Research and Treatment of Cancer; QLQ-C30: Quality of Life Core Questionnaire Core 30; QLQ-CLL16: Quality of Life Questionnaire Chronic Lymphocytic Leukemia Module 16; GHS: global health status; HRQoL: health-related quality of life; MID: minimum important difference; OS: overall survival; PFS: progression-free survival; QoL: quality of life; Wk: week.
At the end of the trial, the overall response rate was 77% (n = 161), with a complete response (CR) or CR with incomplete marrow recovery reported in 19% (n = 39) patients; responses were maintained in the majority of patients, with a 12-month DOR estimate of 92.5% (95% CI: 87.1–95.7%). Median time-to-event endpoints were reached by the end of the study for all endpoints except OS. The median DoR, TTP, and PFS for the overall population were 36.0 months (95% CI: 30.0–38.9), 43.0 months (95% CI: 33.4–47.2), and 35.4 months (95% CI: 32.9–42.9), respectively. At week 108, median OS for the overall population was not reached (95% CI: 53.1 months – not estimable), and a total of 145 patients (69%) were alive at the end of follow-up. The 24-month OS estimate was 80.4% (95% CI: 74.3–85.3%).
The overall safety profile for venetoclax was similar at week 108 as described at week 48. By week 108, 95% patients (n = 199) had experienced an AE (). AEs that led to venetoclax dose reduction, interruption and discontinuation were experienced by 22% (n = 46), 52% (n = 110), and 19% (n = 40), respectively. A second primary malignancy event was experienced by 13% (n = 28) patients. Sixty-five patients died during the study, 11% (n = 24) of whom died within 30 days of the last dose of venetoclax, and 8% (n = 16) of whom died from AEs. Fatal AEs were most frequently reported within the system organ classes infections and infestations and nervous system disorders (2% (n = 5) each). The only fatal AE that was reported for more than one patient was COVID-19 pneumonia (1% (n = 2)).
Table 1. Summary of any grade and grade ≥3 treatment emergent AEs occurring in ≥10% of patients and serious AEs occurring in ≥3% of patients treated with venetoclax.
There is a lack of existing literature with respect to HRQoL for patients with CLL, and our understanding of HRQoL of patients is constantly evolving [Citation3]. This may be due, in part, to the intertrial variability of HRQoL assessment tools, which limits cross-trial comparisons. However, recent studies have identified fatigue as a significant HRQoL burden experienced by patients with CLL [Citation2,Citation4], which highlights the relevance of the sustained meaningful improvement in fatigue seen in VENICE-II from week 48 to week 108. As illustrated in this study, venetoclax initiation is associated with improvements in various aspects of HRQoL. In the VENICE-II trial, venetoclax monotherapy conferred sustained HRQoL improvements in a cohort of patients including many with high disease burden, poor prognostic features (i.e., 20% patients had del17p, 13% patients had mutated TP53, and 38% of patients received ≥3 prior therapies), or who had been previously treated with BCRi (19% patients) [Citation1]. Clinically meaningful important differences in the role functioning and social functioning domains were sustained during venetoclax treatment; these domains are relevant to participants’ self-reported ability to work and to engage in social and family activities. These improvements in HRQoL become particularly important when selecting between therapies of comparable efficacy [Citation4,Citation5]. As such, there is an increasing need to include patient preferences, patient values, and other combinatorial factors in clinical decision-making.
The overall safety profile was consistent with previously published venetoclax data in R/R CLL and no new safety signals were identified. The safety profile at week 108 was also consistent with that at week 48. Pneumonia was the most common serious AE and is a known risk among patients receiving CLL therapy.
The results of this study highlight the need for consideration of patient-centric factors such as HRQoL in making treatment decisions in an evolving CLL treatment landscape. With newer treatments providing increased long-term OS and PFS, HRQoL must be considered in the context of AEs and treatment-related toxicity; future analyses assessing correlations between AE incidence and HRQoL data would be informative. Limitations of the study include that at the end of follow-up, data were not sufficiently mature to generate a median OS estimate, and few events were observed for time-to-event analyses. However, this updated analysis from this phase 3b trial confirms that venetoclax monotherapy is an effective therapy, which confers sustained improvements in several aspects of QoL for patients with R/R CLL.
Acknowledgements
AbbVie and authors thank the patients and their families, investigators, study coordinators, and support staff. Medical writing support was provided by Hayley Ellis, PhD, of Fishawack Communications Ltd, funded by AbbVie.
Disclosure statement
Tara Cochrane: Honorarium: Celgene. Research funding: Beigene. Alicia Enrico: Speaker fees: Bristol, Novartis, and AbbVie. David Gomez-Almaguer: Advisory and speaker boards: AbbVie, Takeda, Celgene, Janssen, Roche, and Amgen. Evgueniy Hadjiev: Advisory and speaker boards: AbbVie, Janssen, Roche, Novartis, Genzyme, Pfizer, and Takeda. Ewa Lech-Maranda: Advisory board: AbbVie, Amgen, Janssen, Roche, Novartis, and Celgene. Tamas Masszi: Advisory board: AbbVie, Janssen-Cilag. Eugene Nikitin: Honoraria and speaker fees: AbbVie. Tadeusz Robak: Research grants: AbbVie, BeiGene, Janssen, Roche, AstraZeneca, UCB, Octapharma, Pfizer, Novartis, and Celgene; honoraria: Roche, AbbVie, BeiGene, Janssen, AstraZeneca, and Novartis; conference travel support: Roche, Janssen, and AbbVie. Robert Weinkove: Speaker fees: AbbVie and Janssen; honoraria and advisory boards: AbbVie and Janssen. Shang-Ju Wu: Speaker fees: AbbVie. Beenish S. Manzoor, Todd Busman, Madhavi Pai, and Viktor Komlosi: Employees of AbbVie and may hold stock or options. Mary Ann Anderson: Honoraria: AbbVie, CSL, Novartis, AstraZeneca, BeiGene, and Janssen; support for attending meetings and/or travel: support from AstraZeneca to attend EHA; employee of the Walter and Eliza Hall Institute, which receives payments in relation to venetoclax. Venetoclax is being developed in collaboration between AbbVie, Inc., and Genentech. AbbVie, Inc., sponsored the study (NCT02980731), contributed to the analysis, and interpretation of the data, and participated in the writing, review, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this manuscript. No honoraria or payments were made for authorship.
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select ‘Home’.
Additional information
Funding
References
- Cochrane T, Enrico A, Gomez-Almaguer D, et al. Impact of venetoclax monotherapy on the quality of life of patients with relapsed or refractory chronic lymphocytic leukemia: results from the phase 3b VENICE II trial. Leuk Lymphoma. 2022;63(2):304–314. doi:10.1080/10428194.2021.1986217
- Youron P, Singh C, Jindal N, et al. Quality of life in patients of chronic lymphocytic leukemia using the EORTC QLQ-C30 and QLQ-CLL17 questionnaire. Eur J Haematol. 2020;105(6):755–762. doi:10.1111/ejh.13503
- Waweru C, Kaur S, Sharma S, et al. Health-related quality of life and economic burden of chronic lymphocytic leukemia in the era of novel targeted agents. Curr Med Res Opin. 2020;36(9):1481–1495. doi:10.1080/03007995.2020.1784120
- Molica S. Quality of life in chronic lymphocytic leukemia: a neglected issue. Leuk Lymphoma. 2005;46(12):1709–1714. doi:10.1080/10428190500244183
- Efficace F, Kemmler G, Vignetti M, et al. Health-related quality of life assessment and reported outcomes in leukaemia randomised controlled trials – a systematic review to evaluate the added value in supporting clinical decision making. Eur J Cancer. 2008;44(11):1497–1506. doi:10.1016/j.ejca.2008.03.017
